UP Board Class 11 Chemistry Chapter 9 Hydrogen Multiple Choice Questions
Question 1. An alkene (molecular formula : C5H10) on ozonolysis forms acetone as one of the products. The alkene is—
- 2-methyl-1-butene
- 3-methyl-1-butene
- 2-methyl-2-butene
- Cyclopentane
Answer: 2. 3-methyl-1-butene
Question 2. Which of the following compounds can be used to prepare both ethylene and acetylene—
- CH3CH2OH
- BrCH2CH2Br
- CH 3CH2Br
- BrCH2CH2OH
Answer: 2. BrCH2CH2Br
Question 3. Alkyl chloride on dehydrochlorination produces 2 alkenes (C6H12) which on ozonolysis form four compounds—
- CH3CHO
- CH3CH2CHO,
- CH3COCH3 and
- (CH3)2CHCHO.
The alkenes are—
- 4-methylpent-2-ene and 2-methylpent-2-ene
- 2-methyl pent-2-ene and 2,3-dimethyl but-2-ene
- 4-methylpent-2-en§ and hex-3-ene
- 2-methylpent-2-ene and hex-3-ene
Answer: 1. 4-methyl pent-2-ene and 2-methyl pent-2-ene
Question 4. The compound that exhibits geometrical isomerism is—
- C2H5Br
- (CH)2(COOH)2
- CH3CHO
- (CH2)2(COOH)2
Answer: 2. (CH)2(COOH)2
Question 5 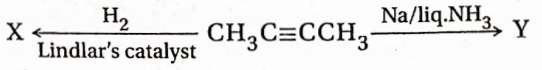
- X: cis-2-butene and Y: frans-2-butene
- X: trans-2-butene and Y :cis-2-butene
- X, Y both are cis-2-butene
- X, Y both are trans-2-butene
Answer: 1. X: cis-2-butene and Y: frans-2-butene
Question 6. An alkene may be formed from a carbocation if—
- One H- ion gets eliminated
- One H+ ion gets added
- One H+ ion gets eliminated
- One H- ion gets added
Answer: 3. One H+ ion gets eliminated
Question 7. The number of moles of water produced when one mole acetylene undergoes complete combustion is—
- 1 mol
- 2 mol
- 3 mol
- 4 mol
Answer: 1. 1 mol
Question 8. 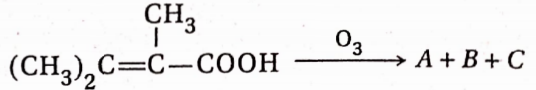 , A, B, C in the above reaction are respectively—
, A, B, C in the above reaction are respectively—
- CH3COCH3, CH3CHO, CO2
- CH3COCOOH, CH3COOH, CO2
- CH3CH2COOH, CH3CHO, CO2
- CH3COCH3, CHgCOOH, CO2
Answer: 4. CH3COCH3, CHgCOOH, CO2
Question 9. The position of the double bond in an alkene can be determined by—
- Hydrogenation
- Ozonolysis
- Hydroxylation
- Hydroboration
Answer: 2. Ozonolysis
Question 20. Heavy water reacts with calcium carbide to form—
- CaD2
- C2D2
- Ca2D2O
- CD2
Answer: 2. C2D2
Question 21. In case of trisubstituted benzene, if the substituents are different, then the number of isomers will be
- 5
- 8
- 6
- 10
Answer: 4. 10
Question 22. The chemical formula ofCetane is—
- C6H12
- (CH3)3C(CH2)11CH3
- CH3(CH2)14CH3
- (C2H5)4C
Answer: 3. CH3(CH2)14CH3
Question 23. Which of the following gets converted into an explosive when it is turned into liquid by applying high pressure—
- Propane
- n-butane
- Isobutane
- Acetylene
Answer: 4. Acetylene
Question 24. The product which is not obtained when ethylene reacts with K3 mixed with Br2/H2O is—
- BrCHCH2Br
- BrCH2CH2OH
- HOCH2CH2OH
- BrCH2CH2I
Answer: 3. HOCH2CH2OH
Question 25. Which of the following does not form a sooty flame—
- Toluene
- Benzene
- Mesitylene
- Butane
Answer: 4. Butane
Question 26. Which of the following statements is incorrect—
- Delocalisation of electrons occur between two n bonds in a propadiene molecule
- Delocalisation of electrons occur between two n bonds in a molecule of 1, 3-butadiene
- Cumulated polyenes with odd number of double bonds exhibit geometrical isomerism if their terminal groups are different
- Cumulated polyenes with even number of double bonds exhibit optical isomerism if their terminal groups are different
Answer: 1. Delocalisation of electrons occur between two n bonds in a propadiene molecule
Question 27. Which of the given is a benzenoid aromatic compound—
- Anthracene
- Pyrrole
- Pyridine
- Cyclopentadienyl anion
Answer: 1. Anthracene
Question 28. Gas used in Hawker’s lamp for emitting bright light is—
- Acetylene
- Ethylene
- Methane
- Propane
Answer: 1. Acetylene
Question 29. The compounds which exist as liquids are—
- C5H12
- C3H8
- C2H6
- C7H16
Answer: 1,4
Question 30. Which of the given can be prepared by Wurtz reaction–
- 2-methylpropane
- 2,3-dimethyl butane
- Hexane
- All of them
Answer: 2,3
Question 31. Which of the following compounds do not produce acetylene on hydrolysis—
- CaC2
- Al4C3
- Be2C
- Zn(CH4)2
Answer: 2,3,4
Question 32. Which of the following options are correct with respect to Friedel-Crafts reaction —
- Alkylation Reagent: CH2=C6H5Cl
- Solvent: C6H5NO2, CS2
- Catalyst: AlCl3 , H2SO4
- All Of the Above
Answer: 2,3
Question 33. Lewisite and its antidote are—
- Lewisite ClCH=CHAsC12
- Antidote 1,1-dimercapto-l-propanol
- Lewisite CH2=CHAsCl2
- Antidote 2,3-dimercapto-l-propanol
Answer: 1,4
Question 34. Halogenation ofan alkene is a or an—
- Substitution reaction
- Elimination reaction
- Addition reaction
- Oxidation reaction
Answer: 1,4
Question 35. During detection of unsaturation in an unknown organic compound disappearance of the violet colour of dilute and cold KMn04 solution indicate—
- Presence of ethylenic unsaturation in the compound
- The presence of a group in the compound which gets easily oxidised by kmn04
- Presence of only single covalent bond in the compound
- All of the above are true
Answer: 1,2
Question 36. Which of the following options is correct—
- Ortho- or para-orienting: — NR2, —NHCOCH3
- Mete-orienting: —NO3, —Cl
- Ortho- or para-orienting: — CF3, —SO3H
- Mete-orienting: —CHO, —COR
Answer: 1,4
Question 37. Which of the following statements are true for Kolbe’s electrolytic method—
- It is an effective method for preparing symmetrical alkanes
- Reduction of carboxylate ion occurs at the anode
- Platinum electrodes are used in this method
- Methane cannot be prepared by this method
Answer: 1,3,4
Question 38. 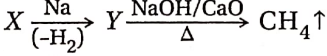 In this reaction, X and Y are-
In this reaction, X and Y are-
- X = CH3COOH
- X = HCOOH
- F = CH3COONa
- Y = C2H5COONa
Answer: 1,3
