UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Concepts
- Types of Crops
- Basic Practices of Crop Production
- Food from Animals
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Chapter In A Nutshell
- The process of growing, cultivating, and harvesting crops is known as agriculture.
- Plants of the same kind that are cultivated in fields on a large scale for food, clothing or commercial purposes are known as crops.
- Several crops like rice, wheat, maize, cotton, vegetables, etc., are grown on a large scale for food, clothing, and commercial purposes.
- On the basis of the season in which they grow, crops grown in India are classified into Kharif and rabi crops.
- Kharif crops are planted in the rainy season and harvested in October. A few examples of Kharif crops are paddy, maize, cotton, etc.
- Rabi crops are planted in the winter season and harvested in March or April. A few examples of rabi crops are wheat, mustard, pea, gram, etc.
- For growing crops, farmers perform several activities known as agricultural practices. They include:
- Preparing the soil: It involves plowing, leveling, and applying fertilizers. Plowing is the process of loosening and turning the soil with a plow, hoe, etc. A wooden leveler is used for pressing the soil and fertilisers or manures are then applied to the soil.
- Selection of good quality seeds: Good quality seeds are clean and healthy seeds of a good variety.
- Sowing of seeds: Planting seeds into the soil is called sowing. Seeds can be sown by broadcasting or scattering: using seed drills or by transplantation. In transplantation, seeds are sown in a nursery. Small plants that grow out of these seeds are transferred to the fields.
- Adding manures and fertilizers to the soil: Manures are organic substances obtained by the decomposition of dead plants and animal wastes. Manures are not nutrient specific. Besides adding nutrients to the soil, manures also improve the texture of the soil and add beneficial soil
- organisms to the soil. Fertilisers are chemicals, which increase the fertility of soil by providing specific nutrients to the soil. Examples of fertilizers are urea, ammonium phosphate, NPK (nitrogen, phosphorous, and potassium), etc.
- Irrigation: It is the artificial supply of water to the soil at regular intervals and in a regular quantity as per the need of the crop. It can be done by traditional or modem methods. Traditional methods of irrigation include canal irrigation, furrow irrigation, chain pumps, etc.
They are cheaper but often lead to a waste of water. Sprinkler irrigation and drip irrigation are j modem methods of irrigation and help in saving water. - Weeding: Weeds are unwanted plants, which grow along the main crops and compete with them for sunlight nutrients, water, and space. Removal of unwanted plants from a field is called weeding. It can be done manually with a hoe or by using chemicals known as weedicides.
- Harvesting: Cutting and gathering of crops upon maturity is called harvesting. It is done manually; using a sickle or with the help of a machine called a harvester.
- Threshing: Harvested crops are separated from the plant bulk by beating the bulk on floors or with the help of machines. This process is called threshing.
- Winnowing: Grains are separated from the chaff by a process called winnowing.
- Storage: Harvested and cleaned grain must be stored in a safe and dry place. Stored grain may; be sprayed with pesticides to keep away pests. The grain is usually stored in jute bags or metallic; bins.
- Large-scale storage of go-as is done in silos and granaries.
- With the increase in population, several techniques are used for increase ng crop yield.
- Mixed cultivation and crop rotation is used so that the nutritional needs of one crop are fulfilled by another crop.
- Atmospheric nitrogen is fixed into the soil by nitrogen-fixing bacteria called Rhizobium, which is present in the root nodules of leguminous plants.
- The nitrogen cycle is the process of the circulation of nitrogen between the soil, organisms, and the atmosphere.
- Rearing of animals on a large scale for products such as milk, meat, eggs, fiber, hide, honey, etc., is known as animal husbandry.
Read and Learn More UP Board Notes for Class 8 Science
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Types of Crops
- Until around 10,000 BC, human beings were nomads and went from one place to another in search of food. They ate raw fruits, vegetables, and meat of animals. It was much later that is culture was discovered and human beings started to grow ing crops. Crops can be classified in many different ways. Based on the type of food they provide, crops can be classified as cereals, fruits, vegetables, oilseeds and nuts, legumes, sugar crops, etc. Crops can also be classified on the basis of the season in which they grow. Crops grown in India are classified as Kharif and rabi crops. Kharif crops are planted in the rainy season and harvested in October. A few examples of Kharif crops are paddy, maize, cotton, etc. Rabi crops are planted in winter and harvested in March or April. A few examples of rabi crops are wheat, mustard, pea, gram, etc. Pulses and vegetables are generally grown in summer.
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Basic Practices Of Crop Production
Farmers perform several activities for producing crops and these are known as agricultural practices. The basic steps of growing crops include the following step:
- Soil preparation which includes plowing, leveling, and applying fertilisers. Plowing is the process of loosening and turning soil using a plow, hoe, etc. Soil is loosened and overturned to make it better ventilated and suitable for the growth of tiny organisms living in it. During dry season, Dloughing turns soil into big mud pieces or crumbs, which are broken down by a park. At times, a wooden leveler is used for pressing the soil
- The process of planting seeds in the soil is called sowing. Before sowing good quality seeds must be selected. Seeds can be sown in fields by hand (broadcasting) or with the help of a seed drill.
- Manure or art facial fertilisers are added to the soil to provide nutrients for the healthy growth of crops.
- Water is supplied to the soil at regular intervals and n regular quantity. This is called inigation and it can be done by traditional or modem methods. Traditional methods of irrigation include canal irrigation, furrow irrigation, chain pumps, etc. They are cheaper but often lead to a waste of water. Sprinklers and drip irrigation are modem methods of irrigation and help in saving water.
- Unwanted plants are removed from fields either manually or by using chemicals called weedicides. Examples of weedicides are linazine, dilation, etc.
- Cutting and gathering of crops upon maturity is called harvesting. It is done manually using a sickle or with the help of a machine called a harvester.
- Grains are obtained from the harvested crops by threshing and winnowing.
- Threshing: Separation of grains from the plant bulk is called threshing. On a large scale, threshing is done using animals. In large farms, a machine called a combine harvester is used for both harvesting and threshing.
- Harvested grains are stored in a safe and dry place before they are made available for consumption. Pesticides may be sprayed to keep away pests from stored grains. Grains are usually stored in jute bags or metallic bins. Large-scale storage of grains is done in silos and granaries. Grains stored at home can be protected from pests by putting dried Neem leaves in them.
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Activity 1
Aim: To show that damaged seeds would float on top of water Procedure:
- Take a beaker and fill half of it with water.
- Put a handful of wheat seeds into it and stir well.
- Leave the beaker undisturbed for a few minutes.
Observation: Few seeds float on top of the water.
Conclusion: Damaged seeds are hollow from inside and thus, they float on top of water.
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Activity 2
Aim: To show that manure or fertilisers are needed for good growth of crops
Procedure:
- Germinate moong or gram seeds in three equal-sized containers.
- In the first container, sow the seeds into the soil.
- In the second container, add some cow dung to the soil.
- In the third container, add urea to the soil.
- Keep the three containers in a warm place and water them every day.
- Observe after 7 to 10 days.
Observation: Growth of seeds in the second and the third container is better than in the first one. Conclusion: Manures and fertilisers help the seeds to grow- better.
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management True Or False.
- Soil preparation involves plowing, leveling, and applying fertilizers.
- Water should be supplied to crops only when the soil seems dry and not at regular intervals
- Tilled and plowed soil is better aerated and lets water and nutrients reach to the roots of the plants.
- Weeds are unwanted plants, which grow alongside crops and must be removed for healthy growth of crops.
- Cutting and gathering of crops upon maturity is called threshing.
- A combined harvester is used for both harvesting and threshing.
- Leguminous plants bear root nodules.
- Winter crops are known as Kharif crops.
- In India, special festivals are held commemorating the harvest.
- Large-scale storage of grains is done in silos or granaries.
- Wheat is a rabi crop.
- Crops can be categorized on the basis of the type of food they provide.
- Gram is a Kharif crop.
- Rearing of honeybees on a large scale is known as apiculture.
- We get hide and meat from poultry birds.
- Animals such as goats and sheep provide us with fibers.
- Cows and buffaloes provide us with milk.
- Honey is obtained from cows
Answer
- True
- False
- True
- True
- False
- True
- True
- False
- True
- True
- True
- True
- False
- True
- False
- True.
- True
- False
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Multiple Choice Questions.
Question 1. In which category of crops would you place cotton?
- Rabi crops
- Kharif crops
- Fruits
- Cereals
Answer: 2. Kharif crops
Question 2. Which of the following is a manual method of sowing seeds directly into the field?
- Using seed drills
- Broadcasting
- Transplantation
- None of these
Answer: 2. Broadcasting
Question 3. Which of the following is a result of adding fertilizers to the soil?
- Addition of specific nutrients to the soil
- Improvement of soil texture
- Addition soil organisms to the soil
- None of these
Answer: 1. Addition of specific nutrients to the soil
Question 4. Which of the following can be used for protecting stored grains at home?
- Weedicides
- Dried Neem Leaves
- Dried Tulsi Leaves
- None of these
Answer: 2. Dried Neem Leaves
Question 5. ‘What is rearing of silkworms for producing silk called?
- Pisciculture
- Sericulture
- Apiculture
- None of these
Answer: 2. Sericulture
Question 6. Which of the following products is provided by cows and buffaloes?
- Milk
- Hide
- Fuel
- All of these
Answer: 4. All of these
Question 7. ‘What is the name of the process in which seeds or grains are separated from the plant bulk Dy beating it on a hard floor?
- Winnowing
- Broadcasting
- Threshing
- Weeding
Answer: 3. Threshing
Question 8. ‘Which of the following is used for removing weeds?
- Weedicides
- Pesticides
- Fertilizers
- Manures
Answer: 1. Weedicides
Question 9. ‘Which of the following is a traditional method of irrigation?
- Canal irrigation
- Furrow irrigation
- Chain pumps
- All of these
Answer: 4. All of these
Question 10. Which of the following is provided to us by animals?
- Wood
- Rubber
- Wheat
- Wool
Answer: 4. Wool
Question 11. ‘What is the name given to crops which are mainly grown for money?
- Food crops
- Cash crops
- Vegetables
- Oilseeds and nuts
Answer: 2. Cash crops
Question 12. What do animals get from crops?
- Fodder
- Oilcake
- Grains
- All of these
Answers: 4. All of these
Question 13. In which category of crops would you place wheat and rice?
- Rabi crops
- Kharif crops
- Fruits
- Cereals
Answer: 4. Cereals
Question 14. Which of the following is a Khar/From?
- Linseed
- Mustard
- Groundnut
- Pea
Answer: 3. Groundnut
Question 15. Which of following constitutes the food that early human beings ate?
- Cereals
- Raw fruits, vegetables, and meat
- Milk products
- None of these
Answer: 2. Raw fruits, vegetables, and meal
Question 16. Under which of the following type of crops would you categorise mustard?
- Cereals
- Fruits and vegetables
- Oilseeds and nuts
- Sugar crops
Answer: 3. Oilseeds and nuts
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Fill In The Blanks.
- A ______________may be used for sowing seeds
- ________and______ irrigation are modern methods of irrigation.
- A___________is used for harvesting crops.
- ____________ are chemicals that kill or destroy weeds.
- When crops in a field are supplied water at regular intervals of time, it is called_______
- __________of crops take place due to lack of water.
- The area of land on which crops are grown is known as a________
- The downward movement of water through the pores of the soil is known as________
- There are two types of fertilizers: and, artificial.
- Rhizobium bacteria fixes__________into the so
- ___________crops are planted in winter and _________in March or April.
- _______and are __________grown in summer
- _____ and ______are milk yielding animals.
- ____ are provided by a hen.
Answers
- Seed drill
- Sprinkler and drip
- Sickle
- Weedicides
- Irrigation
- Shriveling
- Crop field
- Percolation
- Natural
- Atmospheric nitrogen
- Rabi, harvested
- Pulses, Vegetables
- Buffalo, cow
- Eggs
UP Board Notes For Class 8 Science Chapter 1 Crop Production And Management Short Answer Type Questions
Question 1. Before sowing seeds, soil is plowed and tilled. Why?
Answer
Before sowing seeds, soil is plowed and tilled.
Soil is plowed and tilled to aerate and loosen it up. This allows water to percolate down the layers of soil and reach to the roots of plants. Aeration of soil helps roots to grow and breathe. It also aids in the growth of worms and microbes in the soil. Worms and microbes make the soil fertile.
Question 2. What are the different methods of sowing seeds?
Answer
Different methods of sowing seeds
Seeds can be sown into the soil using broadcasting, seed drills, or by transplanting. In broadcasting, seeds are broadcasted or scattered over the plowed soil by hand. A seed drill is a small device that sows seeds at equal distances. In transplantation, seeds are sown in nursery beds and once they grow into young plants, they are uprooted and planted in the field.
Question 3. What are the basic requirements for growing any crop?
Answer
The basic requirements for growing a crop are:
- A proper type of soil as per the need of the crop
- Sowing good quality, healthy seeds at the right time
- Use of manures or fertilizers
- Regular irrigation
- Proper weeding
- Protection from pests
- Harvesting at the right time
Food from Animals
- Animals provide us with many useful products such as milk, eggs, meat, fibers, hide, honey, etc. These animals need to be provided with food, shelter, and care. Rearing of animals on a large scale for food, clothes or other products is known as animal husbandry. Besides providing these products, animals help us in various tasks. Such animals are called draught animals. Examples of draught animals are horses, ox, etc.
Question 4. Explain how soil gets affected by the continuous plantation of the same plant. How can this be prevented?
Answer
By repeated growth of the same plant, specific nutrients get depleted from the soil. To prevent this, different types of crops are grown in succession and this practice is known as crop rotation.
Question 5. Define rabi and Kharif crops with two examples of each.
Answer
Rabi and Kharif crops: Crops which are grown in the winter season and harvested in March or April are known as rabi crops. Examples of rabi crops are wheat and mustard. Crops which are grown in the rainy season and harvested in October are known as Kharif crops Examples of Kharif crops are paddy and cotton.
Question 6. Define agriculture.
Answer
Agriculture
The process of growing, cultivating, and harvesting crops is known as agriculture.
Question 7. What is staple food?
Answer
Staple food
The food which forms the main part of our diet is called staple food, e.g. rice and wheat. They are grown on a large scale in vast fields as they are consumed in large amounts.
Question 8. What are weeds?
Answer
Weeds:
Weeds are unwanted plants that grow alongside crops and compete with crops for food, water, and sunlight. By taking up nutrients, space, water, and sunlight; weeds adversely affect crop yield and hence, should be removed as soon as possible.
Question 9. What is poultry farming?
Answer
Poultry farming
The rearing and breeding of chickens and hens on a large scale is called poultry farming. It is done to produce eggs and meat
Question 10. What is crop rotation? What are its advantages?
Answer
Crop rotation:
Growing the same type of crop on the same land, again and again, depletes the soil of nutrients, deteriorates soil quality, and leads to poor crop yield. Growing crops of different kinds in succession is termed crop rotation. It helps maintain the quality of the soil.
Let us consider the case of nitrogen-fixing bacteria. These are found in the roots of leguminous plants. Nitrogen-fixing bacteria help in fixing atmospheric nitrogen in the soil. Planting legumes and Plants in the soil will result in recovering nitrogen in the soil and increase its fertility. A sample four-year crop rotation plan may be as follows:
Leaf crop —> Fruit crop —> Root crop —> Leguminous crop
UP Board Notes for Class 8 Science Chapter 1 Crop Production And Management Exercises
Question 1. Select the correct word from the following list and fill in the blanks:
float, water, crops, nutrients, preparation
- The same kind of plants grown and cultivated on a large scale at a place are called
- The first step before growing crops is the of soil.
- Damaged seeds would on top of water.
- For growing of crops, sufficient sunlight from the soil are essential.
Answers:
- Crop
- Preparation
- Float
- Water, nutrients
Question 2. Give two examples of each.
- Kharif crop
- Rabi crop
Answers:
- Paddy and Maize
- Wheat and Gram
Question 3. Write a paragraph in your own words on each of the following.
Answer
- Preparation of soil
- Sowing
- Weeding
- Threshing
- Preparation of soil: Soil is loosened and overturned to make it better ventilated and suitable for the growth of tiny organisms living in it. The ent reprocess is called ’tillage and plowing’. During dry season, plowing turns the soil into big mud pieces or crumbs, which are then broken down with a plank. The plowed soil is liable to be removed by wind and water. Therefore, a wooden leveler is used for pressing the soil.
- Sowing: The process of planting seeds into the soil is called sowing. It is the most important part of crop production. Before sowing, good-quality seeds must be selected. Seeds can be sown in fields by hand (broadcasting) or with the help of i seed drill.
- Weeding: It is a process of the removal of unwanted plants, i.e., weeds from fields. It is necessary to remove weeds as they compete with the main crop for water, nutrients, sunlight, etc. Weeds may be removed manually by uprooting or cutting or by using chemicals called weedicides. Examples of weedicides are linazine, dilation, etc. The best time to remove weeds is before they produce flowers and seeds. Hand removal of weeds can be done with burps or a tractor-driven harrow.
- Threshing: Separation of grains from the bulk of harvested plants is called threshing. Animals are used on a large scale for threshing. In large ”arms, a machine, a combined harvester, is used for both harvesting and threshing.
Question 4. Explain how fertilisers are different from manure.
Answer
Fertilisers are different from manure
Fertiliser Manure
a) These are chemical compounds rich in nitrogen, phosphorus, and potassium. a) These are organic substances prepared from the decomposition of plants and animal wastes.
b) They are man-made and manufactured on a large scale in factories. b) They are made by natural processes either on a small or large scale.
c) Chemical fertilisers are nutrient specific, i.e., nitrogenous, phosphatic, etc. c) They contain a mixture of various nutrients recycled from biomass wastes.
Question 5. What is irrigation? Describe two methods of irrigation which conserve water.
Answer
Irrigation:
Applying water to the soil at regular intervals of time and in regular quantity is called irrigation. The time and frequency of irrigation vary from crop to crop, soil to soil, and season to season. The latest irrigation methods that help in conserving water are:
Two methods of irrigation which conserve wate
- Sprinkler system: This system is very useful on uneven land or on sandy soil. The pipes of the sprinkler system have rotating nozzles and are joined to the main pipeline at regular intervals. Water comes out from the rotating nozzles and is sprinkled on the crops as if it raining.
- Drip system: In this system, water is added drop by drop to the soil just at the position of the roots. Water wastage is minimised and that is why the drip system is a boon for regions where availability of water is limited.
Question 6. If wheat is sown in the Kharif season, what would happen? Discuss.
Answer
Wheat is a rabi crop, i.e., it is grown in the winter season If it is sown in the Kharif season, it will get more water, which is harmful to the crop. The wheat crop will neither be healthy nor will it provide a good yield.
Question 7. Explain how soil gets affected by the continuous plantation of crops in a field.
Answer
For better growth of crops, fertilisers are added regularly to a field. Fertilisers are nutrients like nitrogen, phosphorus, potassium, etc. Using them year after may change the nature of the soil. The soil may become too alkaline or acidic and less fertile.
Question 8. What are weeds? How can we control them?
Answer
Weeds
Weeds are undesirable plants that grow naturally along with the crops. Removal of weeds is called weeding. The following methods can be used to control weeds:
- Tilling: The process of loosening and turning of the soil is called till ng or plowing. This is done by using a plow. Tilling uproots and kills weeds.
- Manual removal: This method includes physical removal of weeds by uprooting or cutting them close to the ground. This is done with the help of a khurpi or harrow.
- Chemical method: In this method, weeds are controlled or killed by using chemicals called weedicides. They are sprayed in fields with a sprayer.
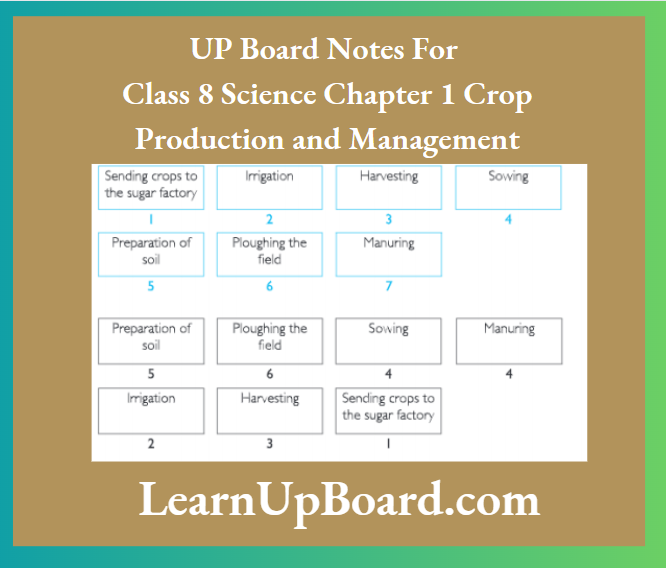
Question 9. Arrange the following boxes in the proper order to make a flow chart of sugarcane crop production.
Answer:
Question 10. Complete the following word puzzle with the help of the clues given below.
Answer:
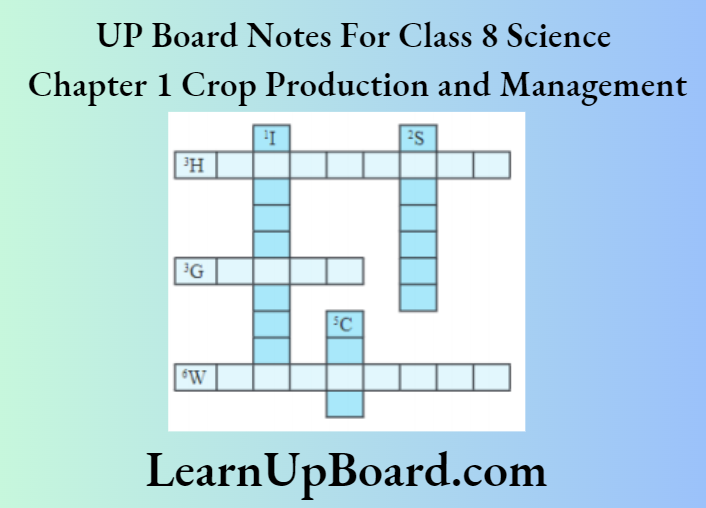
Down
1. Providing water to the crops
2. Keeping crop grains for a long time under proper
5. Certain plants of the same kind that are grown on a large scale Across
Across
3. A machine used for cutting matured crop
4. A rabi crop that is also one of the pulses
6. A process of separating grains from chaff
Answers
Down
1. Irrigation
2. Storage
5. Crop
Across
3. Harvestor
4. Grams
6. WINNOWING
UP Board Notes for Class 8 Science Chapter 1 Crop Production And Management Hots Corner
Question 1. Which irrigation method would you recommend for a region in Rajasthan? Why?
Answer:
Rajasthan is a dry region and hardly gets any rain. The soil found there is sandy and has very poor water-holding capacity. Hence, modem methods of irrigation like drip irrigation and sprinkler irrigation should be used to water crops in such regions.
Question 2. The following table shows the yield of wheat crops grown by Ram Singh between the years 2005 and 2010. Look at the table below and answer the questions that follow.
Answer:
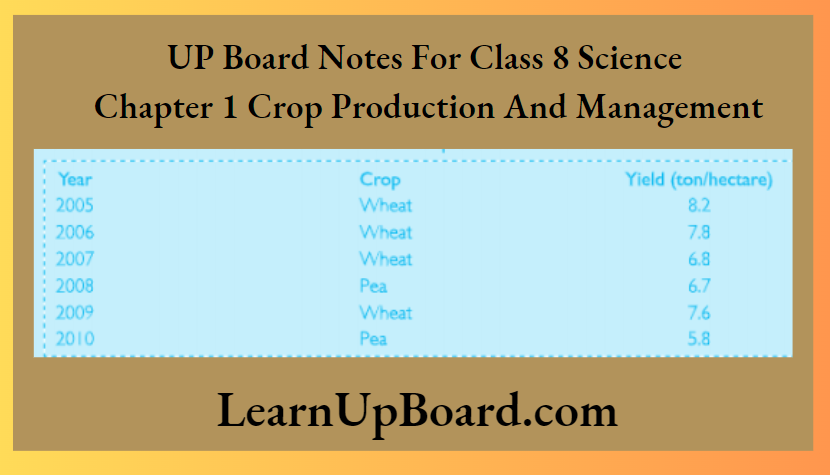
1. What could be the reason for a decrease in the yield of wheat crops in the year 2007?
Answer
Growing the same crop in the same field year after year decreases the fertility of soil. The soil gets depleted of nutrients. Hence, the yield of wheat crops in the year 2007 is low.
2. What could be the reason for all increase in die yield of wheat crops in the year 2009?
Answer
Peas are leguminous plants. Rhizobium bacteria present in the root nodules of leguminous plants fix atmospheric nitrogen into the soil. This probably increased the fertility of the soil and resulted in an increase in the yield of wheat crops in the year 2009.
Question 3. A farmer grew seeds in his field but only a small number of plants germinated. The field was plowed, tilled, leveled, and manured properly. He also irrigated the soil regularly and took care of weeds, pests, and insects. What do you think is the reason behind the poor germination of seeds?
Answer
The reason behind it might be unhealthy seeds. Everything else has been taken care of and hence, this is the only possible reason for poor germination of seeds.
Question 4. In a musk melon field, it was observed that a few fruits would sometimes crack. What do you think is the reason behind it?
Answer
Fruits in a field would sometimes crack due to excess absorption of water. This may happen due to improper drainage or leveling of the field.
UP Board Notes for Class 8 Science Chapter 1 Crop Production And Management Practice Exercise Objective Type Questions
1. Circle the odd one out.
- Wheat, rice, maize, mango, millet
- Sugarcane, jute, cotton, flax, coconut
- ‘Wheat, mustard, gram, soybeans, linseed
- Plowing, tilling, harvesting, leveling, manuring
- Eggs, silk, paper, wool, honey
Answers:
- Mango
- Sugarcane
- Soybeans
- Harvesting
- Paper
2. Give one word for the following.
- Nitrogen-deficient soil can be made nitrogen-rich by applying this farming practice
- Chemicals that are used for killing pests
- The process by which seeds are scattered in the field by hand
- Nitrogen-fixing bacteria, Rhizobium, lives in the root nodules of these plants
- Rearing of fish on a scale
Answers:
- Crop rotation
- Pesticides
- Broadcasting
- Legumes
- Pisciculture
3. The process of cutting and gathering crops is called harvesting.
- Paddy is a rabi crop.
- Tea is a leguminous crop.
- The microorganisms found in the roots of leguminous plants are Rhizobium bacteria.
Answers:
- True
- True
- False
- False
Also Read
- Chapter 1 Crop Production and Management
- Chapter 2 Microorganisms: Friend and Foe.
- Chapter 3 Synthetic Fibres and Plastics
- Chapter 4 Materials: Metals and Non-Metals
- Chapter 5 Coal and Petroleum
- Chapter 6 Combustion and Flame
- Chapter 7 Conservation of Plants and Animals
- Chapter 8 Cell: Structure and Functions
- Chapter 9 Reproduction in Animals
- Chapter 10 Reaching the Age of Adolescence
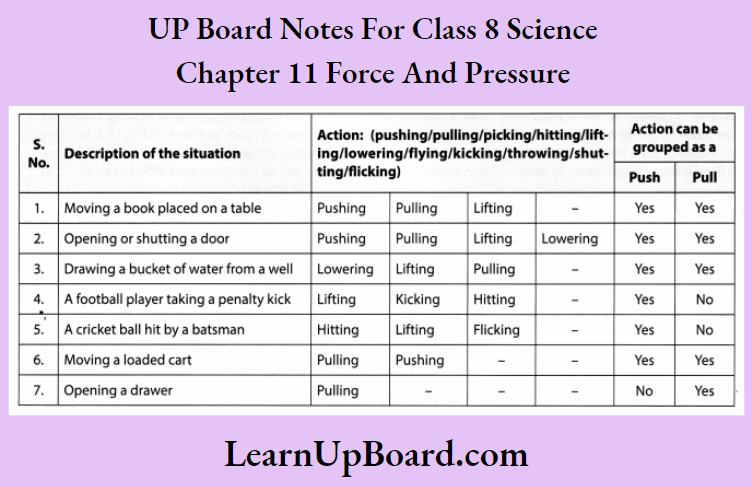
- Chapter 11 Force and Pressure
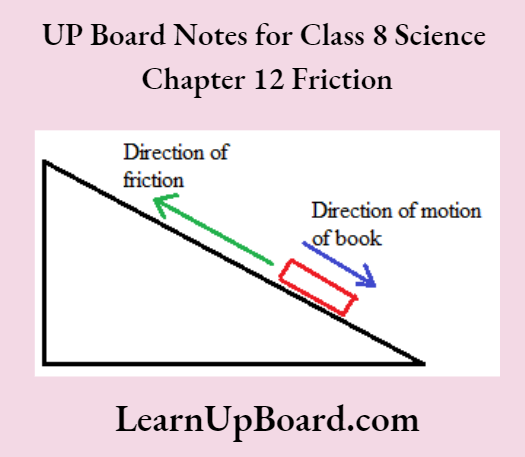
- Chapter 12 Friction
- Chapter 13 Sound
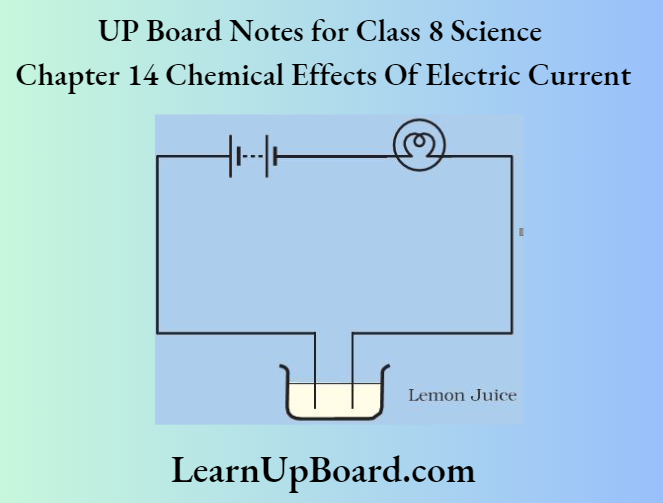
- Chapter 14 Chemical Effects of Electric Current
- Chapter 15 Some Natural Phenomena
- Chapter 16 Light
- Chapter 17 Stars and the Solar System
- Chapter 18 Pollution of Air and Water

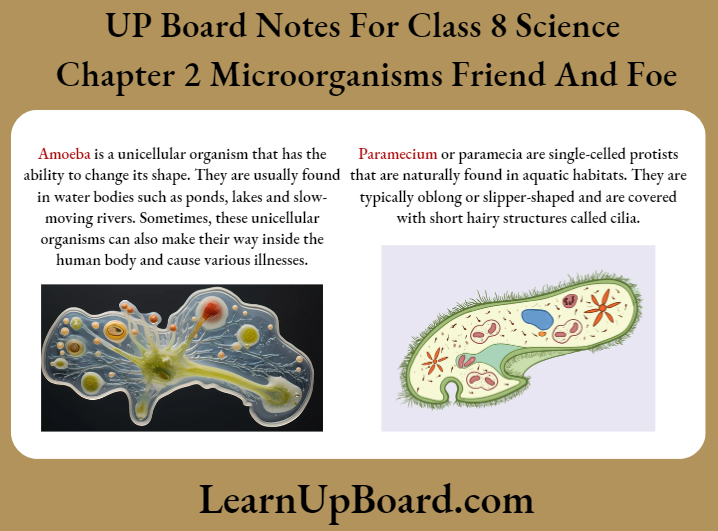
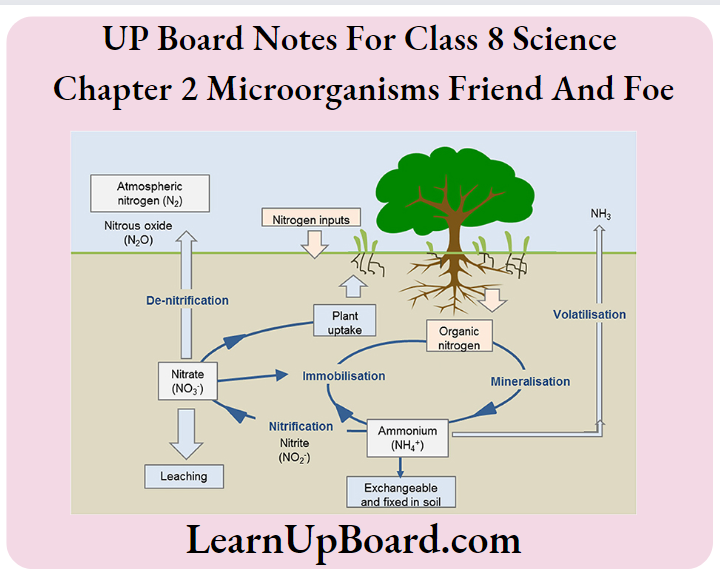
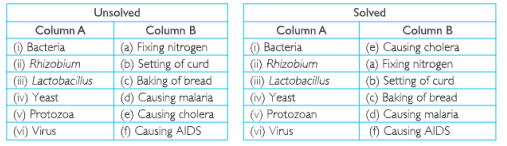 4. Can microorganisms be seen with the naked eye? If not, how can they be seen?
4. Can microorganisms be seen with the naked eye? If not, how can they be seen?


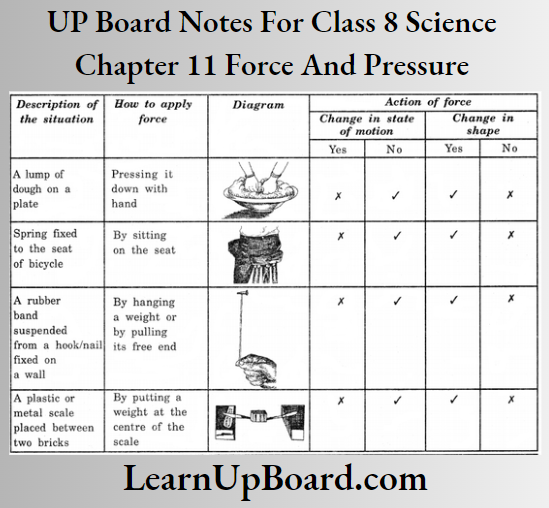




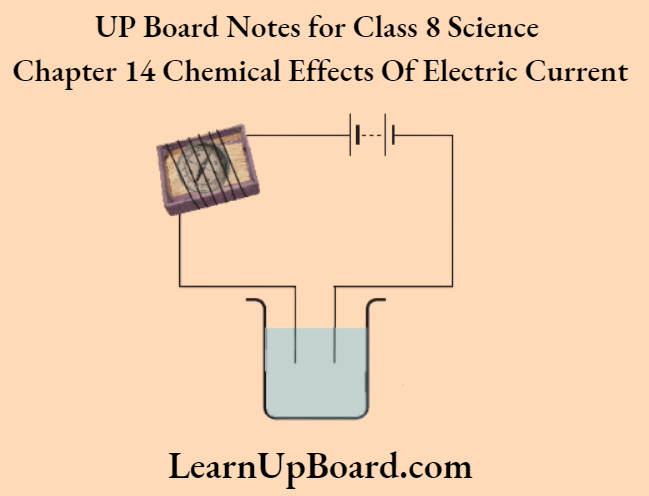
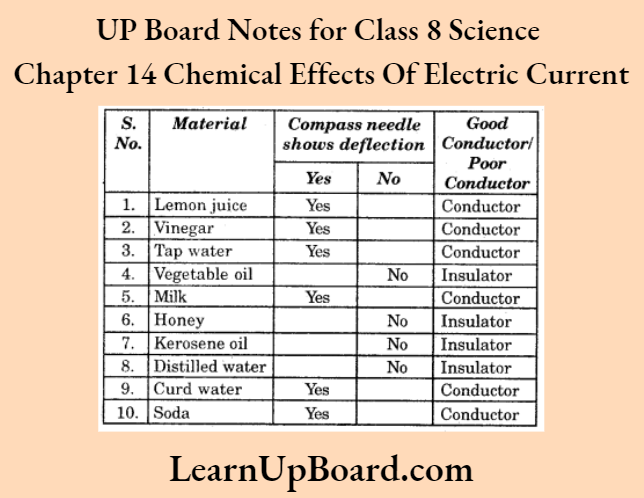
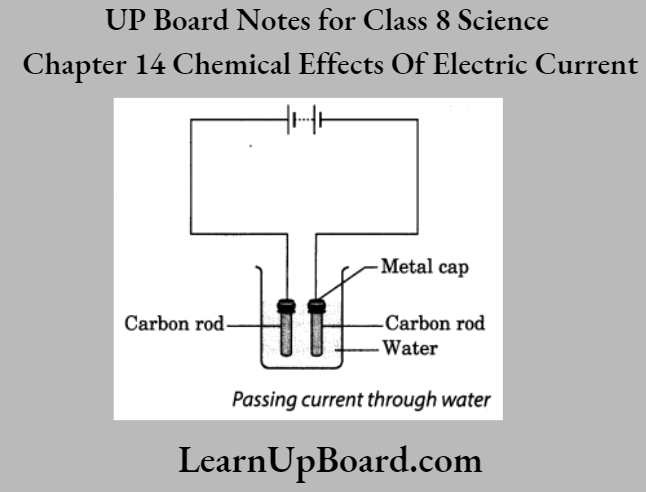
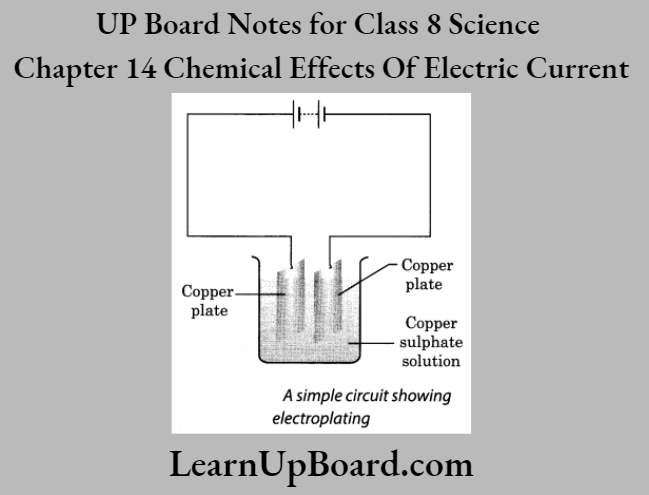










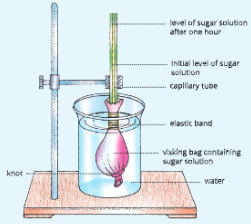
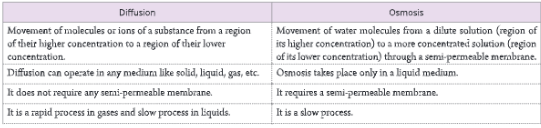
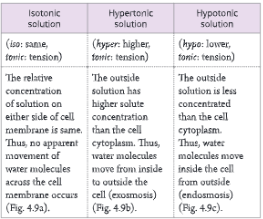
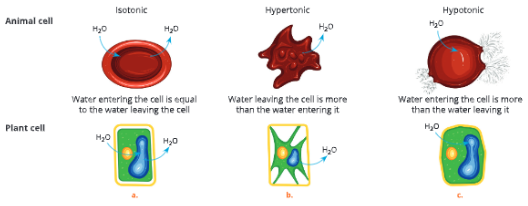
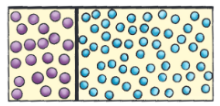 A-barrier-separates-two-kinds-of-molecules
A-barrier-separates-two-kinds-of-molecules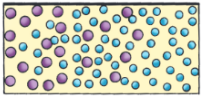


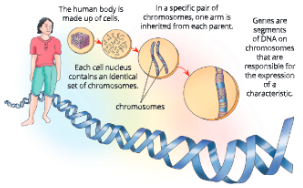
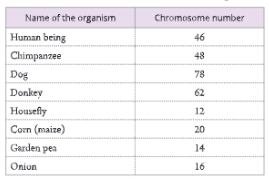
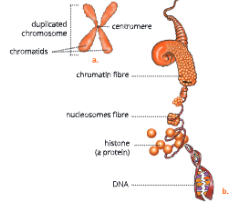
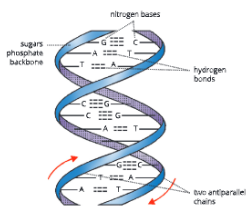
 Human karyotype – Human somatic cells have 22 pairs of autosomes and 1 pair of sex chromosomes (XX or XY); Chromosomes are paired by matching their banding pattern and are arranged by size and shape.
Human karyotype – Human somatic cells have 22 pairs of autosomes and 1 pair of sex chromosomes (XX or XY); Chromosomes are paired by matching their banding pattern and are arranged by size and shape.