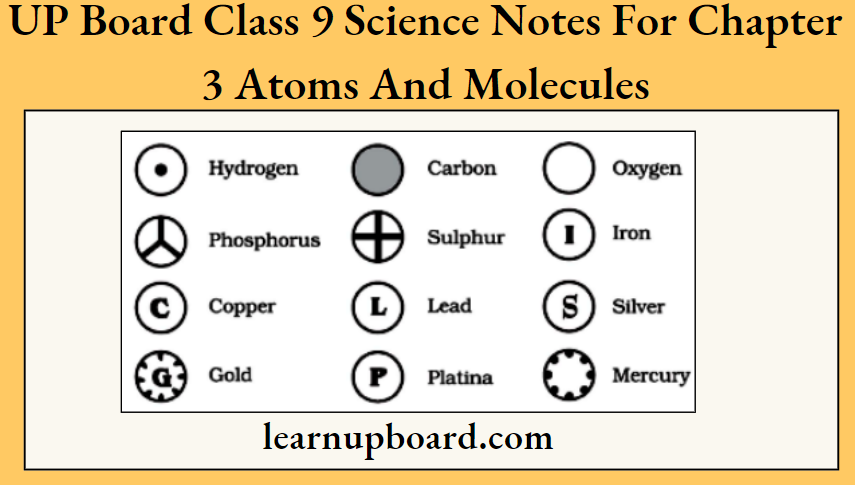
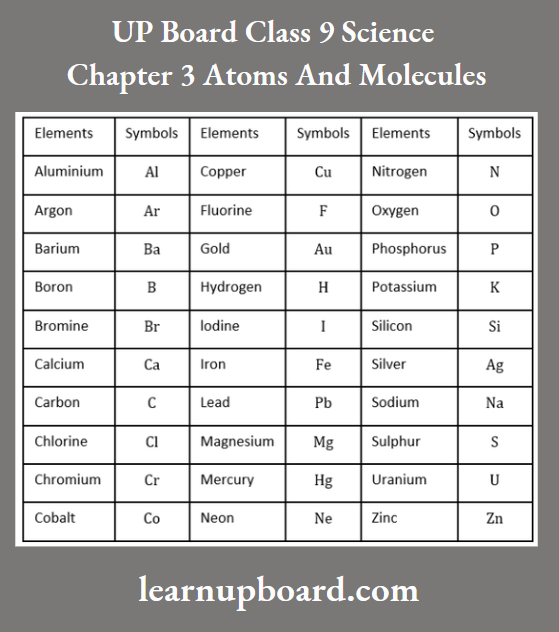
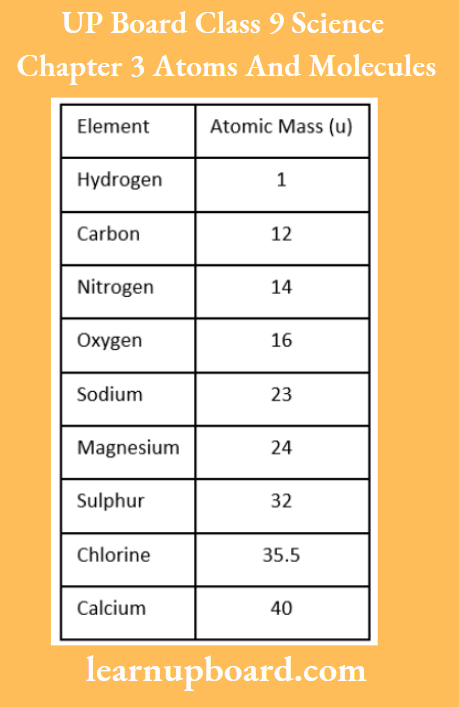
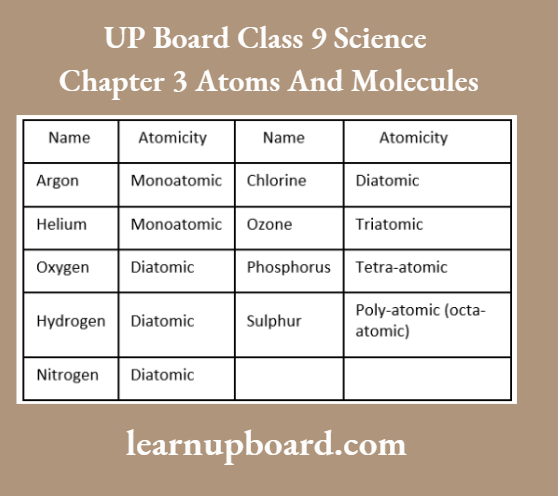
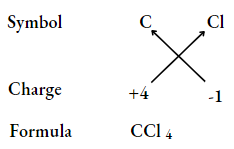
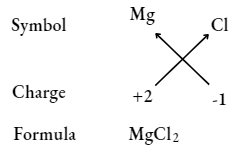
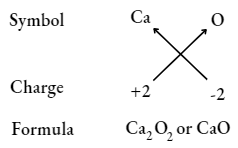
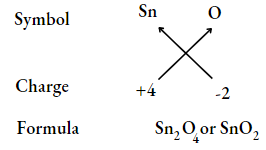
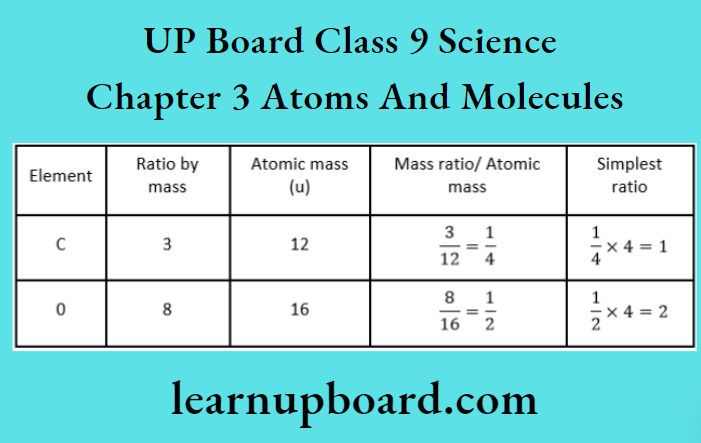
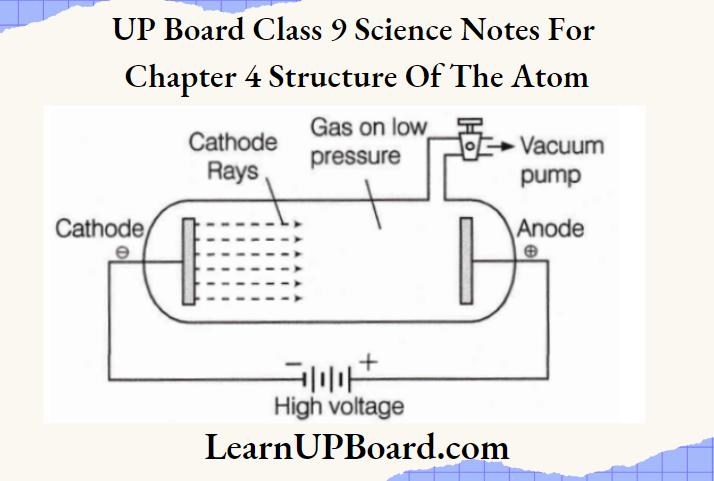
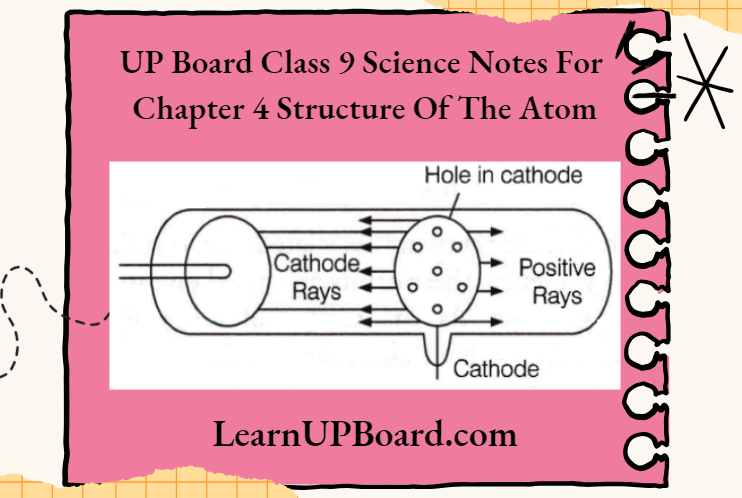
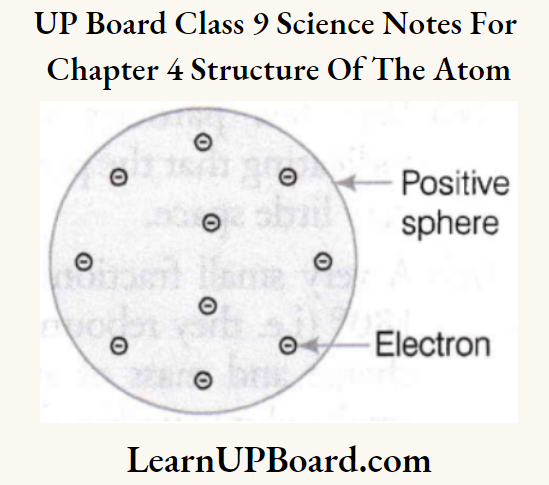
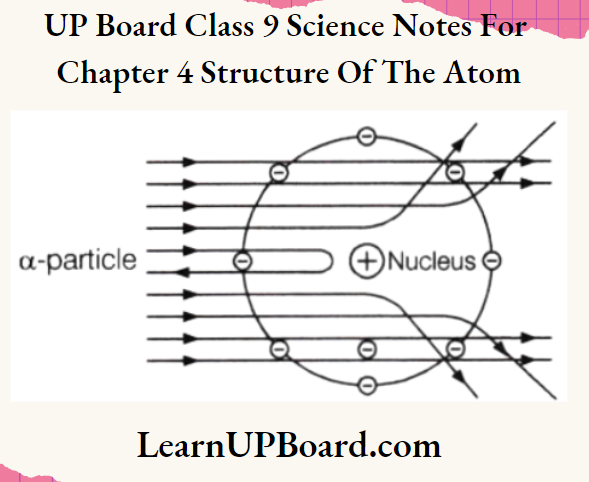
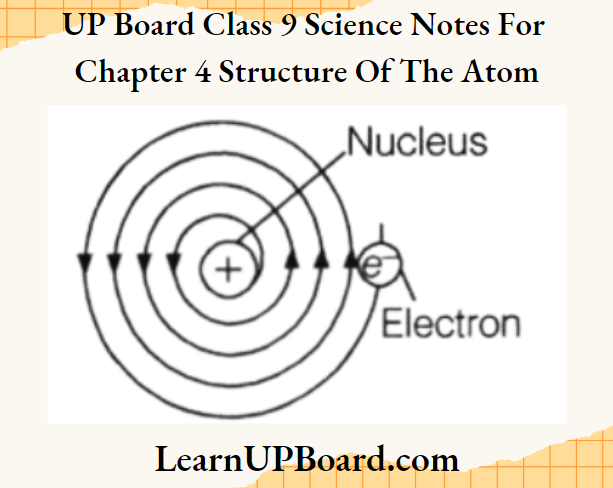
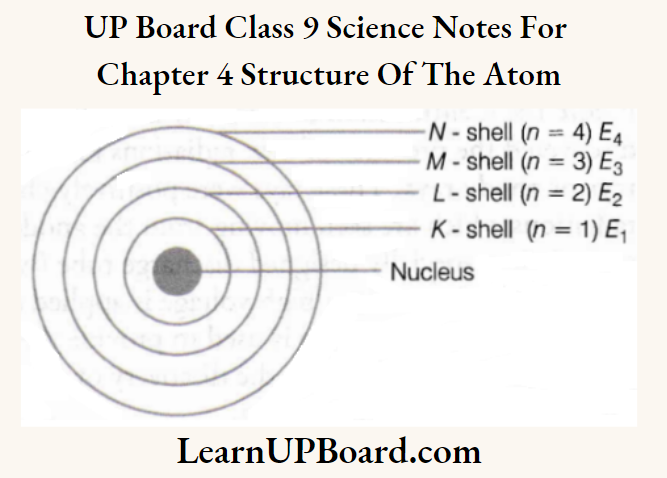
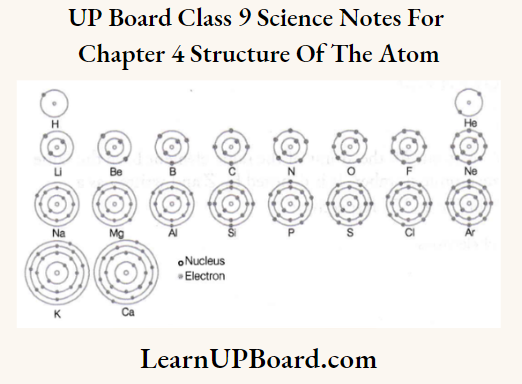
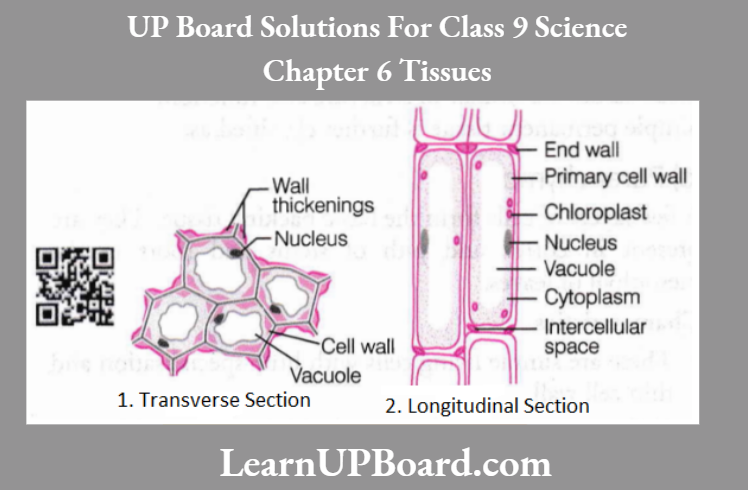
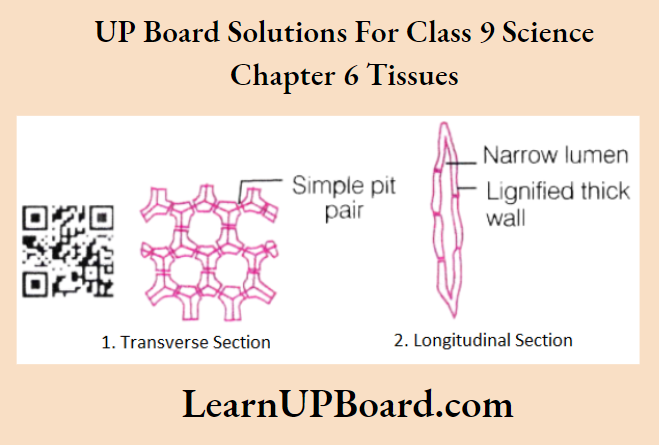
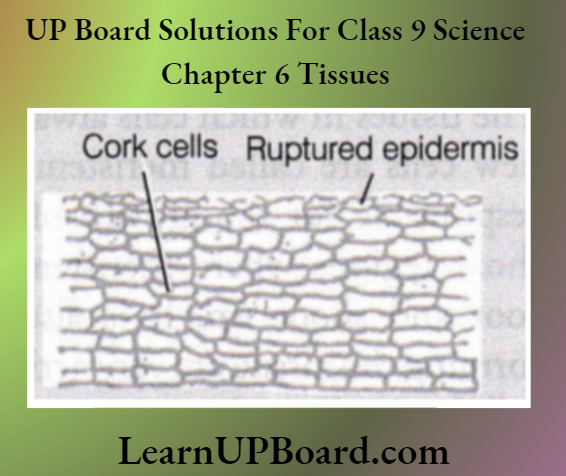
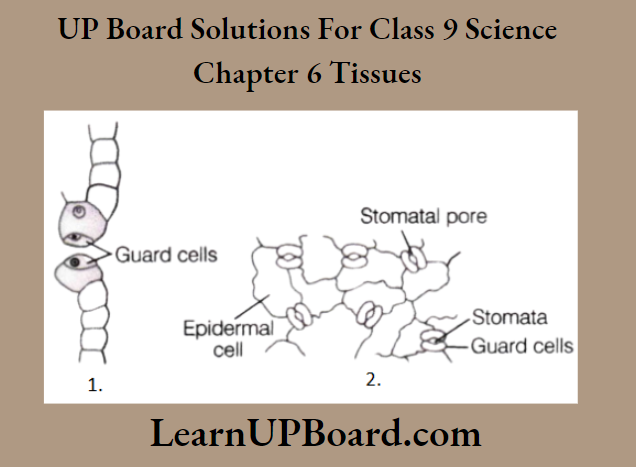
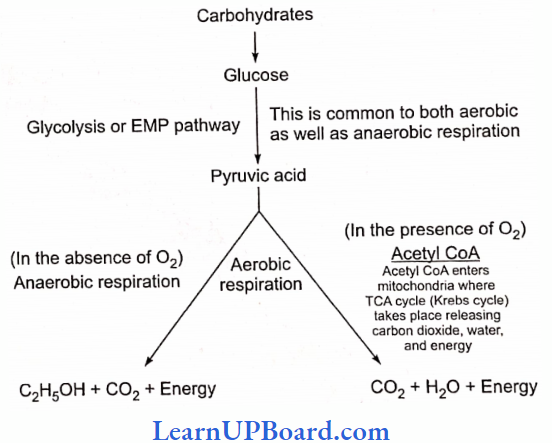
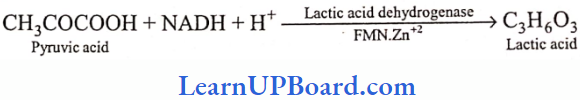
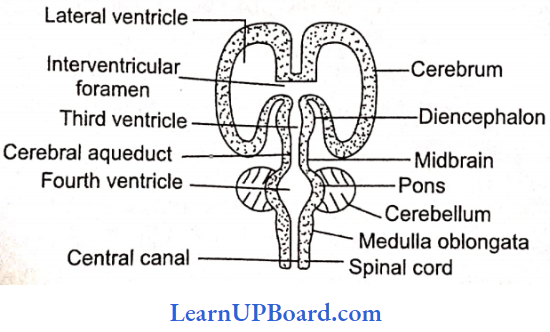
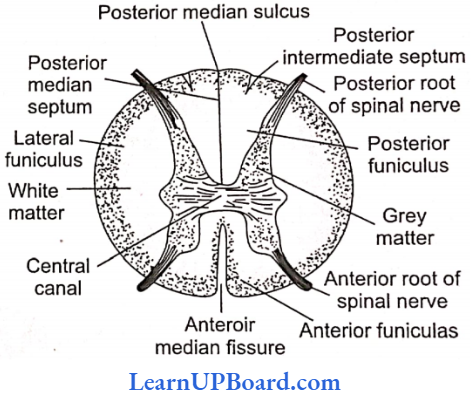
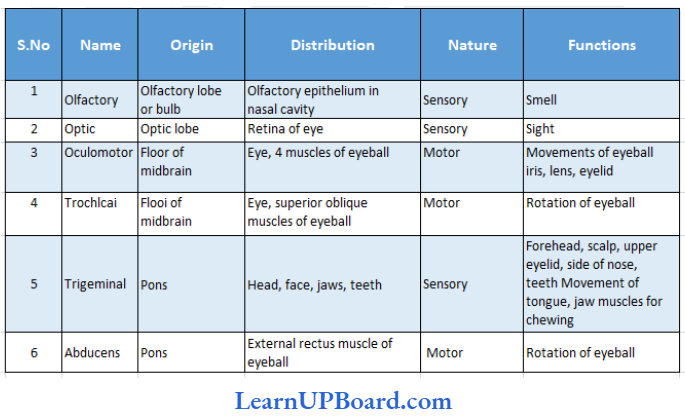
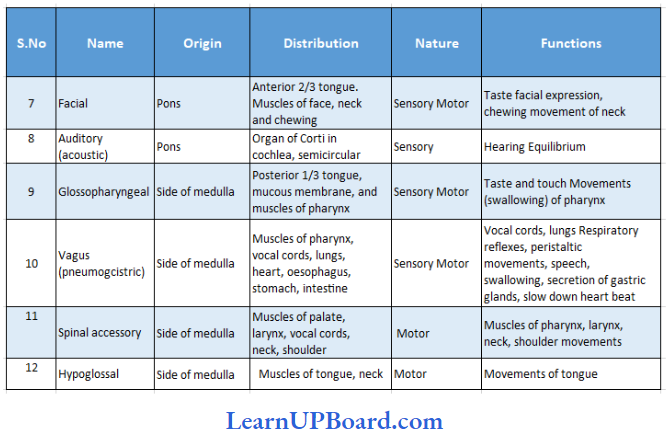
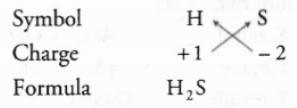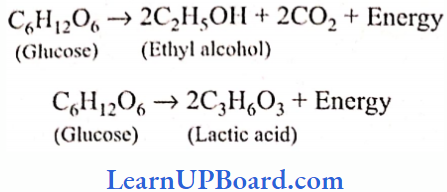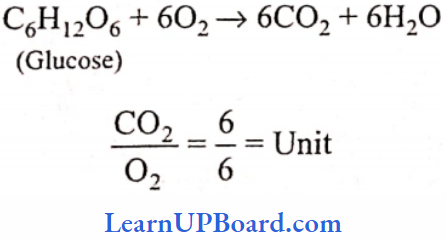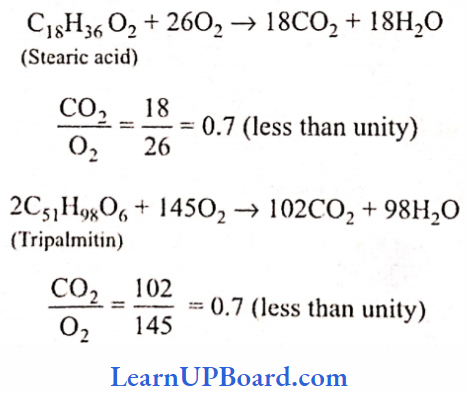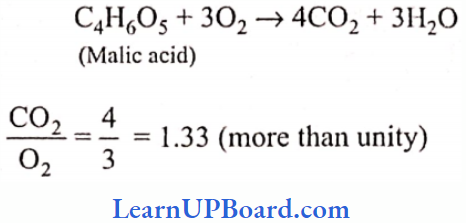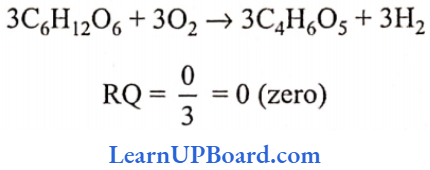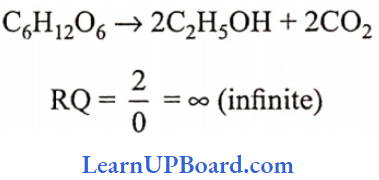UP Board Class 9 Science Notes For Chapter 1 Matter In Our Surroundings
In our surroundings, we see a large variety of objects with different shapes, sizes and textures. All objects everything in this universe is made up of material which scientists have named ‘matter’.
- The air we breathe, the food we eat, stones, clouds, stars, plants and animals, even a small drop of water or a particle of sand— everything is matter. Matter can be seen, tasted, smelled or felt.
- Matter can neither be created nor destroyed, it can only be changed from one form to another. In the modern day, scientists have evolved two types of classification of matter based on their physical and chemical properties. In this chapter, we shall learn about matter based on its physical properties.
UP Board Class 9 Science Notes For Chapter 1 Matter In Our Surroundings
Matter is anything that has mass and volume or we can say that anything that has mass, occupies space and can be felt by one or more sense organs is called matter.
Note: The SI unit of mass is kilogram (kg), volume is cubic metre (m3). The common unit of measuring volume is the litre (L) and 1L = 1 dm3, 1L = 1000 mL, 1 mL =1 cm3
Read and Learn More Class 9 Science Notes
Classification Of Matter In Science
- Early Indian philosophers classified matter into five basic elements, called the Panch-Tatva. These are air, water, earth, sky and fire. According to them everything living or non-living, was made up of these five basic elements.
- Nowadays, matter is classified according to its physical properties and chemical nature.
For example., solid, liquid and gas (based on particle arrangement or physical properties) or elements, compounds and mixtures (based on chemical nature).
Physical Nature Of Matter
If we study the physical composition of matter, we find that:
- Every matter is made up of certain particles which differ in shape, size and nature from other types of matter.
- The particles of matter are tiny (beyond our imagination).
Characteristics Of Particles Of Matter
Some important characteristics of particles of matter are as follows:
- Particles of matter have space between them.
- Particles of matter are in a state of continuous movement. This suggests that they possess some energy, called kinetic energy. As the temperature rises, the kinetic energy of the particles increases and hence, particles move faster.
- The particles of matter tend to diffuse, i.e. to intermix on their own with each other. They do so by getting into the spaces between the particles. The intermixing of particles of two different types of matter on their own is called diffusion.
- Particles of matter attract each other. A force of attraction exists between the particles, which is known as the intermolecular force of attraction. This force keeps the particles together. The strength of this force of attraction varies from one kind of matter to another.
Diffusion And Osmosis Process
Diffusion is the process in which molecules of a substance move from higher concentration to lower concentration and goes on until a uniform mixture is formed. In osmosis, the solvent molecules move from their lower concentration to higher concentration through a semipermeable membrane.
UP Board Class 9 Science Notes For Chapter 1 States Of Matter
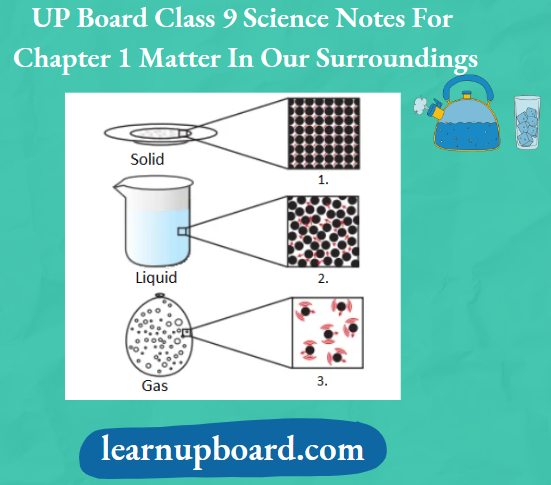
Matter around us exists in three different states which are solid, liquid and gas. These states of matter arise due to the variation in the characteristics of the particles of matter.
The Solid State: A Solid is defined as that form of matter which possesses rigidity, incompressible and hence, has a definite shape and a definite volume.
Some Important Properties Of Solid State Are As Follows:
- Solids have definite shapes, distinct boundaries and fixed volumes, i.e. have negligible compressibility.
- Solids tend to maintain their shape when subjected to outside force.
- Exception A rubber band changes shape under force and regains the same shape when the force is removed. If excessive force is applied, it breaks.
- Sugar and salt also take the shape of the container in which they are placed but are considered solids.
- This is because the shape of each sugar or salt crystal remains fixed.
- Solids either do not diffuse or diffuse at a very slow rate.
- Exception Sponge is compressible but considered as a solid. This is because a sponge has minute holes, in which air is trapped.
- When it is pressed, the air is expelled and we can compress it.
- Solids may break under force, but it is difficult to change their shape, so they are rigid.
- Generally, solids have higher densities as compared to their liquid or gaseous forms.
- Sugar, sand, rocks, stones, and metals like iron, copper, aluminium, gold, silver, etc., are examples of substances which exist in the solid state.
The mass per unit volume of a substance is called its density,
∴ Density =\(\frac{\text { Mass }}{\text { Volume }}=\frac{m}{V}\)
The Liquid State: Liquid is defined as that form of matter, which possesses a fixed volume, but has no fixed shape.
Some Important Properties Of the Liquid State Are As Follows:
- Liquids do not have a definite shape, i.e. they take up the shape of the container in which they are kept.
- Liquids flow and change shape, so they are not rigid but can be called fluid.
- Note: Fluid In science, the common name of gases and liquids is fluid.
- Solids, liquids and gases can diffuse into liquids. The gases from the atmosphere diffuse and dissolve in water. These gases, especially oxygen and carbon dioxide, are essential for the survival of aquatic animals and plants. Aquatic animals can breathe underwater due to the presence of dissolved oxygen in water.
- Liquids are almost incompressible.
- The attraction force between the particles of liquid is greater than that of gases but less than that of solids.
- The rate of diffusion of liquids is higher than that of solids. This is because, in the liquid state, particles move freely and have greater space between each other as compared to particles in the solid state.
- The density of a liquid is generally less than that of its solid form. Some exceptions are also there, for example., solid ice is lighter than water as it floats on water, i.e. the density of the solid form of water (ice) is less as compared to that of the liquid form of water. Water, milk, juice, oil, kerosene, petrol, alcohol, benzene etc., are examples of the substances which exist in the liquid state.
The Gaseous State: Gases can be defined as that form of matter which possesses high compressibility and hence, has neither definite shape nor definite volume.
Some Important Properties Of Gaseous State Are As Follows:
- Gases tend to flow as liquids do. Therefore, they are also considered as fluids.
- Gases show the property of diffusing very fast into other gases due to the high speed of particles and the large spaces between them.
- Due to the high diffusion tendency of gases, the smell of hot cooked food reaches us in seconds. The particles of the aroma of food mix with the particles of air spread, reaching us and even farther away.
- Gases are highly compressible. The Liquefied Petroleum Gas (LPG) cylinder used in our homes for cooking or the oxygen supplied to hospitals in cylinders is compressed gas.
- Compressed Natural Gas (CNG) is used as a fuel these days in vehicles. Due to its higher compressibility, large volumes of gas can be compressed into a small cylinder and transported easily.
- In a gaseous state, the particles move about randomly at high speed. Due to this random movement, gases exert pressure on the walls of the container, in which they are kept. Air is an example of a gaseous state. It is a mixture of gases like oxygen, nitrogen, carbon dioxide, inert gases, etc. Other examples of gases are hydrogen, ammonia, nitrogen dioxide, sulphur dioxide, etc.
- All living creatures need to breathe for survival. So, solids, liquids and gases can diffuse into liquids.
- The density of gases is minimal. A gas is much lighter than the same volume as a solid or a liquid.
Rigidity And Fluidity Definition
Rigid means inflexible. A solid is a rigid form of matter, hence it does not require a container to keep it. Fluid is a material which can flow easily and requires a vessel to keep it. A liquid is a fluid form of matter which takes the shape of a container, while a gas is a fluid form of matter which fills the container.
UP Board Class 9 Science Notes For Chapter 1 Change Of States Of Matter
In your daily life, you come across various substances which exist in three states, i.e. solid, liquid and gas, for example. water, wax, ghee, etc. Water is the most commonly observed example that exists as ice (solid), water (liquid) as well as water vapour (gas).
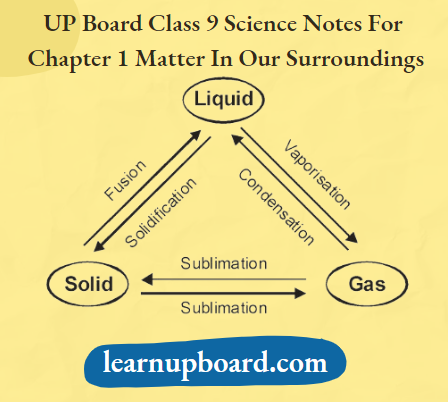
Interconversion Of States Of Matter Definition: The states of matter are interconvertible. The phenomenon of change of matter from one state to another and back to the original state by altering the conditions of temperature and pressure is called the interconversion of states of matter.
The following two factors (or any one of these) make it possible to convert one state of matter into another:
- Change in temperature
- Change in pressure
Terms Involved In Change Of State
The following terms are involved in a change of state:
- Fusion or Melting and Melting Point
- The process of conversion of a matter from its solid state to its liquid state at specific conditions of temperature and pressure is called fusion/melting.
- The definite temperature at which a solid starts melting is called the melting point of that solid, for example., the melting point of ice is 0°C or 273.16 K. The Higher the melting point of a substance, the greater the force of attraction between its particles.
- Boiling and Boiling Point
- The process of conversion of a matter from its liquid state to vapours (gaseous state) at specific conditions of temperature and pressure is called boiling.
- It is a bulk phenomenon. The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
- Sublimation
- The process of changing of solid state directly into a gaseous state without passing through the liquid state upon heating and vice-versa on cooling is known as sublimation. for example., naphthalene, camphor, iodine, ammonium chloride, etc., are the solids that undergo sublimation.
- Vapourisation
- The process of conversion of a matter from its liquid state to a gaseous state at specific conditions of temperature and pressure is called vapourisation.
- Freezing and Freezing Point
- The process of conversion of matter from its liquid state to solid state at specific conditions of temperature and pressure is called freezing.
- It is a reverse process of fusion/melting. The definite temperature at which a liquid changes into a solid state by giving out heat energy at 1 atm is called the freezing point.
- Condensation
- The process of conversion of matter from its gaseous state to liquid state at specific conditions of temperature and pressure is called condensation. It is a reverse process of vapourisation.
UP Board Class 9 Science Notes For Chapter 1 Effect Of Change Of Temperature
When a solid is heated, the kinetic energy of its particles increases. Due to an increase in kinetic energy, the particles start vibrating with greater speed.
- The energy supplied by the heat overcomes the forces of attraction between the particles.
- The particles leave their positions and start moving more freely. At a certain stage (i.e. at a melting point), a solid melts and is converted into a liquid state.
- At a certain temperature, a point is reached when the particles have enough energy to break free from the forces of attraction of each other. At this temperature (i.e. boiling point), the liquid starts changing into gas.
- In contrast, by decreasing the temperature (by cooling), a gas can be converted into a liquid state and a liquid can be converted into a solid state.
The effect of change in temperature on the physical state may be summarised as:

So, it can be concluded that the state of matter can be changed into another by changing the temperature.
Difference Between Gas And Vapour
A substance is said to be a gas if its boiling point is below room temperature, for example., O2, N2, CO2, etc. If the normal physical state of a substance is either a solid or a liquid, but gets converted into the gaseous state either on its own or by absorbing energy, the gaseous state is called the vapour state, for example., vapours of water in air.
Scales of Measuring Temperature
Three scales of measuring temperature are as follows:
1. Temperature on Kelvin scale
= Temperature on Celsius scale +273.16;
T(K)=t(°Q +273.16
Kelvin is the SI unit of temperature, 0 °C =273.16 K
For convenience, we take 0°C =273 K
2. Temperature on Celsius scale
= Temperature on Kelvin scale -273.16;
t(°C=T(K)-273.16
3. Temperature on Fahrenheit scale: Celsius and Fahrenheit temperatures are related to each other by the relation,
∴ \({ }^{\circ} \mathrm{F}=\frac{9}{5}\left({ }^{\circ} \mathrm{C}\right)+32\)
Question 1. Convert the temperature of 200°C to the Kelvin scale.
Answer:
Given
200°C
We know that, the temperature on the Kelvin scale
Temperature on Celsius scale +273.16 = 200 + 273.16 = 473.16 K
Thus, a temperature of 200°C on the Celsius scale is equal to 473.16 K on the Kelvin scale.
Question 2. Convert the temperature of 450 K to the Celsius scale.
Answer:
Given
450 K
We know that, the temperature on the Celsius scale
= Temperature on Kelvin scale – 273.16
= 450-273.16 =176.84°C
Thus, a temperature of 450 K on the Kelvin scale is equal to 176.84°C on the Celsius scale.
Latent Heat Definition:
When heat is given to a substance, its temperature increases. However, when heat is supplied to change the physical state of a substance, there is no increase in the temperature of a substance.
- Thus, the heat energy which has to be supplied to change the state of a substance is called its latent heat. In actuality, the word latent’ means ‘hidden’.
- Latent heat does not raise (or increase) the temperature. However latent heat is always supplied to change the state of a substance.
Latent Heat Is Of The Following Two Types:
Latent Heat Of Fusion (Solid To Liquid Change)
The amount of heat energy that is required to change 1 kg of a solid into liquid at atmospheric pressure and at its melting point is known as the latent heat of fusion. Particles in water at 0°C (273.16 K) have more energy as compared to particles in ice at the same temperature.
Latent Heat Of Vapourisation (Liquid To Gas Change)
The amount of heat energy that is required to convert 1 kg of a liquid into gas (at its boiling point) without any temperature rise is known as the latent heat of vapourisation. Particles in steam, i.e. water vapour at 373 K (100°C) have more energy than water at the same temperature.
Note: It has been found that burns caused by steam are much more severe than those caused by boiling water though both of them are at the same temperature of 100°C.
As particles in steam have absorbed extra energy in the form of latent heat of vapourisation. Thus, when steam falls on our skin and condenses to produce water, it gives more heat than boiling water.
UP Board Class 9 Science Notes For Chapter 1 Effect Of Change Of Pressure
The physical state of a substance can also be changed by changing the pressure. An increase in pressure brings the particles closer and increases the force of attraction between them, which brings about the change, for example., when high pressure is applied to a gas and its temperature is reduced, the gas is converted to a liquid, i.e. the gas is liquefied.
Hence, we can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
The pressure exerted by a gas is measured in the atmosphere (atm) unit. The pressure of air in the atmosphere is called atmospheric pressure.
Atmospheric pressure at sea level is taken as 1 atm which is also normal atmospheric pressure. As we go higher, atmospheric pressure decreases.
1 atm = 1.01 x 105 Pa (Pa = Pascal, SI unit of pressure)
UP Board Class 9 Science Notes For Chapter 1 Evaporation
The process of conversion of a liquid into its vapour state at any temperature below its boiling point is called evaporation. The particles of a liquid have different amounts of kinetic energy.
- The particles present at the surface possess comparatively higher kinetic energy as compared to those present in the bulk.
- Therefore, particles at the surface with higher kinetic energy can break away from the forces of attraction of other particles and get converted into vapour.
- Water, when left uncovered, slowly changes into vapour. Wet clothes dry up, etc., are happen due to evaporation.
Factors Affecting Evaporation
The rate of evaporation of a liquid depends upon the following factors:
- Surface area Evaporation is a surface phenomenon, if the surface area is increased, the rate of evaporation increases, for example., while putting clothes for drying up, we spread them out.
- Temperature The rate of evaporation of a liquid increases with a temperature rise. With the increase of temperature, more particles get enough kinetic energy to go into a vapour state. That is why, evaporation is faster in a hot summer day than in winter or on a cloudy day.
- Humidity It is the amount of water vapour present in air. The air around us cannot hold more than a definite amount of water vapour at a given temperature. If the amount of water in air is already high, the rate of evaporation decreases. That is why, clothes dry up faster on a dry day than on a wet (rainy) day.
- Wind speed It is known that clothes dry faster on a windy day. This is because, with an increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surroundings. That is why, the rate of evaporation of a liquid increases with increasing wind speed.
Note: The liquids which evaporate fast are called volatile liquids.
Evaporation Causes Cooling Effect
In an open vessel, the liquid keeps on evaporating. The particles of liquid absorb energy from the surroundings to regain the energy lost during evaporation. This absorption of energy from the surroundings makes the surroundings cold.
Some daily life examples of the cooling effect of evaporation are given below:
- When ice-cold water is kept in a glass tumbler for some time, water droplets are observed on its outer surface.
- Explanation This occurs as the water vapours present in the air come in contact with the glass tumbler, get cooled and condense to form these small water droplets.
- The formation of drops of water on the outside surface of a tumbler containing crushed ice shows the presence of water vapour in the air.
- Cotton clothes are used to wear during the summer season.
- Explanation Cotton is a good absorber of water, so it helps to absorb sweat from our bodies.
- As it is obvious, the person perspires more during summer due to the auto temperature control mechanism.
- Hence, wearing cotton clothes helps in the easy evaporation of sweat.
- When this sweat evaporates, it takes the latent heat of vapourisation from our body, which in turn, cools the body. Thus, a person feels comfortable.
- People sprinkle water on the roof or open ground on a hot sunny day.
- Explanation When water is sprinkled on a hot surface, it gets evaporates very quickly.
- As evaporated water leaves the surface cool due to the large latent heat of vapourisation of water, this technique is quite effective in summers for cooling the surface.
- Liquids like acetone (nail polish remover) or alcohol placed on your palm give you a feeling of cooling.
- Explanation Acetone and alcohol are volatile liquids. When kept on the palm, their particles gain energy from the palm or surroundings and evaporate causing the palm to feel cool.
UP Board Class 9 Science Notes For Chapter 1 Activity 1
Objective
To prove that matter is made up of tiny particles (and have intermodular space).
Materials Required
Beaker, water, salt or sugar, glass rod and marker.
Procedure
- Take a 100 mL beaker half filled with water and mark the initial water level with the help of a marker.
- Then, add a teaspoonful of sugar (or salt) into it and stir with the help of the glass rod.
- Mark the water level after the disappearance of the solute.

Question 1. What happens to the sugar when it is dissolved in water?
Answer: When sugar is dissolved in water, its crystals separate into very fine particles.
Question 2. Where does the sugar go?
Answer:
The sugar particles go into the spaces present between the particles of water and mix with them to form a sugar solution.
UP Board Class 9 Science Notes For Chapter 1 Activity 2
Objective
To prove that particles of matter:
- Are very small in size (particulate in nature).
- Move (diffuse) faster in a gaseous state as compared to solid or liquid states.
- Diffuse at a slower rate, if the density is higher.
- Diffuse faster at a higher temperature
Materials Required
Potassium permanganate, water, beakers, perfume, blue ink, honey and copper sulphate.
Observation
The water level does not change.
Explanation
- Matter is not continuous and is particulate, i.e. it is made up of particles. When salt is dissolved in water the water level does not change.
- It indicates that there are some vacant spaces among the particles of water. These are known as interparticle spaces. The particles of salt have occupied some of them.
Conclusion
Matter is made up of tiny particles and intermolecular spaces are present in between them. Check Yourself
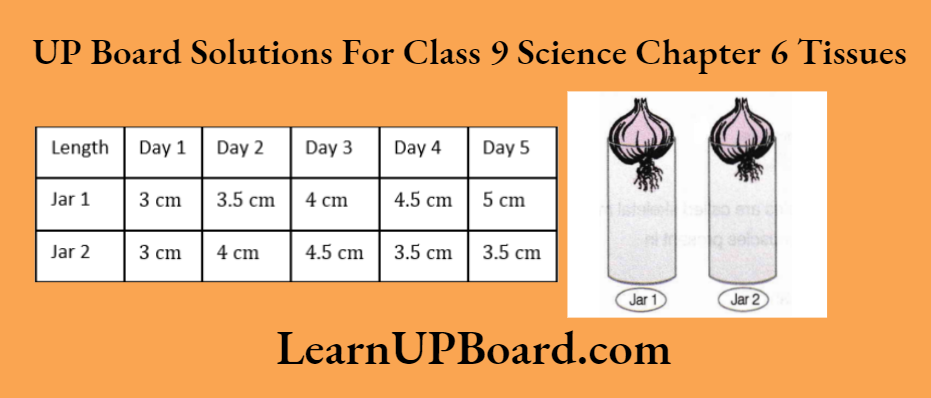
Observation Table
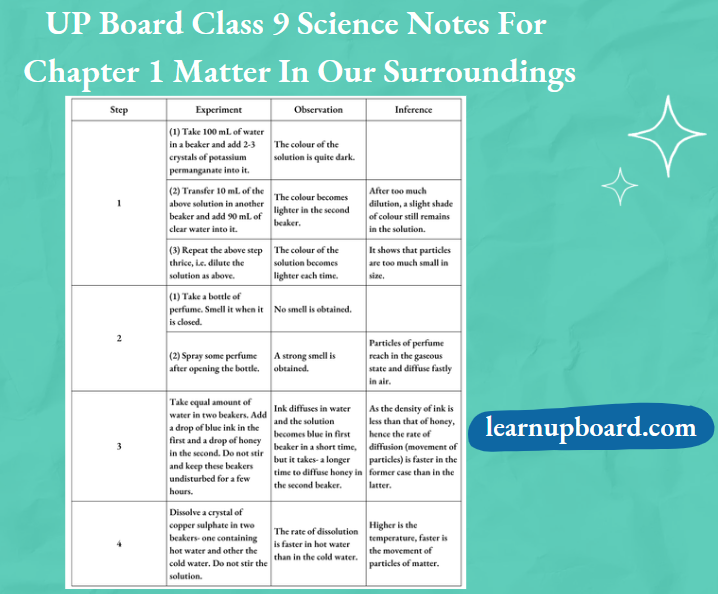
Question 1. What conclusion can you draw after adding 2-3 crystals of KMnO4 in water?
Answer:
After the addition of 2-3 crystals of KMn04 in water, it is concluded that a crystal of KMnO4 is made up of millions of tiny particles. They keep dividing themselves into smaller particles.
Question 2. When someone opens a bottle of perfume in one corner of a room, its smell spreads in the whole room quickly. Why?
Answer:
This happens because the particles of perfume (gas) move rapidly in all directions and mix with the moving particles of air in the room. They do so by getting into the spaces between the air particles.
Question 3. Why honey in step 3 dissolves at a slower rate?
Answer:
In step 3, honey dissolves at a slower rate because it is more viscous i.e. has more density and has strong intermolecular forces of attraction.
Question 4. From step 4, write the effect of temperature on diffusion.
Answer: Diffusion becomes faster at a higher temperature.
Question 5. The rate of diffusion is faster in gases, why?
Answer:
The molecules of gases have large intermolecular space between them and have higher speeds. So they diffuse faster.
UP Board Class 9 Science Notes For Chapter 1 Activity 3
Objective
To study the properties of solids and liquids.
Materials Required
Pencils, books, needles, thread, paper, hammers, some liquids (for example., water, oil, milk etc), containers of different shapes and the same volume.
Procedure
- Take a book, a needle and a piece of thread. Draw their sketch on paper with the help of a pencil. Observe their shapes and judge their volume. Now pull, drop and hammer these things one by one and record your observations.
- Take some liquids (50 mL each) and pour them into containers of different shapes. Put a 50 mL mark on these containers using a measuring cylinder from the laboratory.
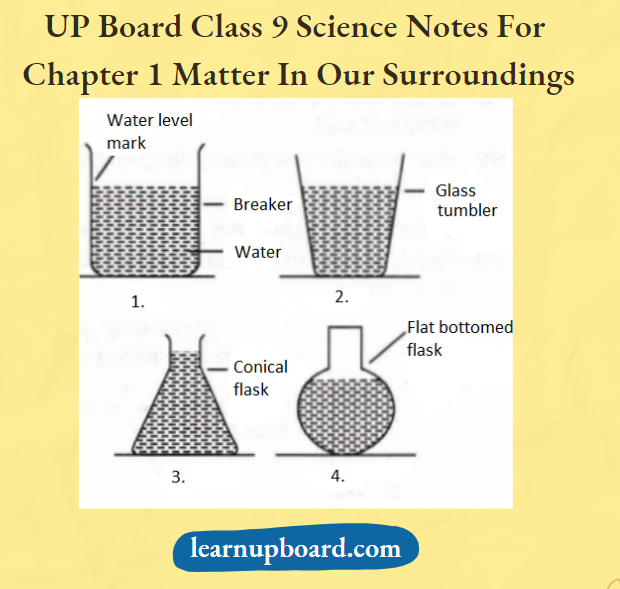
Observe the shapes of each liquid in each container. When 50 mL of liquid is poured into another container, does its volume change?
Observation
- All these things (books, needles and thread) have sharp boundaries, i.e. definite shapes and definite volumes. When pulled, dropped or hammered, these things remain unaffected or do not break.
- Volume of the liquids remains the same but its shape depends upon the shape of the container.
Conclusion
- Solids have a definite shape and definite volume. Solids are hard and rigid and held together with greater force.
- Liquids have fixed volumes but different shapes, as they acquire the shape of the container in which they are kept Liquids tend to flow, i.e. these are fluids.
Question 1. In which states of matter, the arrangement of particles is in the most ordered form?
Answer: In a solid state, the particles are arranged in the most ordered form.
Question 2. State one similarity between solids and liquids.
Answer: Both solids and liquids have a definite volume.
Question 3. What is the nature of shape and volume of liquids?
Answer: Liquids have indefinite shape and definite volume.
UP Board Class 9 Science Notes For Chapter 1 Activity 4
Objective
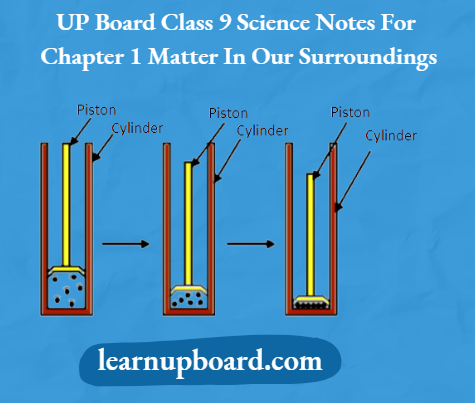
To show that gases can be compressed more easily than liquids and solids.
Materials Required
Three syringes, rubber corks, chalk and Vaseline.
Procedure
1. Take three 100 mL syringes and close their nozzles by rubber corks, as shown in the figure below.

Compressing gas, liquid and solid on applying pressure
2. Remove the pistons from all the syringes.
3. Leaving the first syringe untouched, fill water in the second and pieces of chalk in the third.
4. Insert the pistons back into the syringes. You may apply some Vaseline on the pistons before inserting them into the syringes for their smooth movement.
5. Now, try to compress the content by pushing the piston in each syringe.
Observation
The piston of the first syringe can be moved very easily but less easily in the second and with the most difficulty in the third.
Conclusion
Gases are highly compressible as compared to solids and liquids.
Question 1. Why gases can be compressed more easily than liquids?
Answer:
Cases can be compressed more easily than liquids because of weak intermolecular forces of attraction between particles.
∴ \(\left(\text { Because, Compressibility } \propto \frac{1}{\text { Intermolecular forces of attraction }}\right)\)
Question 2. In this activity, what do you infer from your observations?
Answer: Gases are highly compressible as compared to solids and liquids.
Question 3. Why solids cannot be compressed?
Answer:
Solids cannot be compressed because constituent particles are very closely packed and the movement of constituent particles is restricted.
Question 4. Which form of water is highly compressible?
Answer: The gaseous form of water is highly compressible.
UP Board Class 9 Science Notes For Chapter 1 Activity 5
Objective
To show the effect of temperature on the physical state of matter.
Materials Required
Ice, beaker, laboratory thermometer, glass stirrer, burner and iron stand.
Procedure
- Take about 150 g of ice in a beaker and suspend a laboratory thermometer in it such that the bulb of the thermometer is in contact with the ice.
- Start heating the beaker on a low flame.
- Note the temperature when the ice starts melting.
- Note the temperature when all the ice has converted into water.
- The observations are recorded for the conversion of ice into water.
- Place a glass stirrer in the beaker and heat while stirring till the water starts boiling.
- Note the temperature when most of the water vaporised.

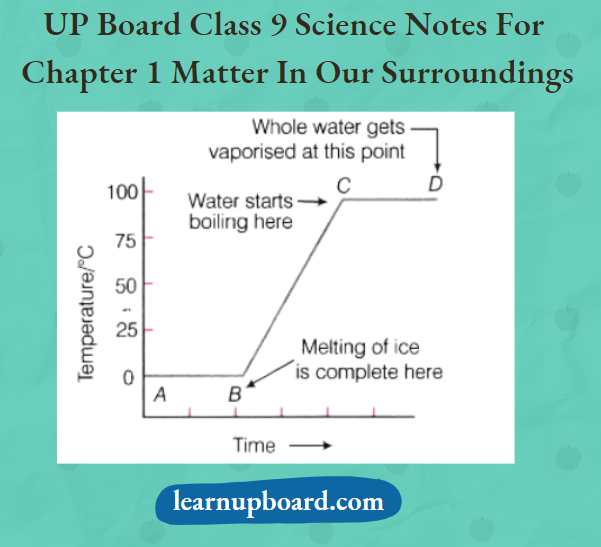
Observation
- There is no change in temperature till all the ice melts though heating continues.
- Temperature remains constant at 0°C. Once the ice is converted to water, the temperature starts rising till the water begins to boil. Once the water starts boiling, the temperature remains constant at 100°C till all the water has changed to vapours.
Conclusion
- During the change of state from solid to liquid or from liquid to gas, the temperature remains constant till all the solid has melted or all the liquid has vaporised.
- The heat energy supplied is used up in overcoming the forces of attraction and hence, the thermometer does not show any temperature rise.
Question 1. What is the change in temperature during this activity?
Answer: The temperature remains constant during the complete melting and boiling process.
Question 2. Is melting an exothermic or endothermic process?
Answer: Melting is an endothermic process because heat is absorbed during this process.
Question 3. Under which conditions, we can boil water at room temperature?
Answer: We can boil water at room temperature under low pressure.
Question 4. Draw a temperature-time graph for the heating of ice.
Answer: The graph of temperature-time for the heating of ice is given below:
Question 5. In an experiment for studying the effect of heating on ice, why do we use crushed ice?
Answer: Crushed ice will cover the thermometer bulb intimately and thus, would give the correct temperature.
Question 6. What do we call the temperature at which liquid starts boiling?
Answer: The temperature at which liquid starts boiling is called its boiling point.
Question 7. What would be the temperature when water starts boiling?
Answer: As the water starts boiling, the temperature will be 100°C or 373K.
Question 8. What is the temperature when all the water has boiled?
Answer: The temperature remains constant, i.e. 100°C during the complete boiling process.
UP Board Class 9 Science Notes For Chapter 1 Activity 6
Objective
To study the process of sublimation.
Materials Required
Camphor or ammonium chloride, China dish, funnel, cotton plug and burner.
Procedure
- Take some camphor or ammonium chloride. Crush it and put it in a China dish.
- Put an inverted funnel over the China dish.
- Put a cotton plug on the stem of the funnel and set the apparatus
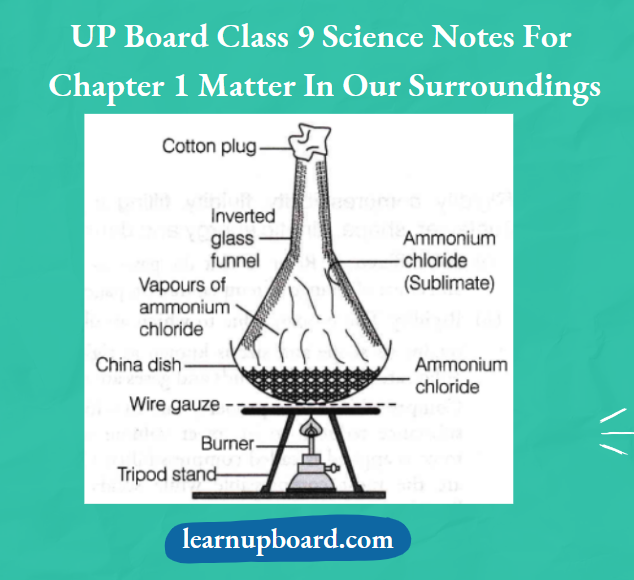
Observation
Solid ammonium chloride changes into vapours without changing into a liquid state and gets condensed on the walls of the funnel.
Conclusion
A change of state directly from solid to gas without changing into liquid state or vice-versa is called sublimation.
Question 1. In an experimental set-up for sublimation, a perforated asbestos sheet is placed between the China dish and funnel. What is its purpose?
Answer: The asbestos sheet prevents direct heating of the funnel.
Question 2. What happens when solid ammonium chloride is heated?
Answer:
On heating, solid ammonium chloride changes into vapours without changing into a liquid state and gets condensed on the walls of the funnel,
Question 3. Name the solid substance which is obtained by cooling the vapour.
Answer: The solid substance obtained by cooling the vapour is known as sublimate.
Question 4. Which substance is sublimated in this activity?
Answer: Sublimate is pure ammonium chloride.
UP Board Class 9 Science Notes For Chapter 1 Activity 7
Objective
To study the factors which affect evaporation.
Materials Required
Test tubes, water, jar, China dish, thermometer and cupboard.
Procedure
- Take 5 mL of water in a test tube and keep it near a window or under a fan.
- Take 5 mL of water in an open China dish and keep it near a window or under a fan.
- Take 5 mL, of water in an open China dish and keep it inside a cupboard or on a shelf in your class.
- Record the room temperature.
- Record the time or days taken for the evaporation process in the above cases.
- Repeat the above three steps of activity on a rainy day and record your observations.
Observation
- In an open China dish kept near a window or on a shelf, water evaporates very fast as compared to a test tube.
- On sunny days, water evaporates very fast as compared to rainy days.
Conclusion
- Surface area is increased the rate of evaporation increases.
- If the temperature is increased, the rate of evaporation increases because with the increase of temperature, more particles get enough kinetic energy to go into the vapour state.
- If wind speed is increased, the rate of evaporation increases because with the increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surroundings.
- If humidity is decreased, the rate of evaporation increases and vice-versa
Question 1. Why does water evaporate faster in a Chinese dish as compared to a test tube?
Answer:
China dish has more surface area as compared to a test tube. So, evaporation is faster in the case of China dishes.
Question 2. Why evaporation is fast when water is kept under a fan?
Answer:
When water is kept under a fan, the rate of evaporation increases because with the increase in wind speed, the particles of water vapour move away with the wind.
Question 3. What is the effect of humidity on evaporation?
Answer: If humidity is decreased, the rate of evaporation increases and vice-versa.
Question 4. What do you infer about the effect of temperature on evaporation?
Answer: If the temperature is increased, the rate of evaporation increases.
Question 5. Why evaporation is slow on a rainy day?
Answer: On a rainy day, humidity is increased, so the rate of evaporation decreases.
UP Board Class 9 Science Notes For Chapter 1 Matter In Our Surroundings Summary
Matter is anything that has mass and occupies volume. The SI unit of mass and volume is kilogram (kg) and cubic metre (m3), respectively.
- Matter is classified based on their physical and chemical properties, i.e. physical properties (solid, liquid and gas) and chemical properties (elements, compounds and mixtures).
- Every matter is made up of certain particles which differ in shape, size and nature.
- The particles of matter tend to diffuse.
- Solids have definite shapes, distinct boundaries and fixed volumes.
- Liquids do not have a definite shape.
- Gases have neither definite shape nor volume.
- The state of matter can be interchanged by changing temperature or pressure.
- At specific conditions of temperature and pressure; the conversion of a matter from its solid to its liquid state is called fusion.
- The conversion of a matter from its liquid state to vapour (gaseous state) is called boiling.
- It is a bulk phenomenon.
- The conversion of a matter from its liquid to solid state is called freezing.
- The conversion of a matter from its liquid to gaseous state is called vapourisation.
- The conversion of matter from its gaseous to liquid state is called condensation.
- The process of changing of solid state directly into a gaseous state without passing through the liquid state upon heating and vice-versa on cooling is called sublimation. The heat energy which has to be supplied to change the state of a substance is called latent heat.
- The latent heat of vapourisation is the heat energy required to change 1kg of a liquid to gas at atmospheric pressure at its boiling point.
- Latent heat of fusion is the amount of heat energy required to change 1 kg of solid into liquid at its melting point.
- The process of conversion of a liquid into its vapour state at any temperature below its boiling point is called evaporation.
- Apart from the solid, liquid and gaseous states, scientists have discovered two more states, i.e. plasma and Bose-Einstein condensate.