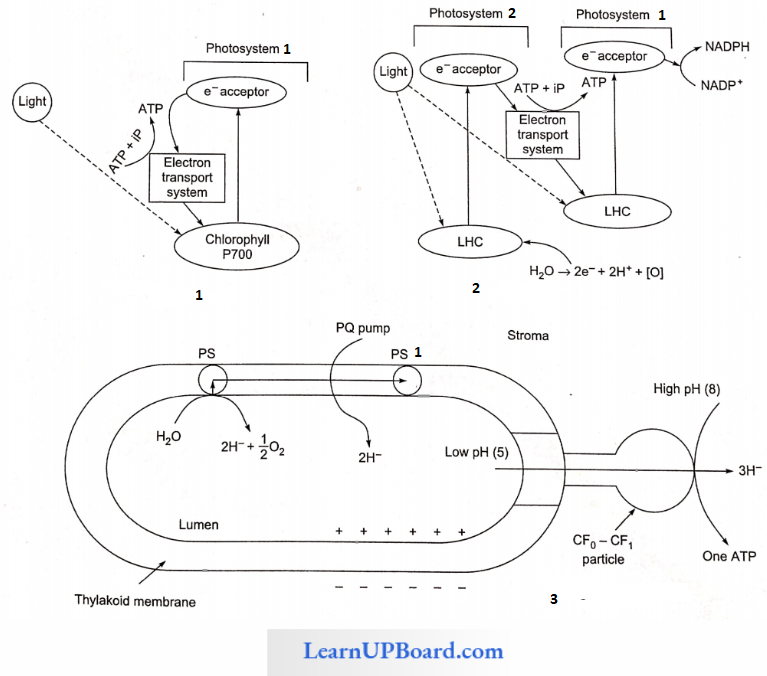
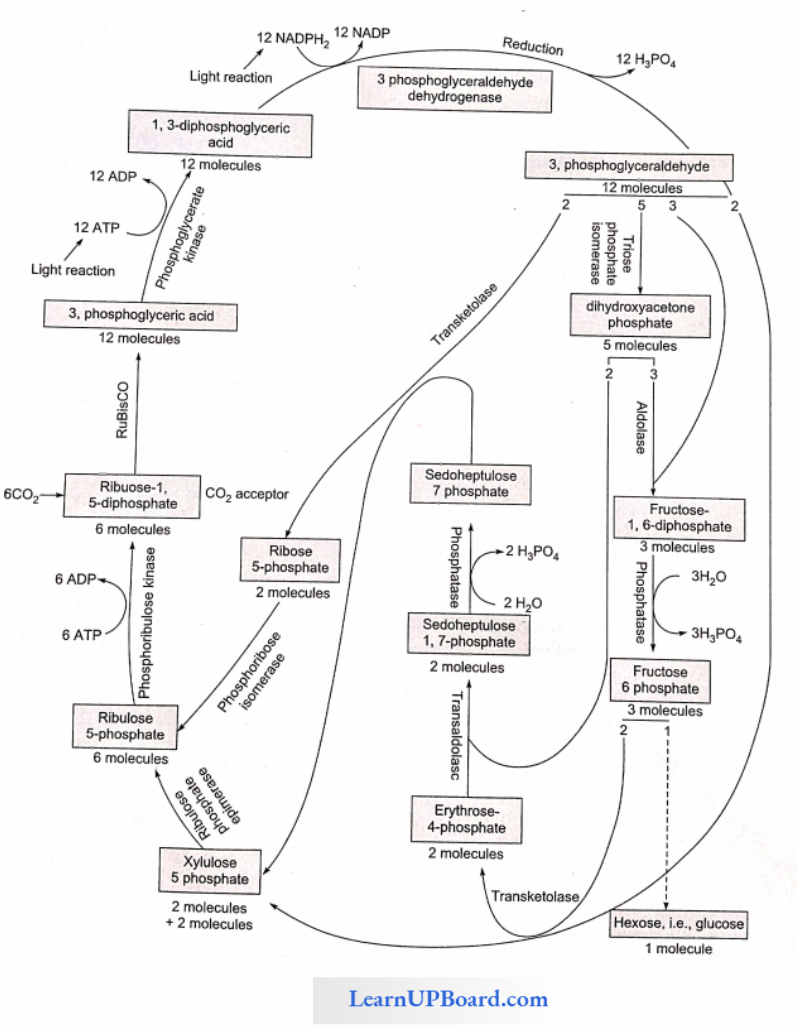
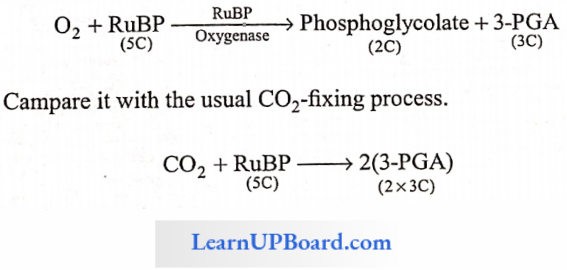
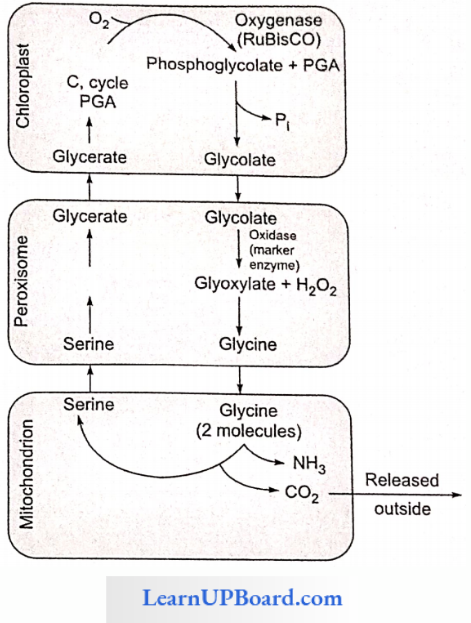
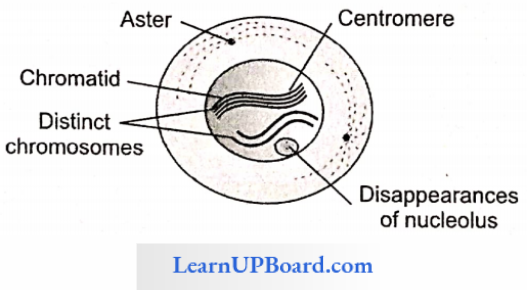
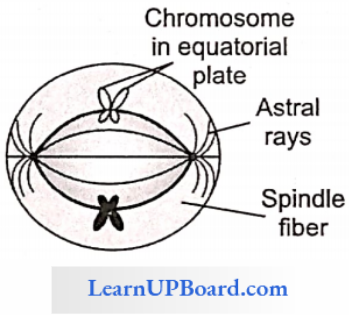
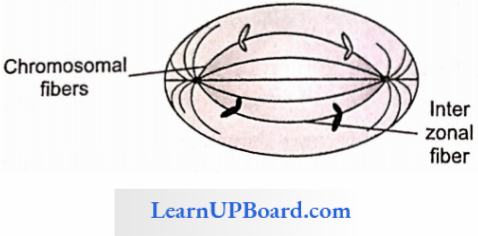
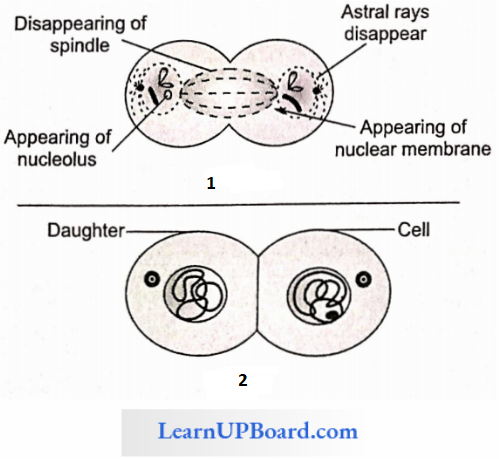
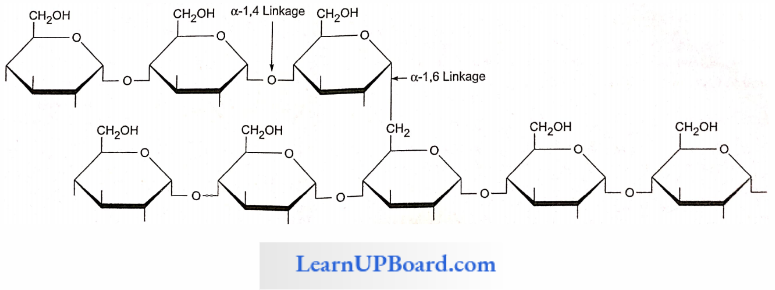
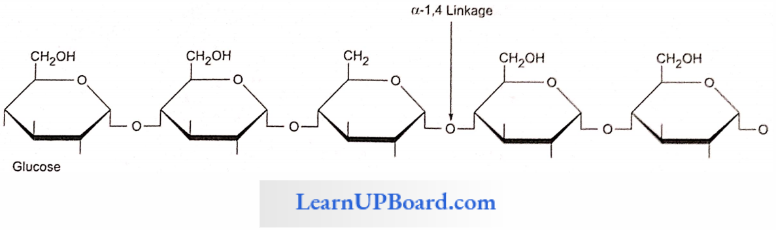
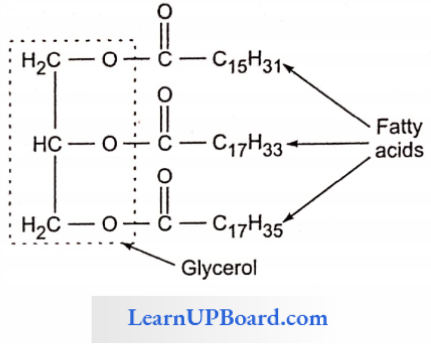
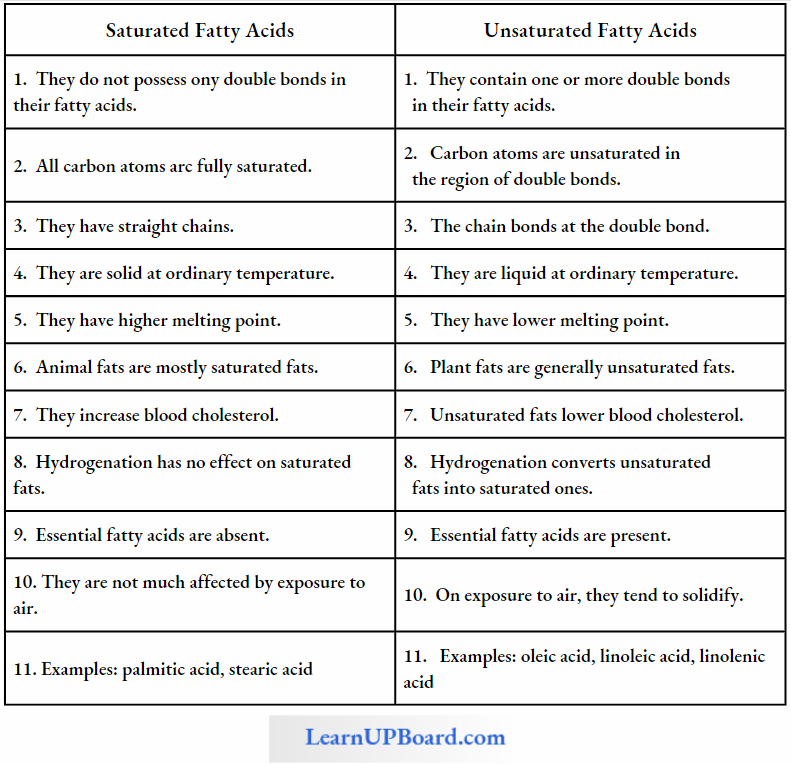
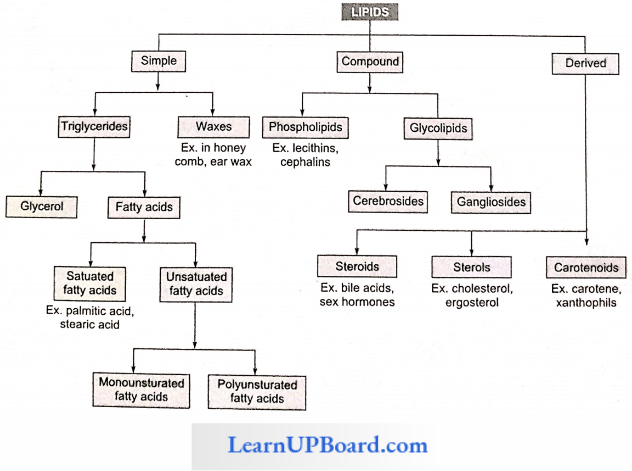
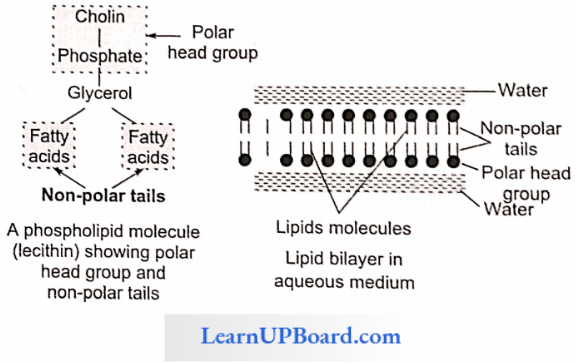
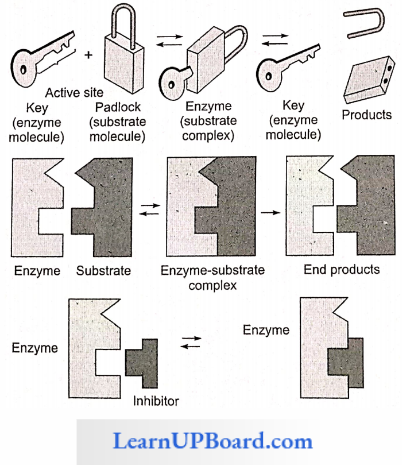
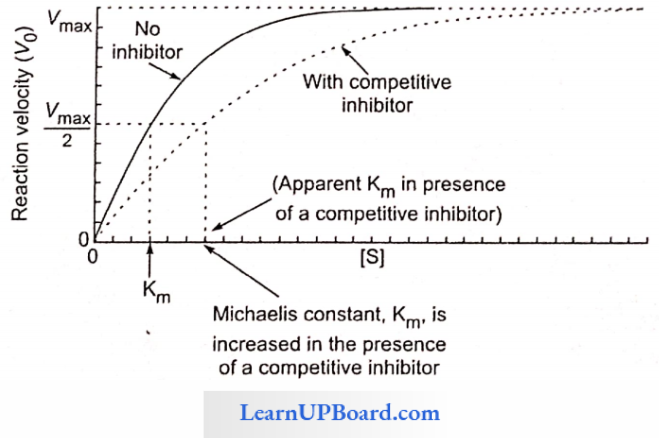
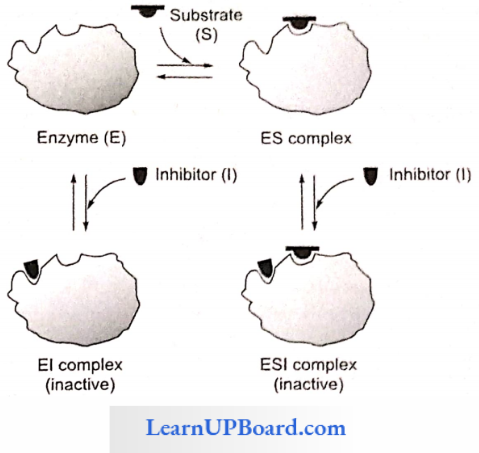
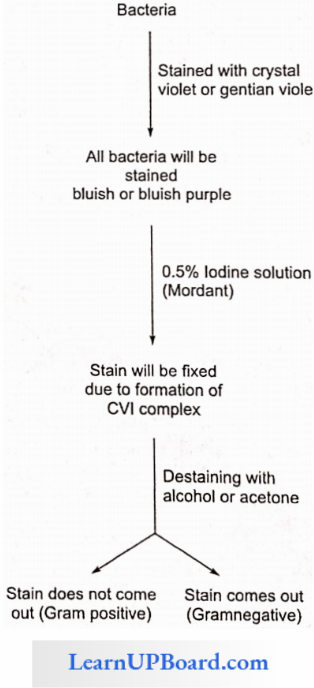
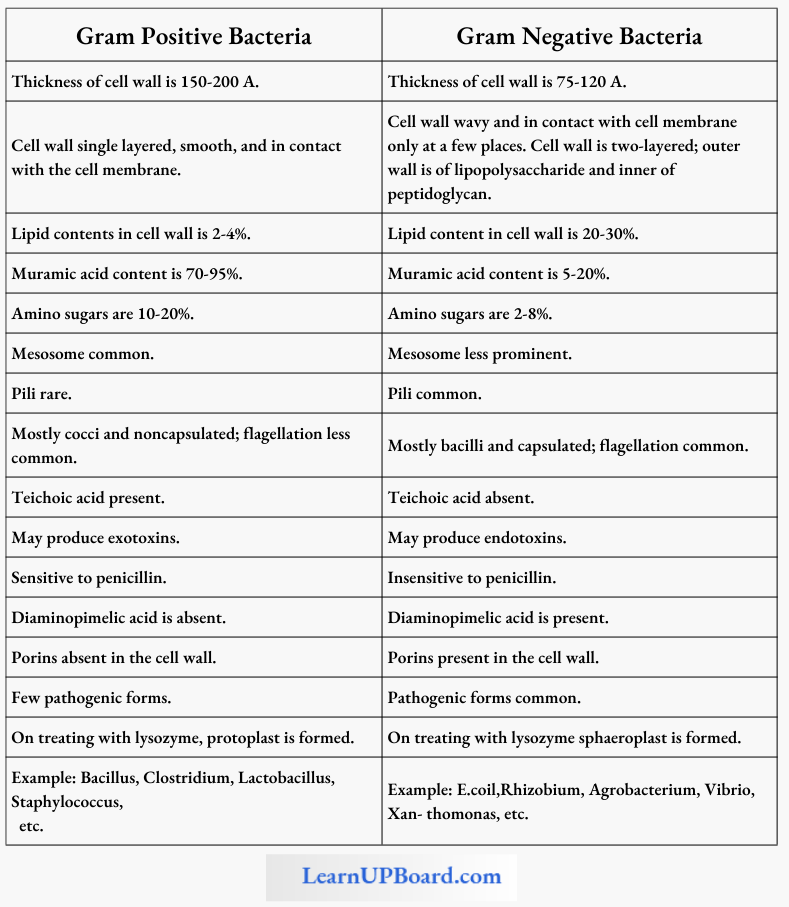
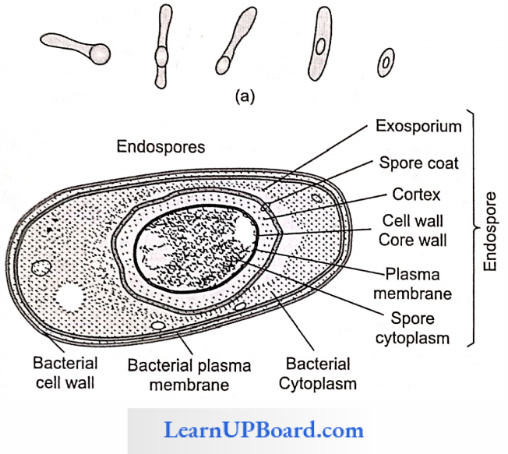
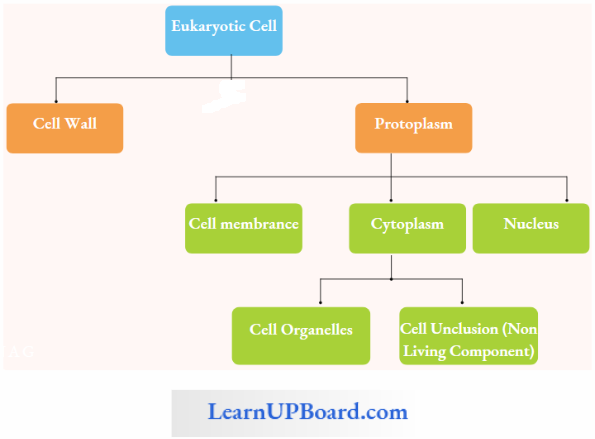
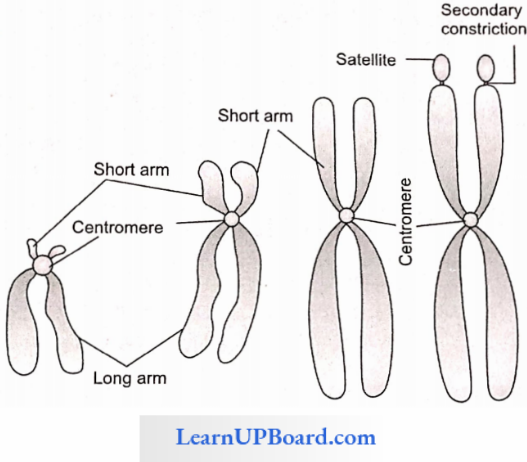
NEET Biology Notes Transport In Plant Importance Of Water
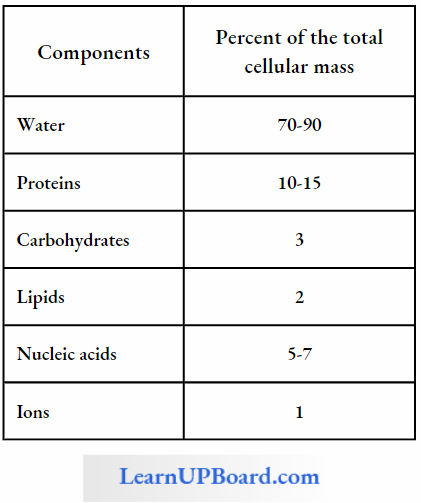
Water is one of the most important constituents of the protoplasm. It makes up 80-90% of the fresh weight of most herbaceous plants and over 50% of the fresh weight of woody plants. It acts as a solvent in which the oases, minerals, and other solutes enter the plant cells and move from cell to cell and from organ to organ.
- Water is a reactant or reagent in many important processes such as the hydrolysis of starch to sugar. Water maintains turgidity which is essential for cell enlargement, growth, and maintaining the form of herbaceous plants.
- Turgor is also important in the opening and closing of stomata, movement of leaves, flower petals, and various specialized plant structures. Water is also needed for the hydration of protoplasm, in the mobility of gametes, dehiscence, etc.
Diffusion: Diffusion is the movement of particles (or molecules or ions) of a substance from a region of its higher concentration to the region of lower concentration down the concentration gradient until the molecules are evenly distributed throughout the available space.
The rate and direction of a diffusing substance depend upon the concentration of that substance at different spots and is independent of the presence of other diffusing substances. The rate of diffusion is affected by many factors, for example,
transport plants
Rate ∝ Temperature
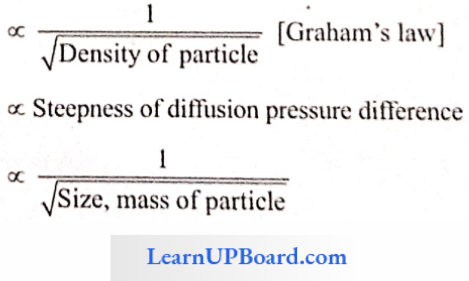
Diffusion Pressure (DP): It is the pressure exerted by a substance due to the tendency of its particles to diffuse. It is also defined as the potential ability of a molecule or ion to diffuse. It is directly proportional to the diffusing particles.
DP ∝ Concentration
DP ∝ Temperature
The DP of pure water is maximum.
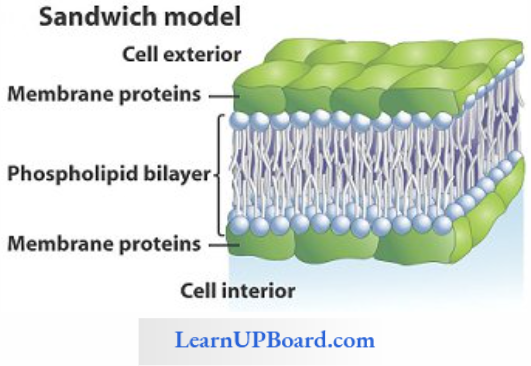
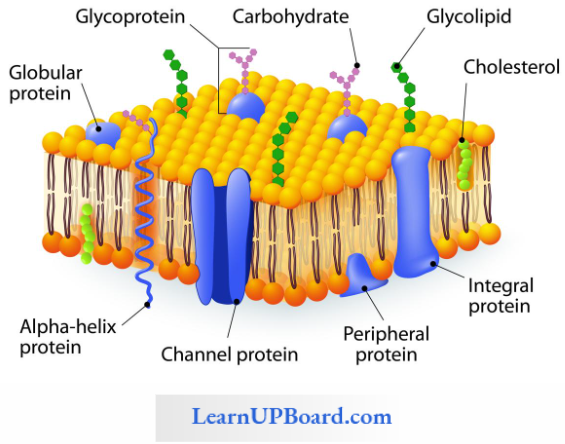
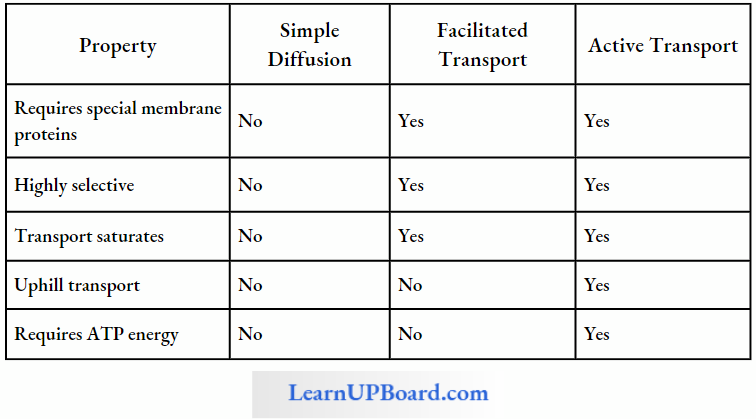
Facilitated Diffusion: Particles that are lipid soluble can easily pass directly through the cell membrane as it is mainly made of it. The hydrophilic solutes find it difficult to pass through the membrane. Their movement has to be facilitated. For this, the membranes possess aquaporins and ion channels.
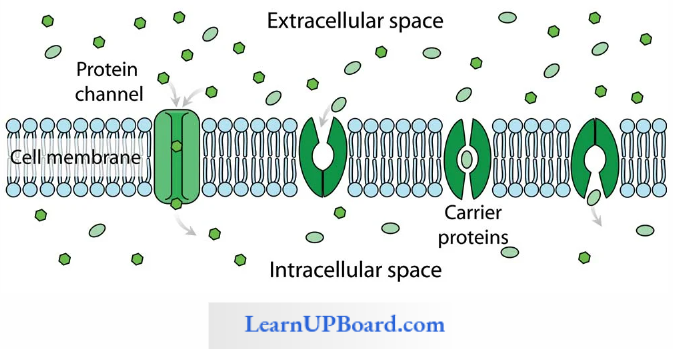
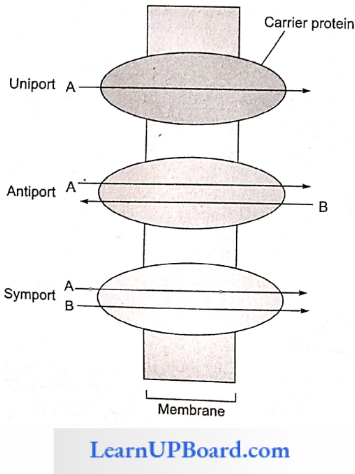
Some carrier proteins allow transport only if two types of molecules move together. This is called cotransport. It is of two types. In the symport method of cotransport, both molecules cross the membrane in the same direction at the same time. In the antiport method of cotransport, both molecules move in opposite directions. When a molecule moves across a membrane independent of another molecule, the process is called uniport.
Read and Learn More NEET Biology Notes
NEET Biology Notes Transport In Plant Osmosis (Diffusion Through Membrane)
Osmosis may be defined as “the passage of solvent molecules from a region of their higher concentration to a region of their lower concentration through a semipermeable membrane.” In all biological systems, the solvent is water.
Types Of Membranes
- Permeable Membranes: Such membranes allow diffusion of both solvent and solute molecules or ions through them, for example, the cellulose wall of cells. Lignified cell walls are also quite freely permeable to water.
- Impermeable Membranes: Such membranes prohibit the diffusion of both solvent and solute particles through them, for example, heavily cutinized or suberized cell walls in plants.
- Semipermeable Membranes: Such membranes allow the diffusion of solvent molecules but do not allow the passage of solute molecules. Such membranes form a perfect partition between two osmometers, for example, membranes of collodion, parchment paper, and copper ferrocyanide membranes.
Differentially Permeable Membranes: However, all membranes found in plants allow some solutes to pass through them along with the solvent molecules. Such membranes are called differentially permeable membranes, for example, all biological membranes.
NEET Biology Notes Transport In Plant Osmotic Pressure
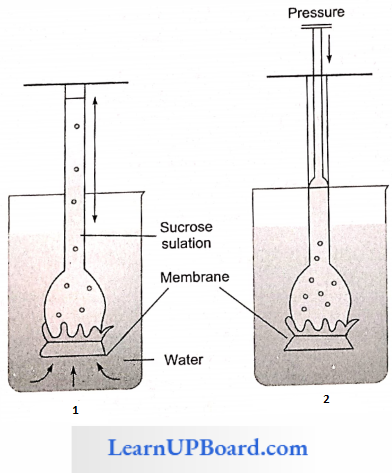
Osmotic pressure (OP) is the “maximum pressure which develops in a solution when it is separated from pure water by a semipermeable membrane.” It is also defined as “the pressure needed to prevent the passage of pure water into an aqueous solution through a semipermeable membrane thereby preventing an increase in the volume of the solution”.
The osmotic pressure depends upon
- The concentration of solute particles,
- Ionization of solute particles,
- Hydration of solute particles, and
- Temperature.
An increase in the concentration of solutes in the solution increases the osmotic pressure. If the solute ionizes in the solution, the number of particles increases, thus raising the osmotic pressure. If the solute molecules are hydrated, the water molecules bound with the solute will be unable to diffuse and hence increase the osmotic pressure.
” transportation in plants”
- An increase in the temperature raises the osmotic pressure of the solution.
- Plant cells exhibit a considerable range of variations in osmotic pressure. In land plants, it varies from 5 to 30 atm.
- In aquatic plants, it varies from 1 to 3 atm. Plants of arid regions possess high osmotic pressure. The highest osmotic pressure is recorded in the halophytic plant, Atriplex confertifolia, i.e., 202.5 atm.
- The osmotic pressure of an electrolyte is two to three times greater than a non-electrolyte.
- Osmotic pressure can be calculated by OP = miRT where m is the molar concentration, i is the ionization constant, R is the gas constant, and T is the temperature (273 °C) (π). Osmotic pressure is numerically equal to osmotic/solute potential (ψs) but has a positive value ψs = π.
Measurement Of Osmotic Pressure: The methods for measuring osmotic pressure are
- Plasmolytic method by de Vries
- Pleffer osmometer
- Berkeley/Hartley osmometer
- Cryoscopic osmometer
NEET Biology Notes Transport In Plant Turgidity And Turgor Pressure
If a plant cell is placed in a hypotonic solution or pure water (i.e., a solution of higher water potential), the water starts moving into the cell by osmosis. As the volume of the protoplast increases, it begins to exert pressure against the cell wall and thus stretches it.
- The pressure exerted by the protoplast against the cell wall is called turgor pressure (TP). The cell wall, being rigid, exerts an equal and opposite pressure on the protoplast, which is called wall pressure (WP).
- The two pressures are equal and opposite in direction. As the turgor pressure of the cell increases, the cell becomes turgid. However, a stage is reached when osmotic pressure is exactly balanced by turgor pressure.
- At this point, the amount of water leaving the cell equals that entering the cell. Hence, there is no further net movement of water and the cell is said to be in equilibrium with the exterior solution.
NEET Biology Notes Transport In Plant Diffusion Pressure Deficit (Suction Pressure)
The diffusion pressure of a pure solvent is maximum. The addition of solutes results in a decrease in the diffusion pressure. This deficit is termed diffusion pressure deficit (DPD). The greater the concentration of a solution, the greater is its DPD.
- Such a solution will tend to take up water and become normal; therefore, water moves from low DPD to high DPD. The value of DPD for a cell is always positive. DPD for a cell is always positive and is calculated as (QP – TP).
- DPD is an index of the water-absorbing capacity of cell. In a flaccid cell, TP = 0. Therefore, DPD = OP.
- In a turgid cell, the entry of water into the cell causes the development of TP. At a point, TP = OP. Therefore, DPD = 0. Therefore, in a fully turgid cell, there is no net movement of water into the cell.
NEET Biology Notes Transport In Plant Chemical Potential
Chemical potential is the free energy of one mole of a particular chemical in a multi-component system. The lamer the chemical potential of a substance, the greater its tendency to undergo a chemical reaction. The chemical potential of water is called water potential and is given the value of zero at prevailing temperature and pressure.
NEET Biology Notes Transport In Plant Water Potential
Currently, the term water potential is used by biologists to describe the tendency of water molecules to move from one place to another. It is denoted by the symbol “ψ” (the Greek letter, psi).
Components Of Water Potential: Water potential is the difference in the free energy per unit molal volume of water in the pure state and in a system. For a solution, the value of water potential is always less than zero or negative. Water potential is determined by the following relation
Water Potential (ψ) = Solute potential (ψs) + Pressure potential (ψp)
Solute Potential (ψs): Also called osmotic potential, it is defined as the amount by which Tyr is reduced as a result of the addition of solute. It is always negative.
Pressure Potential (ψp): It is equal to TP and is positive except in plasmolyzed cell and in xylem vessels where it is negative. In terms of water potential, osmosis can be redefined as “the movement of water molecules from a region of higher water potential to the region of lower water potential through a differentially permeable membrane.”
Water potential may be regarded as the tendency of water to leave a system. The more the water potential, the greater is the tendency to leave. If two systems are in contact, water moves from the system with higher water potential to that with lower water potential or less negative ψw to more negative ψw.
Importance Of Osmosis: Osmosis is involved in
- Water absorption by roots
- Maintenance of turgidity
- Turgor movements in Mimosa and Desmodium.
- Stomatal movement
- Self-dispersal of fruits (autochory)
- Drought and frost resistance
NEET Biology Notes Transport In Plant Plasmolysis
When a cell is placed in a hypertonic solution, exosmosis occurs. Due to the loss of water to the external solution, TP of the cell decreases, hence the cell shape slightly changes. This stage is called limiting plasmolysis.
- If the cell continues to be in a hypertonic solution, more and more water is lost, therefore protoplast starts shrinking. It first leaves the comers of the cell and this stage is called as incipient plasmolysis.
- If more water is lost, the protoplast shrinks but remains in contact with the wall in one or two places. The space between the cell wall and the protoplast is occupied by the outer solution. This stage is called evident plasmolysis. The turgor pressure of a cell at limiting plasmolysis is equal to zero, whereas at incipient plasmolysis and evident plasmolysis, TP of the cell is negative.
NEET Biology Notes Transport In Plant Imbibition
The adsorption as well as absorption of a liquid by a solid without forming a solution is called as imbibition. The solid sub¬stance is called imbibing, whereas liquid is called as imbibe. The liquid particles are held in between the particles of solid by adsorption and capillarity.
The phenomenon depends upon the affinity of imbibant to imbibe. As a result of imbibition, the following events occur
Volume Change: The volume of the system increases, i.e., swelling. The total volume of water imbibed, plus the imbibing material is less after imbibition than before.
Heat Release: This is due to the compact arrangement of water molecules which liberate some of their kinetic energy.
Development Of Pressure: If the imbibing is confined, pressure may be developed. For example, in dry pea seeds, it is 1000 bars.
“transport in plants “
Imbibants present in plants are generally hydrophilic in nature. Among plant imbibing, hydrocolloids (for example, agar-agar, etc.) are the best imbibing followed by proteins, starch, and cellulose. Imbibition is affected by a number of factors such as temperature, pressure, etc., against the imbibing surface, the texture of imbibing, electrolytes, and pH.
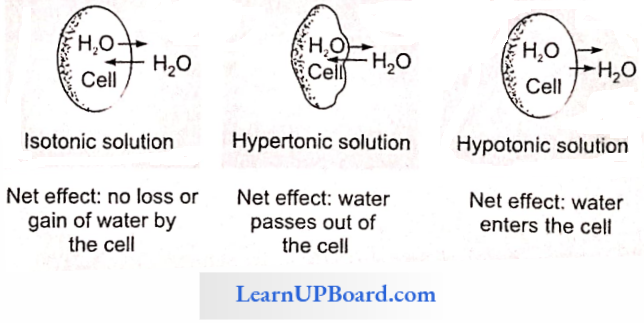
NEET Biology Notes Transport In Plant Hypotonic Hypertonic And Isotonic Solutions
A solution with a higher concentration of solutes is said to be the hypertonic solution. A lower concentration of solutes in a solution is called a hypotonic solution, and two solutions having the same concentration of solutes are called isotonic solutions.
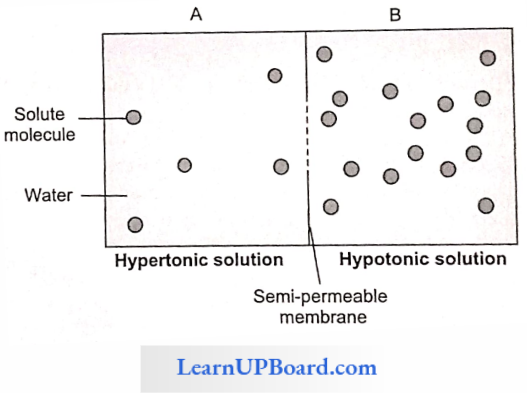
If a cell is placed in pure water or hypotonic solution, there is a net movement of water into the cell (endosmosis); if it is placed in a hypertonic solution, there is a net movement of water from the cell outwards (exosmosis); if the cell is placed in an isotonic solution, the amount of water leaving the cell equals that entering the cell and therefore, there is no net movement of water.
NEET Biology Notes Transport In Plant Classification Of Soil Moisture
The amount of water that can be held by soil depends upon the total pore space in the SCA. Water is present in the spaces between the soil. Various types of groundwater are
Run-off Water: Water that flows along the surface of the soil and reaches to the nearest water body constitutes run-off water. This water is not available to the plants.
Gravitational Water: Water that percolates down through the soil macropores (50 piM diameter) under the influence of gravity and reaches to the water table is called gravitational water. This type of water is also not available to the plants.
Capillary Water: Water retained by soil micropores (20 pm diameter) in the form of fine capillaries constitutes capillary water. This type of water is available to the plants.
Hygroscopic Water: Water held in the form of a very thin film around the soil particles due to adsorption constitutes hygroscopic water. This type of water is not available to the plant.
Chemically Combined Water: Water that combines with inorganic salts of the soil in the form of water of hydration constitutes chemically combined water. This type of water is also not available to the plants.
Water Vapors: They are present in soil air spaces. Normally, they are not available to the plants. Under certain conditions, they are useful in the phenomenon of night recovery.
NEET Biology Notes Transport In Plant Absorption Of Water And Pathway Of Water Across The Root
Water is mainly absorbed by the root hair zone. Root hairs are tubular elongations of the external wall of epiblema cells. They range in length from less than a millimeter to about a centimeter and are usually 10 pm in diameter. They have a vacuole filled with salt, sugars, and organic acid.
Hence, the osmotic pressure of the root hair cell is high (approx. 3-8 atm) as compared to that of soil sap (approx, less than 1 atm). The movement of water from the root hair cell to the xylem may occur by two methods.
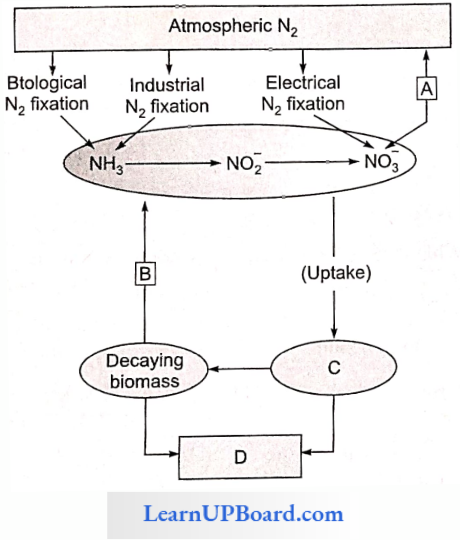
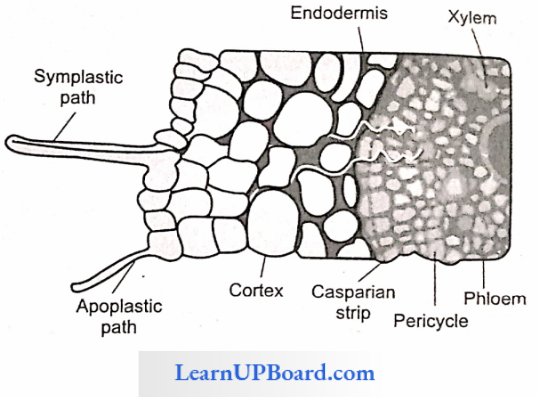
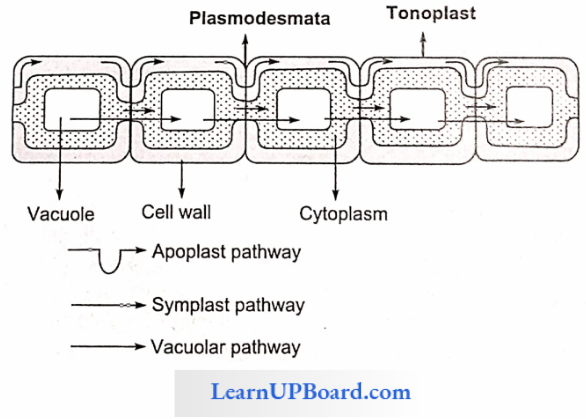
- Apoplast Pathway: In this method, water passes from the root hair cell to the xylem through the walls of intervening cells without crossing any membrane or cytoplasm. The apoplastic movement of water beyond the cortex is blocked due to the presence of Casparian strips in the endodermal cells. The major movement of water through cortical cells occurs by this method, as cortical cells offer the least resistance.
- Symplast Pathway: In this method, water passes from cell to cell by crossing the plasma membrane. Therefore, it is also known as a transmembrane pathway. This may occur by two methods
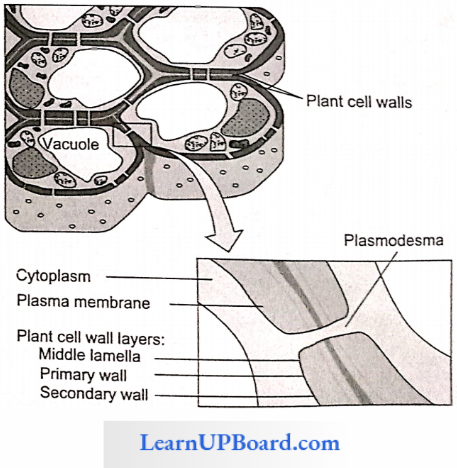
- Non-Vacuolar Symplast Pathway: In this method, water passes between adjacent cells through plasmodesmata. It does not enter into the vacuoles.
- Vacuolar Symplast Pathway: In this method, water crosses the tonoplast surrounding the vacuole. This pathway offers a lot of resistance. Beyond the cortex through the endodermis and pericycle water is forced to move through the symplast pathway.
Mechanism Of Water Absorption: Water is mainly absorbed by two different mechanisms
- Passive (water is absorbed through roots)
- Active (water is absorbed by roots)
Water absorption by rapidly transpiring plants is called passive absorption because forces responsible for water uptake develop in shoots and is transmitted to roots through which water enters. Active absorption depends on forces developing in roots and is found in low-transpiring plants. The table describes the differences between passive and active absorption
Difference Between Passive Absorption And Active Absorption

Factors Affecting Water Absorption: The rate of water absorption decreases with
- Increased salt concentration in soil
- Decreased soil temperature
- Decreased soil aeration (O2)
- Decreased soil moisture
Ascent Of Sap: The water absorbed by roots has to be conducted upwards so as to meet the needs of tissues there. This vertical conduction of water from the root to aerial parts of the plant is called the ascent of water or ascent of sap. Various theories explaining the ascent of sap have been put under three categories
- Vital Force Theories: These theories explain the ascent of sap by the force developing in living cells.
- Relay Pump Theory Or Clambering Theory (By Godlewski): According to it, water rises in the stem by the rhythmic changes in osmotic pressure of the xylem parenchyma and medullary rays. The water is relayed in a staircase-like manner to the next higher cell. Now it has been established that water moves through tracheids and vessels. Hence, this theory has been rejected.
- Pulsation Theory (By J.C. Bose): According to this theory, water rises in the stem due to pulsatory activity in the innermost layer of the cortex. This theory is not accepted nowadays, as neither these pulsations are universal in occurrence nor they are able to explain the magnitude of the ascent of sap.
- Root Pressure Theory (By Priestley): Due to the movement of water from the soil into the root hairs and from there to xylem cells, hydrostatic pressure develops. This is called root pressure (the term given by Stephan Hales).
- This pressure pushes the water up in the xylem vessels. The root pressure can be easily observed and measured when a freshly cut stump continues to exude water (bleeding) from its xylem vessels. The development of root pressure is an active process.
- Stocking has defined root pressure as a pressure developing in tracheary elements of the xylem as a result of the metabolic activity of roots. Root pressure depends upon the active secretion of salts or other solutes into the xylem sap, thereby lowering its osmotic potential.
- This involves the utilization of metabolically produced energy and is inhibited by respiratory inhibitors such as cyanide, lack of oxygen, and low temperatures. The positive hydrostatic pressure generated by root pressure (maximum 2 atm) is not sufficient to push up water to more than a few meters.
- It cannot account for water movement up the xylem in tall trees. Also, actively transpiring plants and tall trees, especially conifers, do not generate root pressure. But it is a contributing factor in many plants. It may be sufficient for the transport of water in slowly transpiring herbaceous plants.
- Physical Force Theories: According to these theories, some physical force or dead cells are responsible for the ascent of sap.
- Capillarity Theory (By Boehm): This theory explains the rise of the water column due to the joint force of capillarity and atmospheric pressure, as the xylem vessels have a narrow diameter and act as capillaries. This theory has been rejected as it does not explain the ascent of sap in tall trees because capillary force developed by a narrow vessel of 0.03 mm diameter would support a water column of about 1 m only.
- Imbibition Theory (By Such): This theory explains the rise of the water column due to the force of imbibition. If it were true, water must rise through the walls of xylem vessels and not through their lumen as it always occurs. Hence, the concept was rejected.
- Cohesion-tension Or Transpiration Pull Theory: This is the most widely accepted theory proposed by Dixon and Joley (1894). The main features of the theory are as follows
- Right from the root hairs to the leaves on top, water forms a continuous column in the plants.
- Water molecules have high cohesive forces between them, i.e., these tend to stick to each other, because, being polar, they are electrically attracted to each other by hydrogen bonds. The high cohesive force means that a relatively large tension is required to break the water column.
- In other words, the water column has a great tensile strength. The magnitude is generally 10-30 MPa.
- The lignin cellulosic cell walls of xylem vessels have a strong affinity (adhesion) for water molecules. Therefore, a strong adhesive force exists between the walls of the xylem vessels and water, i.e., water tends to “stick” to the vessel wall.
- As the water is lost from the leaf surface by transpiration, the DPD of the leaf cells increases.
- As a result, the cells develop low water potential and water from the leaf veins (xylem) moves into leaf cells. The xylem vessels, in turn, draw water from the xylem of the main stem. A negative (pulling) pressure is thus exerted by all the leaves on the stem. The combined pressure, called transpiration pun, is strong enough to pull up the column of water to great heights.
- The whole column of water moves. Water potential as low as -3 MPa or -30 bars has been measured in the leaves borne on tree tops.
- Such a low water potential is sufficient to create pulling pres-sure which can overcome the gravitational pull and resistance offered by the capillaries of xylem vessels.
- Criticism Of Transpiration Pull Theory: The theory assumes tracheids to be more efficient than vessels. Dixon believed that partition walls of the tracheids confer stability on tensile stressed transpiration stream.
NEET Biology Notes Transport In Plant Transpiration
Less than 5% of absorbed water is used by plants and the rest is lost in transpiration.
Types Of Transpiration
- Cuticular: Loss of water vapor from the general surface through the cuticle. Commonly, it is 3-10% of the total but in herbs and ferns it may be up to 50%.
- Lenticular: Loss of water vapor from lenticels or aerating pores in the bark of trees or fruits, etc. It is hardly 0.1% of the total transpiration.
- Bark: It is approximately 1% of the total transpiration.
- Stomatal: Major form of transpiration constituting 50-97% of the total transpiration.
Number And Distribution Of Stomata: In most plants, there are 1000-60000 stomata per square centimeter of leaf surface. The total pore area is approximately 1-2% of the total leaf area. On the basis of their distribution, stomata are divided into the following categories
- Apple Type (Mulberry Type): Stomata are present only on the lower surface of the leaf, for example, apple.
- Potato Type: Stomata are present on both surfaces of the leaf but more on the lower surface, for example, potato, pea, tomato, and many other dicot plants.
- Oat Type: The number of stomata is equal on both surfaces of the leaf, for example, oat and many other monocots.
- Water Lily Type: The stomata are present only on the upper surface of the leaf, for example, water lily and most floating plants.
- Potamogeton Type: Stomata arc cither absent or vestigial, for example, Potamogeton.
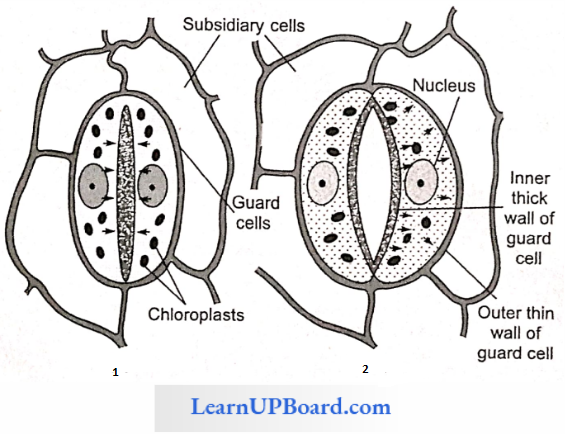
Structure Of Stomata: Each stoma is surrounded by two specialized cells called guard cells. They have chloroplasts and arc green and are surrounded by two or more specialized epidermal cells called accessory or subsidiary cells. Guard cells are of two types: kidney-shaped and dumbbell-shaped. Dumbbell-shaped guard cells are found in the family Gramineae and Cyperaceae. Kidney-shaped guard cells have an inner thick wall and an outer thin and elastic wall.
Dumbbell-shaped guard cells have thin-walled ends and thick-walled middle regions. The cell wall bordering the stomatal pore is thicker than that of next to the surrounding cells. Lotfield classified stomata as follows on the basis of their daily opening and closing movement
- Alfalfa Type: Open the whole day, closed in night. For example, pea, radish, mustard, turnip, apple, grapes.
- Potato Type: Open the whole day and night except for a few hours in the evening. For example, onion, potato, banana.
- Barley Type: Open only for a few hours in day. For example, maize, wheat, and other cereals.
- Equisetum Type: Always open.
Mechanism Of Opening And Closing Of Stomata: Stomata function as turgoroperated valves. When the osmotic concentration of guard cells increases, water comes in guard cells becomes turgid and stomata are open. Whenever the osmotic concentration of the guard cell decreases, water moves out, and guard cells become flaccid, and hence become closed. This increase and decrease in osmotic concentration is explained by a number of theories mentioned below.
Photosynthetic Theory (von Mohl And Schwendener): This theory proposes that in the morning, as soon as light is available, the chloroplasts of guard cells start photosynthesis. As a result, sugars are produced, which increase the osmotic concentration of guard cells. Water comes in, guard cells become turgid, and stomata open. This theory was not accepted because the photosynthetic activity of guard cell chloroplasts seems to be negligible and sugar does not occur in detectable quantity in guard cells.
Starch ⇔ Sugar Interconversion Hypothesis (Classical Theory): The theory was proposed by Sayre and Scarth and was modified by Steward on the basis of the activity of the phosphorylase enzyme.

According to this theory, guard cells contain starch and phosphorylase enzyme, which causes the conversion of starch into glucose at higher pH which is developed by the utilization of CO2 in photosynthesis.
Glucose increases the osmotic concentration of guard cells, raising the turgor pressure and causing the opening of stomata. At night, CO2 accumulates in cell and intercellular spaces, thus lowering the pH at which phosphorylase causes the conversion of glucose to starch. The osmotic concentration of guard cells decreases. Hence, water moves out and, therefore, stomata are closed.
Objections: In some families, starch is absent, for example, onion. Glu¬cose does not appear in detectable quantity in the guard cells of open stomata, rather malic acid accumulates.
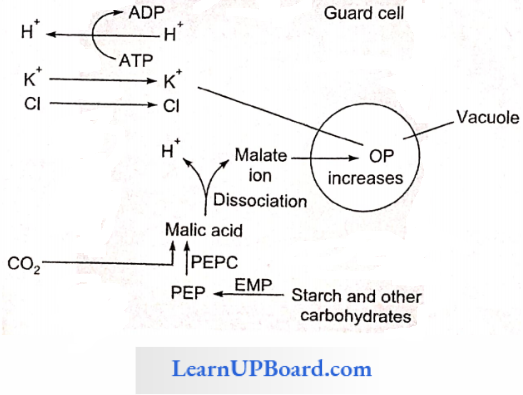
Active K+ Ion Uptake Theory (Levitt 1974): According to this theory, the pH of guard cells rises in the day due to the assimilation of CO2 in the photosynthesis and the uptake of H+ ions by chloroplast and mitochondria from cytoplasm. At this higher pH, starch is converted into phosphoenolpyruvate (PEP). PEP combines with CO2 with the help of PEPCase (phosphoenol pyruvate carboxylase) and forms oxaloacetic acid which gets changed into malic acid. Malic acid dissociates into malate and H+ ions.
- There occurs an efflux of H+ ions and the influx of K+ ions, which forms potassium malate after reacting with malate. Potassium malate is a highly osmotically active substance which is stored in vacuoles. This raises the osmotic concentration of guard cells, water moves in, cells become turgid, and stomata are open. In the night, the process is reversed.
- The potassium, chloride, and malate ions have a prominent role in stomatal opening. The ions accumulate in the vacuole of guard cells, lowering the water potential and thereby increasing water uptake and subsequently opening the stomata.
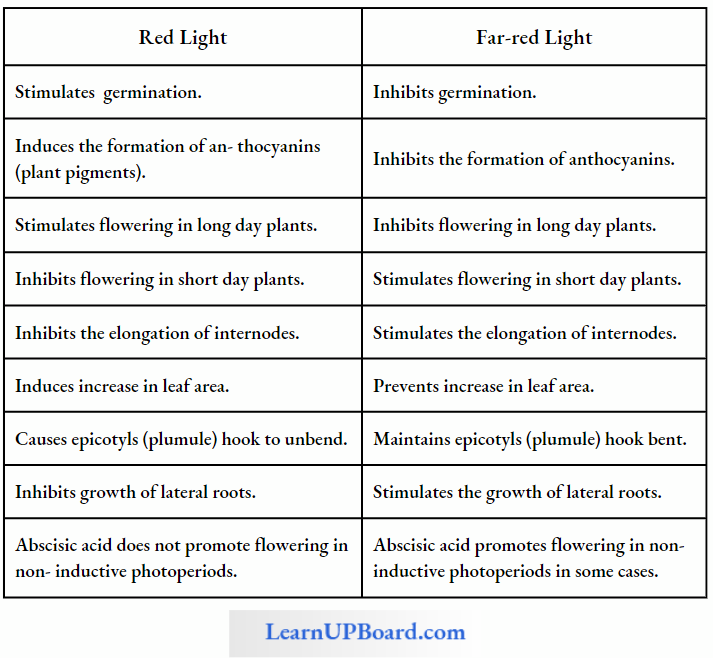
Factors Affecting Transpiration: Blue light induces maximum opening of stomata. Red and blue lights constitute the action spectrum of transpiration.
A rise in the temperature of the leaf increases the vaporization of water within the leaves resulting in a greater DP gradient of vapors, which diffuses out into the air rapidly. The rate of transpiration is generally doubled with about every 10° = C rise in temperature. High CO2 closes stomata and cytokinins open stomata.
- Wind Speed: If the wind velocity is high, the rate of transpiration is also high.
- Relative Humidity: If relative humidity is low, there would be a high rate of transpiration and vice versa.
- Root/shoot Ratio: An increase in the root/shoot ratio causes an increase in the rate of transpiration. Suolt plants have a high root/shoot ratio.
- Plant Factors: Number and distribution of stomata, number of open stomata, water status of the plants, canopy structure, etc.
Significance Of Transpiration: The following roles have been given in relation to transpiration by different biologists
- Since plants absorb far more amounts of water than is actually used by plants, transpiration helps in the removal of excess water.
- Removal of water in the form of vapors has a cooling effect on the leaves. Transpiration, thus, does not allow the leaf temperature to rise to detrimental levels.
- The transpiration pull created by transpiration is responsible for the ascent of water in tall trees.
- Passive absorption of water through the roots takes place due to the negative pressure developing in the shoots as a result of transpiration.
- Development of mechanical tissues, growth of root system, increasing ash and sugar content of fruits, and development of resistance are other beneficial effects of transpiration.
- Many chemicals (antitranspirants) have been found to reduce the rate of transpiration without affecting CO2 uptake, for example, phenylmercuric acetate (PMA) abscisic acid (ABA), and CO2. Silicon emulsion and low viscosity wax cover stomata as a film, allowing CO2 and O2 but resisting diffusion of water.
NEET Biology Notes Transport In Plant Guttation
Plants growing under humid conditions in moist warm soil often exhibit droplets of water along the margins of their leaves. This phenomenon is commonly seen in oats, tomatoes, cucumbers, garden nasturtium, saxifrage, etc. The loss of water in the form of liquid is called guttation.
In moist and humid conditions, the rate of absorption of water greatly exceeds transpiration. The root pressure is built up which pushes the water up in the xylem ducts, from where it comes out on the leaf surface through special structures called hydathodes.
Hydathodes are present at the tips of veins in the leaves. A hydathode consists of a pore in the epidermis followed by large intercellular spaces and loosely arranged parenchyma called epithem and blindly ending xylem elements. Guttated water contains inorganic and organic salts and is not pure.
NEET Biology Notes Transport In Plant Translocation Of Organic Solutes
Organic solutes such as glucose, and sucrose produced during photosynthesis are translocated through phloem tissue. The transport of photosynthates from the production centers (leaves) to the consumption centers (apices, roots, fruits, tubers) through the phloem is called translocation of organic solutes or long-distance transport. Translocation through phloem occurs in upward, downward, and radial directions from the source (leaves) to the sink (apices, roots, fruits, tubers, etc.)
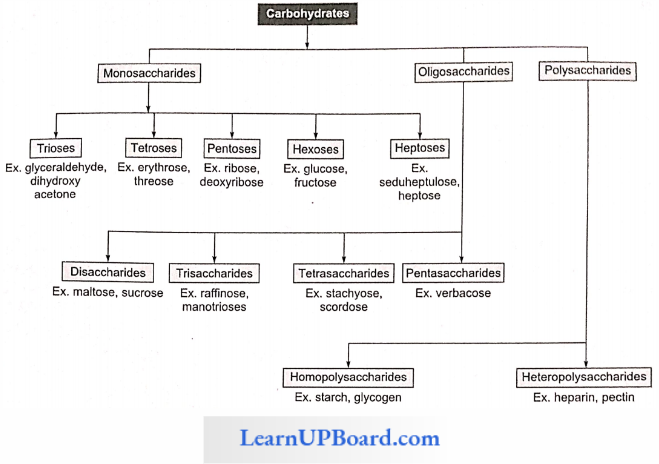
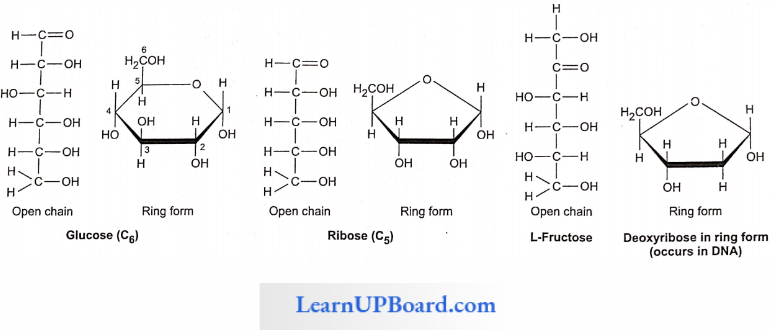
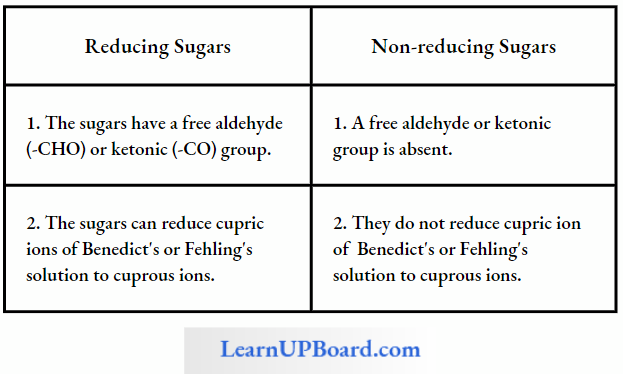
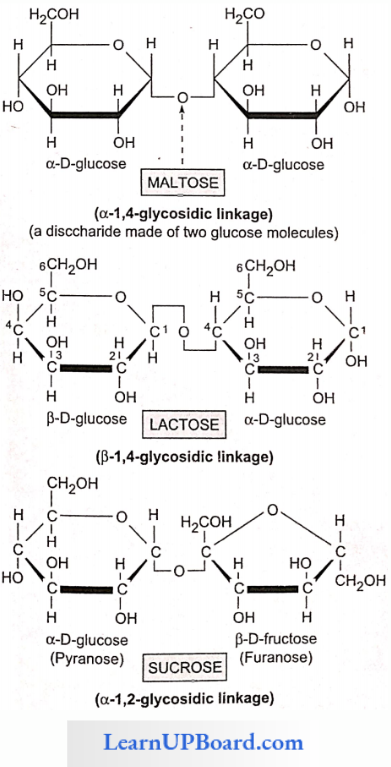
Chemical analysis of the phloem sap revealed the presence of sugars up to 90%. Sucrose constitutes 5-15% of the total sugars. Other sugars such as raffinose (triose). staehyose (tetrose), and verbascose (pentose) are also present in small quantities.
- This analysis strongly suggests that the phloem is the tissue concerned with the translocation of organic solutes. Phloem sap may be collected by using sap-sucking aphids.
- The tracer technique (Rabideau and Bun, 1945) supplied 14CO2 to a leaf during photosynthesis. Sugars synthesized in this leaf were labeled with 14C (tracer). The presence of labeled sugars (radioactivity) in the phloem showed that solutes are translocated through the phloem.
Theories Of Translocation Of Organic Solutes: Protoplasmic Streaming Hypothesis: This theory was proposed by a Dutch botanist, Hugo de Vries, in 18S5 and was supported by C.F. Curtis (1935). According to this theory, the protoplasm of the sieve tubes shows continuous streaming from one end to the other.
- Organic solutes (sugars) that enter the sieve tube are passively carried by the streaming protoplasm from one end of the sieve tube to the other. Solutes move from one sieve tube to the next sieve tube by diffusion through the pores of the sieve plate.
- Thus, the streaming protoplasm acts as a conveyor belt or two-way escalator. Different substances move in different directions at the same time in the same sieve tube.
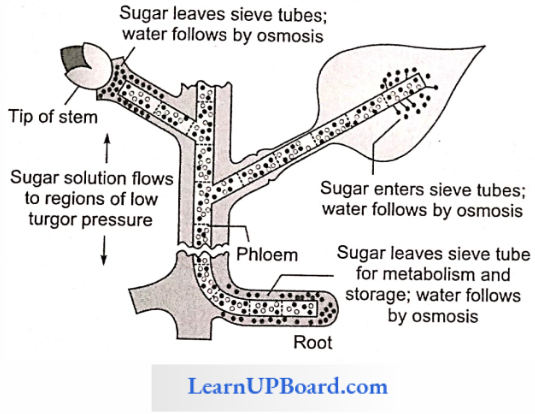
Pressure Flow Or Mass Flow Hypothesis: According to this theory proposed by Munch (1929) and elaborated by Crafts (1938), organic solutes are translocated “en masse’’ through the sieve tubes from the supplying end or source leaves to the consumption end or sink (roots, fruits, tubers).
- Mesophyll cells synthesize sugars during photosynthesis. As these get dissolved in the cell sap, the osmotic concentration and DPD of mesophyll cells increase (ψw decreases). Water enters the mesophyll cells from the xylem. The turgor pressure or pressure potential (ψp) of the mesophyll cells increases.
- Sugars dissolved in water move from mesophyll cells into the symplast system of sieve tubes. Solutes are carried “en masse” through the symplast to finally reach the consumption centers.
- At the consumption end, food materials (solutes) are either used up (as in roots) or are stored in an insoluble form (as in fruits, or tubers). Hence, the osmotic concentration and. consequently, the turgor pressure in these cells will be low. Thus, a continuous turgor pressure gradient gets established across the symplast between the cells of the source (leaf) and the cells of the sink (root).
- Water returns to the source (leaf) through the apoplast system.
Object To Mass Flow Hypothesis: The bidirectional transport of solution in the same sieve tube needs explanation. The slime content and other fibrils of the sieve tube reduce the speed of the flow of solutes even under high pressure.
Mass flow is not a purely physical process as described by Munch Phloem cells utilize 0.1 0.5% of sucrose translocated through them. This is evidence to show that phloem translocation is an active process and requires metabolic energy.
Factors Affecting Translocation Of Solutes
- Temperature: The optimum temperature for translocation is between 20° and 30°C. The file rate of translocation increases with an increase in temperature. The temperature influences the root more than the shoot since it acts as a sink for the sugars.
- Light: The root/shoot dry weight ratio increases with increased light intensities. This indicates that translocation to root increases as compared to shoot when light intensity is increased.
- Metabolic Inhibitors: The metabolic inhibitors inhibit carbohydrate translocation. These include dinitrophenol (DNP), arsenite, azide, fluoride, and hydrogen cyanide.
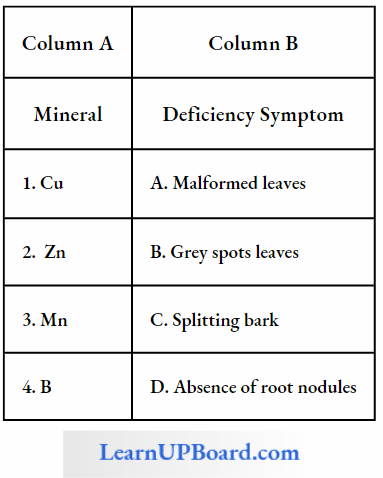
- Mineral Deficiencies: The absorption and translocation of sucrose by a leaf is facilitated by boron. It helps sucrose to move easily through the cell membranes in the form of the boron-sucrose complex.
- Hormones: Sucrose is much more efficiently translocated when growth regulators are applied, such as kinetin, IAA, and gibberellic acid.
NEET Biology Notes Transport In Plant Points To Remember
The unit for water potential is bar or Pascal (1 MPa = 10 bar) The osmotic pressure of 1 molar solution of a non-electrolytic would be 22.4 atm at 0°C. The equimolar concentrations of two solutions of non-ionizing substances will have the same osmotic pressure.
- The value of the osmotic potential of an electrolyte will be greater by the degree of its dissociation into ions at a given temperature.
- Bacteria do not survive in salted pickles because these get plasmolyzed.
- Common salt kills weeds by plasmolysis.
- Plasmolysis can be demonstrated in the epidermal peel of the Rhoeo discolor leaf.
- A plasmolyzed cell regains normal condition if placed in a hypotonic solution. It is called deplasmolysis.
- The government of H2O occurs from the high value of ψw to low value of ψw, i.e., from less negative value to more negative value of ψw.
- The auxin-treated cells show an increase in their metabolism. Respiration in these cells increases and more energy is provided for the absorption of water.
- At low temperatures, water in the intercellular spaces freezes into ice, thus having higher OP. It causes exosmosis of water from cells causing desiccation.
- If a freshwater plant is transferred to marine water, it dies due to exosmosis.
- The approximate water potential of root hairs is -3 to -43 bar.
- Ringing experiments to prove the ascent of water through the xylem were first conducted by Malpighi (1672), Stephan Hales (1727), and Hartig.
- Root pressure is measured by a manometer.
- Stocking (1956) considered root pressure as an active process responsible for guttation and bleeding in plants.
- Maximum root pressure recorded in plants is 2 bar which is sufficient to raise the water column to a height of 20 m.
- Root pressure is absent in gymnosperms (some of the tallest trees are gymnosperms).
- Photometers are used for measuring/comparing the rates of transpiration.
CoCl2 paper method is also used to compare the rates of transpiration. Moisture coming out of stomata turns blue CoCl2 paper to pink. Porometers are used for assessing the total pore area (stoma).
- Generally, stomata are photoactive (open during the day and close at night). But in succulents such as Bryophyllum, Opimtia, and cacti, stomata close during the day and open at night (scotoactive).
- Transpiration in old stems and fruits occurs through lenticels.
- The fresh weight of a plant or leaf would be maximum in the morning and minimum in the afternoon.
- If half of the total number of stomata on a leaf closes down, the rate of transpiration is not reduced by half.
- Stomata are absent or non-functional in submerged hydrophytes.
- Cytokinins help in the opening of stomata while ABA (abscisic acid) and low O2 close stomata.
- Curtis (1926) considered transpiration “as a necessary evil” in plants.
- Plants growing at high altitudes show xeromorphy (adaptation to minimize transpiration).
Transpiration Ratio: the amount of water lost per unit of dry matter produced during the growing season of a plant.
- In Saxifraga, the rate of guttation is high during flowering.
- In Colocasia antiquarian, the rate of guttation is very high.
- Mechanical shock causes stomatal closure.
Stomatal index = \(\frac{E}{E + S}\) x 100 where E is the number of epidermal cells and S is the number of stomata.
Transpiration index = Leaf test tuneAVater test time.
A psychrometer is used to measure relative humidity and rate of transpiration.
The diameter of tree decreases during the day. It is due to the narrowing of tracheary elements due to the development of negative pressure. It is measured by dendrograph.
Matric Potential, ψm: It is used for surfaces that bind water. It is also negative. For example, soil particles, cell wall, cytoplasm, etc.
Gravity Potential, ψg: It denotes the effect of gravity on ψw. It depends on the height (h) of water above the reference state of water, the density of water, and acceleration due to gravity. The value of ψm negligible up to a height of 5 m from the reference level and also the value of ψm is ignored.
∴ ψw = ψs + ψp
NEET Biology Notes Transport In Plant Multiple Choice Questions And Answers
Question 1. What determines the diffusion of water from one cell to another?
- OP
- WP
- DPD
- TP
Answer: 3. DPD
Question 2. The term “water potential” was used for the first time by
- Slatyer and Taylor
- Stocking
- Sachs
- Boehm
Answer: 1. Slatyer and Taylor
Question 3. In a hypertonic solution, the water potential of a cell
- Increases
- Decreases
- First increases and then decreases
- No chance occurs
Answer: 2. Decreases
Question 4. As a result of endosmosis, of a cell
- Increases
- Decreases
- Remains same
- Become zero
Answer: 1. Increases
Question 5. Which of the following equations is wrong?
- ψs = -π
- DPD = OP + TP
- ψw = ψs + ψp
- OP = CRT
Answer: 2. DPD = OP + TP
Question 6. When a cell is fully turgid, which of the following will be zero?
- Osmotic pressure
- Turgor pressure
- Wall pressure
- Suction pressure
Answer: 4. Suction pressure
Question 7. Osmotic potential is numerically equal to
- TP
- DPD
- OP
- WP
Answer: 3. OP
Question 8. The first sign of shrinkage of cell is detectable at
- Limiting plasmolysis
- Incipient plasmolysis
- Evident plasmolysis
- Permanent plasmolysis
Answer: 2. Incipient plasmolysis
Question 9. Osmotic pressure depends upon
- Concentration of solutes
- Temperature
- Ionization
- All of these
Answer: 4. All of these
Question 10. The water potential of a plasmolyzed cell will be
- ψw = -ψs + ψp
- ψs = ψp
- ψw = 0
- ψw = -ψs – ψp
Answer: 4. ψw = -ψs – ψp
Question 11. The water potential of soil at field capacity and wilting point are, respectively,
- —1.5 MPa and-0.01 MPa
- -0.1 MPa and -0.01 MPa
- -0.01 MPa and-1.5 MPa
- -0.01 MPa and -0.1 MPa
Answer: 3. -0.01 MPa and-1.5 MPa
Question 12. Which is not a characteristic of imbibition?
- It is a reversible phenomenon.
- Heat is generated.
- Involve capillarity and adsorption.
- It is a property of hydrophobic and lyophobic colloids.
Answer: 4. It is a property of hydrophobic and lyophobic colloids.
Question 13. The concept of apoplast and symplast imbibition was given by
- Munch
- Kramer
- Renner
- Dixon
Answer: 1. Munch
Question 14. The type of soil water that is available to roots is
- Gravitational water
- Hygroscopic water
- Capillary water
- Chemically combined water
Answer: 3. Capillary water
Question 15. Passive absorption is controlled by
- Transpiration
- Capillarity
- Presence of solutes in soil
- Temperature of atmosphere
Answer: 1. Transpiration
Question 16. If the texture of soil becomes fine, then the rate of movement of water through it will be
- Faster
- Slower
- Nil
- Same
Answer: 2. Slower
Question 17. At low temperatures, the rate of water absorption decreases due to
- Decreased viscosity of water
- Increased permeability
- Reduced rate of diffusion
- Increased root growth
Answer: 3. Reduced rate of diffusion
Question 18. Water absorption in plants is enhanced by
- Increased transpiration
- Decreased transpiration
- Decreased salt absorption
- Increased photosynthesis
Answer: 1. Increased transpiration
Question 19. The phenomenon of water uptake at the expense of energy by the cell and usually again osmotic phenomenon is known as
- Osmosis
- Active absorption
- Passive absorption
- Imbibition
Answer: 2. Active absorption
Question 20. Root pressure is measured by
- Osmometer
- Manometer
- Barometer
- Auxanometer
Answer: 2. Manometer
Question 21. When the temperature of soil is 0°C, then
- The absorption of water increases
- The absorption of water is not affected by temperature
- The absorption of water decreases
- The soil will lose capillary water
Answer: 3. The absorption of water decreases
Question 22. The osmotic theory for active water absorption was given by
- Bennet Clarks
- Thimann
- Atkin and Priestley
- Dixon
Answer: 3. Atkin and Priestley
Question 23. Root pressure is maximum when
- Transpiration is high and absorption is very low
- Transpiration is very low and absorption is high
- Transpiration is very high and absorption is also high
- Transpiration and absorption both are low
Answer: 2. Transpiration is very low and absorption is high
Question 24. The pulsation theory of the ascent of sap was given by
- Godlewski
- Dixon
- Tansley
- Sir J.C. Bose
Answer: 4. Sir J.C. Bose
Question 25. Which is not true for root pressure?
- Positive hydrostatic pressure
- Maximum during the day and minimum during night
- Magnitude is 1-2 bars
- Develops due to the metabolic activity of root.
Answer: 2. Maximum during the day and minimum during night
Question 26. A tension (transpiration pull) of 1 atm can pull water to a height of approx
- 10 feet
- 10 m
- 1 m
- 1 feet
Answer: 2. 10 m
Question 27. Amphistomatic leaves are generally found in
- Dicots
- Monocots
- CAM plants
- Aquatic plants
Answer: 2. Monocots
Question 28. The cutinized wall of epidermal cells is
- Permeable
- Semipermeable
- Impermeable
- Selective permeable
Answer: 3. Impermeable
Question 29. The stomata are widely open in
- Red light
- Blue light
- Greenlight
- Yellow light
Answer: 2. Blue light
Question 30. When transpiration is rapid
- ψw of epidermal cells decreases
- A negative pressure develops in the xylem vessel
- Water is absorbed through the root passively
- All of these
Answer: 4. All of these
Question 31. Which is not true regarding stomata?
- They are turgor-operated valves.
- They have differentially thickened walls in guard cells.
- They open when OP of the guard cell decreases.
- They show photoactive openings in CAM plants.
Answer: 3. They open when OP of the guard cell decreases.
Question 32. Cuticular transpiration is approx
- 50% in herbs and ferns
- 97% in most of the plants
- 1% of the total transpiration
- 50% in most of the plants
Answer: 1. 50% in herbs and ferns
Question 33. The rate of transpiration and stomatal movement are measured by, respectively,
- Porometer and photometer
- Potometer and pyrometer
- Potometer and tensiometer
- Hygrometer and porometer
Answer: 2. Potometer and pyrometer
Question 34. According to starch ⇔ sugar interconversion theory of stomatal opening, a stomatal opening is preceded by
- Increase in H+ concentration
- Decrease in the pH of the guard cell
- Increase in the pH of guard cell
- Inactivation of phosphorylase enzyme
Answer: 3. Increase in the pH of guard cell
Question 35. When stomata open in night only, they are called
- Photoactive stomata
- Scotoactive stomata
- Hydathodes
- All of these
Answer: 2. Scotoactive stomata
Question 36. Which of the following does not happen during stomatal opening?
- Accumulation of K+ ions in guard cell
- Lowering of osmotic pressure of guard cell
- Creation of water potential gradient between guard cell and subsidiary cell
- Increased thickening of the inner wall of the guard cell
Answer: 4. Increased thickening of the inner wall of the guard cell
Question 37. High amount of malate in guard cell during stomatal opening is due to
- Import from subsidiary cell
- Hydrolysis of starch
- Photosynthesis in guard cell
- Hydrolysis of proteins
Answer: 4. Hydrolysis of proteins
Question 38. Active K+ exchange mechanism for the opening and clos¬ing of stomata was given by
- Darwin
- Levitt
- Scarth
- Fujino
Answer: 2. Levitt
Question 39. Which of the following is a metabolic antitranspirant?
- PMA, CO2
- Colorless plastics and waxes
- Aspirin, ABA
- Both (1) and (3)
Answer: 4. Both (1) and (3)
Question 40. Which is not an advantage of transpiration?
- Development of mechanical tissues
- Development of root system
- Distribution of minerals
- Fixation of nitrogen
Answer: 4. Fixation of nitrogen
Question 41. The plant factor which affects the rate of transpiration is
- Leaf area
- Temperature
- Humidity
- Wind speed
Answer: 1. Leaf area
Question 42. In guttation, water is lost in the form of
- Water vapors
- A dilute solution of sugars
- Pure liquid water
- Dilute solution of salts and organic substances
Answer: 4. Dilute solution of salts and organic substances
Question 43. Which of the following is an effective adaptation for better gaseous exchange in plants?
- Presence of multiple epidermis
- Presence of hair on the lower epidermis
- The presence of a waxy cuticle covering the epidermis of the leaves
- Location of stomata on the lower surface of leaves and side turned away from direct sun rays
Answer: 4. Location of stomata on the lower surface of leaves and side turned away from direct sun rays
Question 44. The conditions under which transpiration would be most rapid are
- Excess of water in soil
- Low humidity, high temperature, turgid guard cells, and moist soil
- Low velocity of the wind
- High humidity
Answer: 2. Low humidity, high temperature, turgid guard cells, and moist soil
Question 45. The transpiration index is equal to
- \(\frac{\text{Leaf test time}}{\text{Water test time}}\)
- \(\frac{\text{Water test time}}{\text{Leaf test time}}\)
- The amount of water lost per unit of dry matter produced during the growing season
- \(\frac{\text{PWP}}{\text{Field capacity}}\)
Answer: 1. \(\frac{\text{Leaf test time}}{\text{Water test time}}\)
Question 46. Translocation of photosynthates occurs in the form of
- Starch
- Glucose
- Sucrose
- 3PGA
Answer: 3. Sucrose
Question 47. Although a girdled (up to bast) tree may survive for some time, it will eventually die because
- Water will not move upward
- Water will not move downward
- Sugars and other organic solutes will not move downward
- Sugars and other organic solutes will not move upward
Answer: 3. Sugars and other organic solutes will not move downward
Question 48. When stomata open, the pH of guard cells.
- Increases
- Decreases
- Remains same
- Both (1) and (2)
Answer: 1. Increases
Question 49. Water lost in pulsation is
- Pure water
- Impure water
- In vapor form
- Either (1) and (2)
Answer: 2. Impure water
Question 50. What will happen if plant cells are placed in a hypertonic solution
- Turgid
- Plasmolyzed
- Deplasmolyzed
- Lysed
Answer: 2. Plasmolyzed
Question 51. Loss of water from the tips of leaves is called
- Bleeding
- Gustation
- Respiration
- Transpiration
Answer: 2. Gustation
Question 52. Root pressure is measured by
- Manometer
- Potomcter
- Auxanomcter
- Osmometer
Answer: 1. Manometer
Question 53. Which of the following apparatus is commonly used to measure the rate of transpiration?
- Porometer
- Altimeter
- Potomcter
- Luxmeter
Answer: 3. Porometer
Question 54. Leaves of the Nehnnbo plant are
- Epistomatic
- Hypostomatic
- Amphistomatic
- None of these
Answer: 1. Epistomatic
Question 55. 0.1 M solution has the water potential of
- -2.3 bars
- 0 bar
- 22.4 bars
- +2.3 bars
Answer: 1. -2.3 bars
Question 56. A small mesophytic twig with green leaves is dipped into water in a big beaker under sunlight. It demonstrates
- Photosynthesis
- Respiration
- Transpiration
- None of the above
Answer: 3. Transpiration
Question 57. Which one is not related to transpiration?
- Regulation of plant body temperature
- Absorption and distribution of mineral salt
- Circulation of water
- Bleeding
Answer: 4. Bleeding
Question 58. Stomata can open at night also in
- Xerophyte
- Gamelophyte
- Hydrophyte
- None of these
Answer: 1. Xerophyte
Question 59. Who had said that “transpiration is a necessary evil”?
- Curtis
- Steward
- Andersen
- J.C. Bose
Answer: 1. Curtis
Question 60. Stomata open during day because the guard cells have
- Thin outer walls
- Kidney shape structure
- Chlorophyll
- Large nuclei
Answer: 1. Thin outer walls
Question 61. Stomata open and close due to
- Turgor pressure change
- Hormone change
- Temperature change
- All of the above
Answer: 1. Turgor pressure change
Question 62. In plasmolyzed cell, the space between cell wall and protoplasm is occupied by
- Hypotonic solution
- Hypertonic solution
- Isotonic solution
- Distil water
Answer: 2. Hypertonic solution
Question 63. In CAM plants, stomata are
- Closed at night and open during the day
- Closed during the day and open at night
- Never closes
- Never opens
Answer: 2. Closed during the day and open at night
Question 64. The real force responsible for the movement of water from cell to cell is
- OP
- TP
- DPD
- WP
Answer: 3. DPD
Question 65. Which of the following have sunken stomata?
- Nerium
- Mangifera
- Hydrilla
- Zea mays
Answer: 1. Nerium
Question 66. When a plasmolyzed cell is placed in a hypotonic solution then water will move inside the cell. Which force causes this?
- DPD
- OP
- WP
- None of these
Answer: 1. DPD
Question 67. Rate of transpiration is measured by
- Manometer
- Auxanometer
- Potometer
- Barometer
Answer: 3. Potometer
Question 68. If a cell shrinks when placed in a solution, the solution becomes
- Hypotonic
- Hypertonic
- Isotonic
- Pure solvent
Answer: 2. Hypertonic
Question 69. If cell A with DPD 4 bar is connected to cell B, C, and D whose osmotic pressure and turgor pressure are, respectively, 4 and 4, 10 and 5, 7 and 3 bar, the flow of water will be
- B to A, C, and D
- A to D, B, and C
- C to A, B, and D
- A to B, C, and D
Answer: 1. B to A, C, and D
Question 70. Guard cell controls
- The intensity of light entering
- Photosynthesis
- Closing and opening of stomata
- Change in green color
Answer: 3. Closing and opening of stomata
Question 71. Active transport
- Releases energy
- Requires energy
- Produces ATP
- Produces a toxic substance
Answer: 2. Requires energy
Question 72. Velamen tissues are associated within
- Haustorial function
- Assimilation
- Absorption of moisture
- Nutrition
Answer: 3. Absorption of moisture
Question 73. Cohesion tension theory regarding the ascent of sap was given by
- Dixon and Jolly
- J.C. Bose
- Cristian Wolf
- Godlewski
Answer: 1. Dixon and Jolly
Question 74. Velamen tissue is found in
- Mesophytes
- Epiphytes
- Hydrophytes
- Xerophytes
Answer: 2. Epiphytes
Question 75. In a fully turgid plant cell, which one is zero?
- Turgor pressure
- Wall pressure
- Suction pressure
- None of these
Answer: 3. Suction pressure
Question 76. Who proposed the cohesion theory of the ascent of sap?
- Strasburger
- Godlewski
- Western
- Oixon and Jolly
Answer: 4. Oixon and Jolly
Question 77. The most accepted theory for ascent of sap is
- Relay pump theory
- Pulsation theory
- Root pressure theory
- Transpiration pull cohesion theory
Answer: 4. Transpiration pull cohesion theory
Question 78. The transport of water and salt is mediated by
- Xylem
- Sieve tubes
- Sclerenchyma
- Phloem
Answer: 1. Xylem
Question 79. The removal of a ring of tissue outside the vascular cambi¬um from the tree trunk kills it because
- Water cannot move up
- Food does not travel down and roots become starved
- Shoots become starved
- Annual rings are not produced
Answer: 2. Food does not travel down and roots become starved
Question 80. Wilting of the plant is present in
- Moss
- Fern
- Algae
- Angiosperm
Answer: 4. Angiosperm
Question 81. Root hair absorbs water from the soil on account of
- Turgor pressure
- Osmotic pressure
- Suction pressure
- Root pressure
Answer: 3. Suction pressure
Question 82. Increased humidity in the atmosphere decreases the rate of
- Transpiration
- Photosynthesis
- Glycolysis
- Growth
Answer: 1. Transpiration
Question 83. In osmosis, there is movement of
- Solute only
- Solvte only
- Both (1) and (2)
- Neither solute nor solvent
Answer: 2. Solvte only
Question 84. Guttation takes place through
- Lenticles
- Pneumatophores
- Stomata
- Hydathodes
Answer: 4. Hydathodes
Question 85. Which of the following statements is correct?
- Cell membrane is involved only in exosmosis
- Cell membrane is involved only in endosmosis
- Cell membrane is involved both in exosmosis and endosmosis
- None of the above
Answer: 3. Cell membrane is involved both in exosmosis and endosmosis
Question 86. The root hairs absorb which of the following types of water?
- Capillary water
- Hygroscopic water
- Gravitational water
- All of the water
Answer: 1. Capillary water
Question 87. If flowers are cut and dipped in dilute NaCl solution, then
- Transpiration will become low
- Endoosmosis occurs
- No bacterial growth takes place
- Absorption of solute inside flower cell takes place
Answer: 1. Transpiration will become low
Question 88. A plant cell is plasmolyzed in a solution that is
- Hypotonic
- Hypertonic
- Isotonic
- Concentration no means
Answer: 2. Hypotonic
Question 89. Turgidity in guard cells is controlled by
- Chloride
- Malic acid
- Potassium
- Potassium, chloride, and malic acid
Answer: 4. Potassium, chloride, and malic acid
Question 90. Stomata are not found in
- Algae
- Mosses
- Ferns
- Liverworts
Answer: 1. Algae
Question 91. In which of the following, the rate of transpiration is high?
- CAM plant
- C3 plants
- C2 and C4 plants
- C4 plants
Answer: 1. CAM plant
Question 92. Cell sap is found in which cell organelle?
- Nucleolus
- Chloroplast
- Vacuole
- Golgi apparatus
Answer: 3. Vacuole
Question 93. Which one of the following fixes nitrogen?
- TMV
- Yeast
- Nostoc
- Denitrifying bacteria
Answer: 3. Nostoc
Question 94. Active transport of ions by the cell requires
- High temperature
- ATP
- Alkaline pH
- Salts
Answer: 2. ATP
Question 95. To initiate cell plasmolysis, the salt concentration must be
- Isotonic
- Hypotonic
- Hypertonic
- Atonic
Answer: 3. Hypertonic
Question 96. The basis of stomatal opening is
- Endosmosis
- Plasmolysis of guard cells
- Decrease in cell up concentration
- Exosmosis
Answer: 1. Endosmosis
Question 97. Plants absorb carbon dioxide from
- Vegetative
- Heterocyst
- Both vegetative and heterocyst
- None of these
- Millets
- Cereals
- Carbohydrates present in the soil
- Atmosphere
Answer: 4. Atmosphere
Question 98. Transpiration will increase with the increase of
- Humidity
- Temperature
- Carbon dioxide
- Sulfur dioxide
Answer: 2. Temperature
Question 99. If it is possible to drop a small particle through the stomata of a leaf, what will you conclude?
- It will fall on the earth surface.
- It will stop on the lower epidermis.
- It will stop on mesophyll cells.
- It will stop on vascular tissue.
Answer: 3. It will stop on mesophyll cells.
Question 100. During transpiration, turgidity in guard cells is controlled by
- Potassium
- Bromine
- Sodium
- Oxalic acid
Answer: 1. Potassium
Question 101. Apparatus used for measuring the transpiration
- Evapometer
- Potometer
- Osmometer
- Tensiometer
Answer: 2. Potometer
Question 102. Which of the following theories gives the latest explanation for the closure of stomata?
- ABA theory
- Munch theory
- Starch-glucose theory
- Active KT transport theory
Answer: 1. ABA theory
Question 103. The sugarcane plant has
- Dumbbell-shaped guard cells
- Pentamerous flowers
- Reticulate venation
- Capsular fruits
Answer: 1. Dumbbell-shaped guard cells
NEET Biology Notes Transport In Plant Assertion Reasoning Questions And Answers
In the following questions, an Assertion (A) is followed by a corresponding Reason (R). Mark the correct answer.
- If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
- If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.
- If Assertion is true, but Reason is false.
- If both Assertion and Reason are false.
Question 1. Assertion: Xerophytes have high water-retaining capacity.
Reason: They have high OR.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 2. Assertion: There is an indirect relationship between the rate of respiration and water absorption.
Reason: Increased metabolism increases mineral uptake.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 3. Assertion: Root pressure is a dynamic and always a positive hydrostatic pressure.
Reason: It is a universal phenomenon and develops under absorption lag.
Answer: 3. If Assertion is true, but Reason is false.
Question 4. Assertion: Stomata has delegated the task of providing food while preventing thirst.
Reason: They are made for gaseous exchange.
Answer: 2. If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.
Question 5. Assertion: During stomata opening, there is a relative change in TP of the guard cell and subsidiary cell.
Reason: TP of the subsidiary cell decreases during the opening.
Answer: 2. If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.