UP Board Solutions For Class 9 Science Chapter 4 Structure Of The Atom Long Answer Type Questions
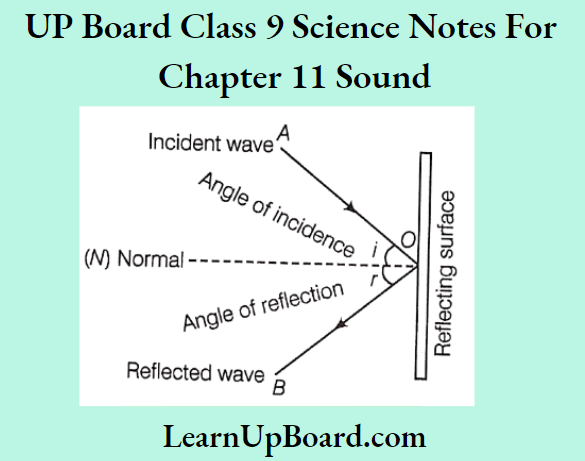
Question 1. Which popular experiment is shown in the figure?
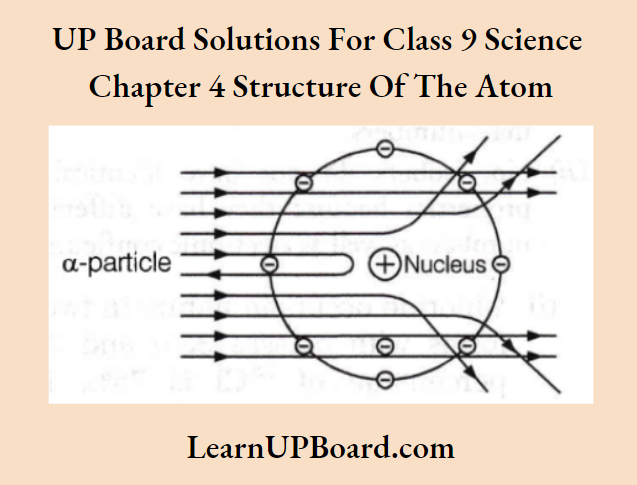
Answer:
This figure shows the scattering of a-particles by a gold foil which is known as the Rutherford experiment or a-particle scattering experiment.
Question 2.
- What is an octet? How do elements attain an octet?
- Make a schematic atomic structure of magnesium and phosphorus.
[Given number of protons of magnesium =12 and that of phosphorus =15 ]
Answer:
- An outermost shell, which has eight electrons is said to possess an octet. Elements attain their octet by sharing, gaining, or losing electrons.
- Atomic structure of Mg
Read and Learn More Class 9 Science Solutions
12Mg = \(\begin{aligned}
& K L M \\
& 2,8,2
\end{aligned}\)
The atomic structure of P
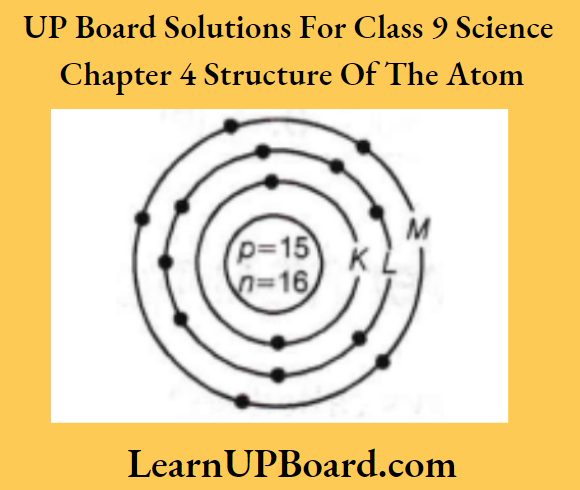
15P = \(\begin{aligned}
& K L M \\
& 2,8,5
\end{aligned}\)
Question 3.
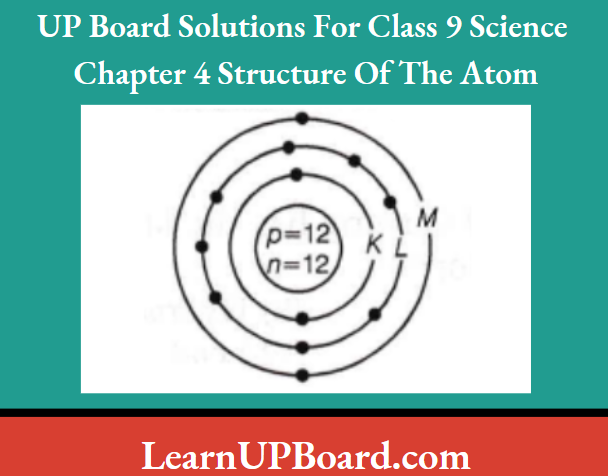
- An element X has an atomic number =12 and a mass number =26. Draw a diagram showing the distribution of electrons in the orbits and the nuclear composition of the neutral atom of the element. What is the valency of the element and why?
- If this element X combines with another element Y whose electronic configuration is 2, 8, 7. What will be the formula of the compound thus formed? State how you arrive at this formula.
Answer:
Atomic number =12. Mass number =26
Atomic structure of X
Electronic configuration = 2, 8, 2
Nuclear composition
Number of protons =12
Number of neutrons =26 -12 =14
Valency = 2
Because it can donate 2 electrons easily to complete its octet and become stable.
The valency of the element E would be 1, i.e. it can gain 1 electron to become stable. When it combines with the element X of valency 2, the compound formed will be XY2.
The formula of the compound would be XY2.
Question 4.
- Rutherford’s a-particle scattering experiment gives the experimental evidence for deriving the conclusion that
- Most of the space inside the atom is empty.
- The nucleus of an atom is positively charged.
- An element has mass number =32 and atomic number = 16, find
- The number of neutrons in the atom of the element.
- The number of electrons in the outermost shell of the atom.
- Based on Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer:
- Most of the a-particles passed straight through the gold foil.
- A few of the a-particles which are positively charged, deflected due to the positive charge of the nucleus.
- Number of neutrons = Mass number – atomic number =32-16=16
- The electronic configuration of the element will K L M be as follows: 2, 8, 6
- Hence, the number of electrons in the outermost shell is 6.
- According to Rutherford’s model of an atom, positively charged protons are present in the nucleus of an atom.
Question 5.
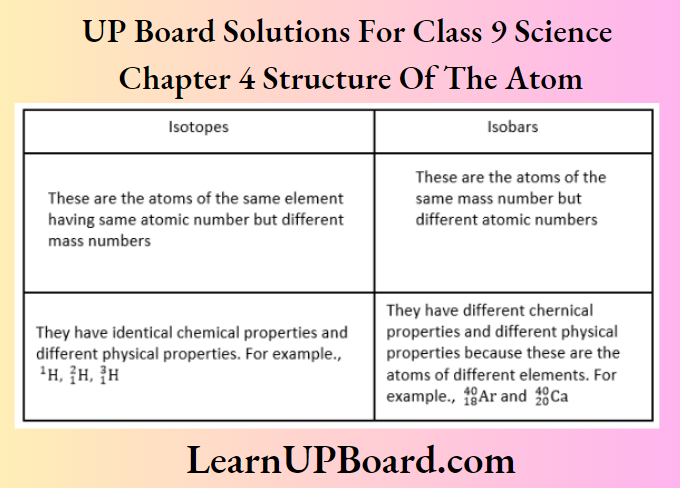
- Write two differences between isotopes and isobars.
- Write uses of Co-60 and U-235.
Answer:
Difference between isobars and isotopes:
- The isotope of cobalt (Co-60) is used in the treatment of cancer.
- The isotope of uranium (U-235) is used as a fuel in a nuclear reactor.
Question 6. Which of the following is a correct electronic configuration of sodium?
- 2, 8
- 8, 2, 1
- 2, 1,8
- 2, 8, 1
Answer: 4. 2, 8, 1
The atomic number of sodium atom is 11, therefore,
its electronic configuration is \(\begin{gathered}
K L M \\
2,8,1 .
\end{gathered}\).
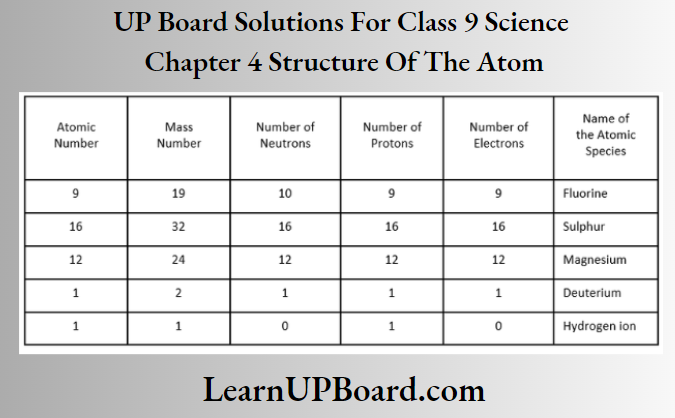
Question 7. Complete the following table.
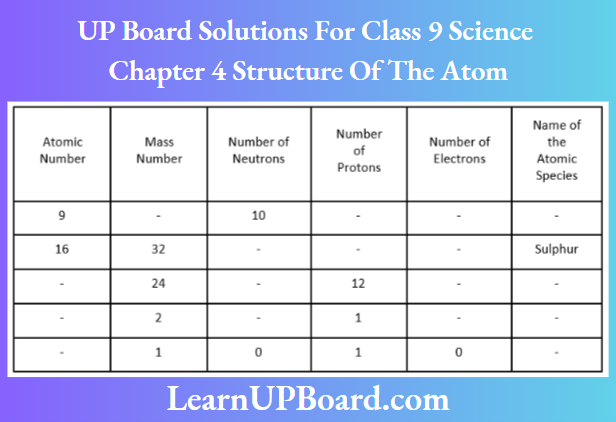
Answer:
Explanation
Fluorine (9F19) Given, atomic number = 9 and number of neutrons =10
Mass number =Atomic number + number of neutrons
= 9 + 10
=19
Number of protons = Atomic number
= Number of electrons = 9
Sulphur (16S32) Given, atomic number =16
Number of protons = Number of electrons =16 Number of neutrons
= Mass number — atomic number – =32-16=16
Magnesium (12Mg24) Number of protons =12
Atomic number = Number of protons = 12
Number of electrons = Number of protons =12
Number of neutrons = Mass number – atomic number
= 24 -12 = 12
Deuterium (1D2) Number of protons = 1
and Number of electrons = 1
Atomic number = 1, mass number = 2
Number of neutrons = 2-1=1
Hydrogen ion (1H+) Mass number = 1
Number of protons = 1
Number of neutrons = 0
Number of electrons = 0
Atomic number = Number of protons =1
Because several electrons are zero, i.e. not equal to that of protons, the species is a hydrogen ion, not a hydrogen atom.
Question 8. Define valency by taking examples of silicon and oxygen.
Answer:
Valency of an element
The combining capacity of the atom of an element with the atom(s) of the same or other elements (s) to complete its octet is called the valency of that element. The number of valence electrons present in the atom of that element determines the valency of an element.
If the number of valence electrons of the atom of an element is less than or equal to four then the valency of that element is equal to the number of valence electrons, e.g. the atom of silicon has four valence electrons
[ ∵ atomic no of silicon is 14, therefore its electronic configuration is \(\begin{aligned}
& K L M \\
& 2,8,4
\end{aligned}\) ]
Thus, the valency of silicon is four.
On the other hand, if the number of valence electrons of the atom of an element is greater than four, the valency of that element is obtained by subtracting the number of valence electrons from eight, e.g. the atom of oxygen has six valence electrons
[ ∵ atomic no. of oxygen is 6, therefore, its electronic configuration is 2 (K), 6 (L)]. Thus, the valency of oxygen is two [8-6 = 2].
Question 9. Explain with examples.
- Atomic number
- Mass number
- Isotopes
- Isobars
Answer:
Give any two uses of isotopes
Atomic number The number of protons present in the nucleus of the atom of an element is called its atomic number.
Atomic number = Number of protons
For example., a sodium atom has 11 protons. Thus, its atomic number is 11. It is represented as 11Na.
Mass number The sum of several protons and neutrons (together called nucleons) present in the nucleus of an atom is called its mass number.
Mass number = Number of protons + number of neutrons
For example., a sodium atom has 11 protons and 12 neutrons. Its mass number is 23. It is represented as 23Na.
Isotopes Atoms of an element having the same atomic number but different mass numbers are called isotopes of that element.
For example., hydrogen has 3 isotopes 1H1,1H2 and 1H3.
Isobars Atoms of different elements with different atomic numbers but the same mass number are known as isobars.
For example., \({ }_{18}^{40} \mathrm{Ar} \text { and }{ }_{20}^{40} \mathrm{Ca}\) are isobars.
Uses of isotopes are:
An isotope of uranium (U-235) is used as a fuel for the production of electricity in nuclear reactors.
An isotope of cobalt is used in the treatment of cancer.
Question 10. Na+ has completely filled K and L shells. Explain.
Answer:
Electronic configuration of Na (atom) = \(\begin{array}{ccc}
K & L & M \\
2, & 8, & 1 .
\end{array}\)
When an atom loses one electron, it acquires a positive charge. Thus, when Na-atom loses one electron from its Af-shell it gets converted into Na+ion.
Electronic configuration of Nation) = \(\begin{array}{ll}
K & L \\
2, & 8 .
\end{array}\)
As the maximum capacity of the K-shell to occupy electrons is 2 and that of the Z-shell is 8 electrons, so, Na+ has filled K and L-shells.
Question 11. If a bromine atom is available in the form of, say, two isotopes \({ }_{359}^{79} \mathrm{Br} \quad(49.7 \%) \text { and }{ }_{35}^{81} \mathrm{Br}\), calculate the average atomic mass of the bromine atom.
Answer:
Given
A bromine atom is available in the form of, say, two isotopes \({ }_{359}^{79} \mathrm{Br} \quad(49.7 \%) \text { and }{ }_{35}^{81} \mathrm{Br}\),
The average atomic mass of an element
⇒ \(\begin{aligned} & =\left[\binom{\text { Atomic mass of isotope I }}{\left.\times \frac{\text { percentage of isotope I }}{100}\right)}\right. \\ & \left.+\left(\text { Atomic mass of isotope II } \times \frac{\text { percentage of isotope II }}{100}\right)\right] \end{aligned}\)
Average atomic mass of bromine
⇒ \(=\left[\left(79 \times \frac{49.7}{100}\right)+\left(81 \times \frac{50.3}{100}\right)\right]\)
= 39.263+ 40.743 = 80.006 u.
Question 12. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 168X and 18X in the sample?
Answer:
Given
The average atomic mass of a sample of an element X is 16.2 u.
Let, the percentage of 8X16 = x%
Then, the percentage of 8X18 = (100 – x) % … (1)
The average atomic mass of X =16.2 u
According to the given data,
⇒ \(16 \times \frac{x}{100}+18 \times \frac{(100-x)}{100}=16.2\)………(2)
⇒ \(\frac{16 x}{100}+\frac{1800-18 x}{100}=16.2\)
⇒ \(16 x+1800-18 x=1620\)
⇒ \(-2 x=-1800+1620 \Rightarrow \)
⇒ \(x=\frac{180}{2}=90 \%\)
Placing the value of x in Eq.(1)
Percentage of ls8X =100 -90 =10%
∴ Isotope 8X16 =90% , isotope 8X18 =10%
Question 13. The number of valence electrons in CF ions is
- 16
- 8
- 17
- 18
Answer: 2. 8
Valence electrons are the electrons present in the outermost shell of an atom. Cl– ion is formed when the Cl atom gains an electron. The atomic number of Cl is 17, it has 1 7e–, and when it gains one e–, it becomes Cl– ion and has 18e–.
Thus, the electronic configuration of Cl– atom and Cl– ion are as follows:
Cl (atom) = 2, 8 7;
Valence electrons = 7
Cl– (ion) = 2, 8, 8;
Valence electrons = 8
Hence Cl– ion has 8 valence electrons.
Question 14. Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
Isotopes, for example., \({ }_{17}^{35} \mathrm{Cl} \text { and }{ }_{17}^{37} \mathrm{Cl}\)
The electronic configuration of both the isotopes of chlorine is the same as their atomic number are the same.
17Cl = \(\begin{array}{ccc}
K & L & M \\
2, & 8, & 7
\end{array}\)
Isobars, for example., \({ }_{20}^{40} \mathrm{Ca} \text { and }{ }_{18}^{40} \mathrm{Cl}\)
The electronic configuration of both isobars would be different as both have different atomic numbers but have the same mass number.
⇒ \(\begin{array}{rccc}
K & L & M & N \\
{ }_{20} \mathrm{Ca}=2, & 8, & 8, & 2
\end{array}\)
⇒ \({ }_{18} \mathrm{Ar}=2,8,8\)
Question 15. Two ions having 3 negative and 3 positive charges are found to have 7 and 14 neutrons respectively. If the electronic configuration of these ions is similar to that of neon (Ne), then find the mass number of both ions.
Answer:
Given
Two ions having 3 negative and 3 positive charges are found to have 7 and 14 neutrons respectively.
The electronic configuration of Ne = \(\begin{array}{cc}
K & L \\
2, & 8
\end{array}\)
Atom, which forms ion similar to neon with 3 negative charges, will have an electronic configuration as = \(\begin{array}{cc}
K & L \\
2, & 5
\end{array}\)
Hence, the given atom is nitrogen.
Mass number = Number of protons + number of neutrons
= 7+7 = 14
Similarly, an atom that forms an ion similar to neon with 3 positive charges, will have an electronic configuration as = \(\begin{aligned}
& K L M \\
& 2,8,3 \\
&
\end{aligned}\)
Hence, the given atom is aluminum.
Mass number = Number of protons + number of neutrons
= 13 + 14
= 27
UP Board Solutions For Class 9 Science Chapter 4 Structure Of The Atom Short Answer Type Questions
Question 1. How can you justify that cathode rays originate from the cathode whereas anode rays do not?
Answer:
- On applying high potential on a discharge tube, any metal used as a cathode-electrode emits a beam of electrons having (-)ve charge, moving towards the oppositely charged (+)ve plate (anode electrode) known as cathode-rays.
- Anode rays are gaseous (+)ve-ions (kept in discharge tube) produced due to the knock-out of electrons from gaseous atoms that move towards (-)ve plate. This shows that cathode rays originate from the cathode whereas anode rays do not.
Question 2. Write the conclusions drawn by Rutherford when he observed the following.
- Most of the a-particles pass straight through the gold foil.
- Some a-particles getting deflected from their path.
Answer:
- Most of the space inside the atom is empty.
- Some negatively charged particles (called electrons) are present around the center (called the nucleus).
Question 3. List down three different names given to the path in which electrons revolve around the nucleus. Also, explain why are they called so.
Answer:
The three different names are:
- Discrete orbit It is called so because electrons revolve in certain distinct paths and not just in any orbit.
- Energy level (energy shell) The energy associated with different orbits (with which electron revolves) is distinct for each orbit, hence it is called so.
- Stationary state Since the energy associated with an orbit is fixed. Hence, the electron revolving in a particular orbit has stationary energy. That’s why, the orbit is also called a stationary state,
Question 4.
- What will be the maximum number of electrons that can be filled in the Z-shell of an imaginary atomic model?
- Why is it almost impossible to find such an atom in nature?
Answer:
According to the Bohr and Bury rule, the maximum number of electrons in. Z-shell (16th shell, starting from K)
= 2n2 = 2 × 16 × 16
= 512
Atoms with greater number of electrons have greater number of protons and neutrons therefore they have unstable nucleus which radiates energy or divide itself into smaller stable nuclei. Hence, elements with atomic number greater than 100 are rarely found in nature.
Question 5.
- Write the name of an element whose atom has the same number of sub-atomic particles. Draw the atomic structure of the atom.
- Draw the atomic structure of an atom with the same number of electrons in L and M-shells.
Answer:
1. Name of element: Oxygen (8O16)
Number of electrons = 8
Number of protons = 8
Number of neutrons =16-8=8
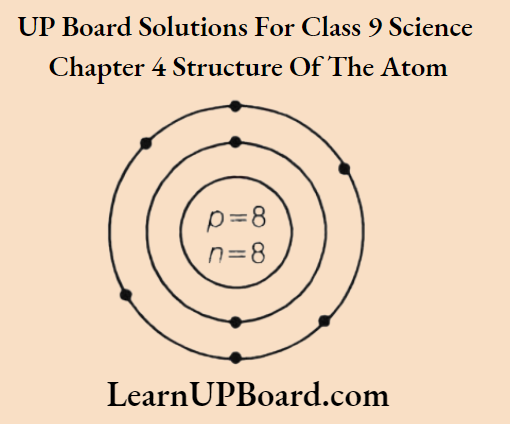
2. Name of element: Argon Atomic number
⇒ \(\begin{array}{r}
K L M \\
(18)=2,8,8
\end{array}\)
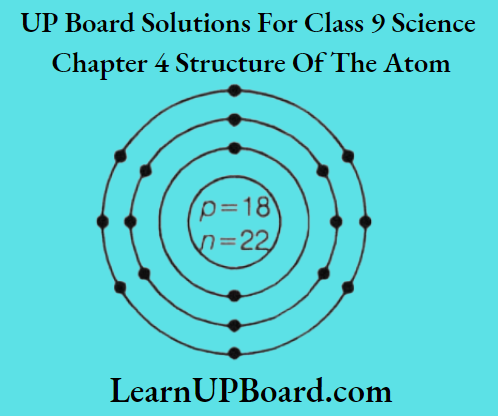
Number of electrons in L-shell = 8
Number of electrons in M-shell = 8
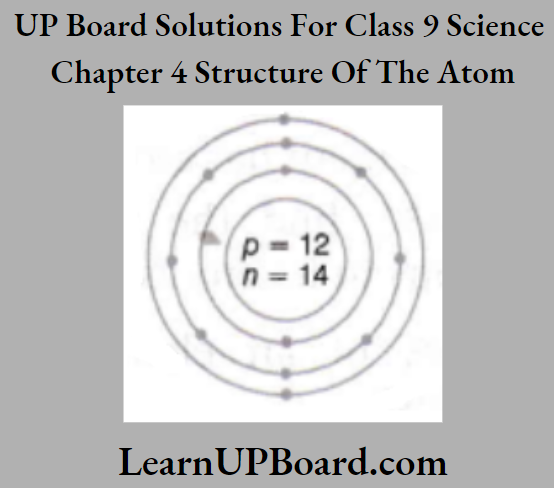
Question 6. Given below is the atomic structure of an atom of element 11A23, according to Bohr’s
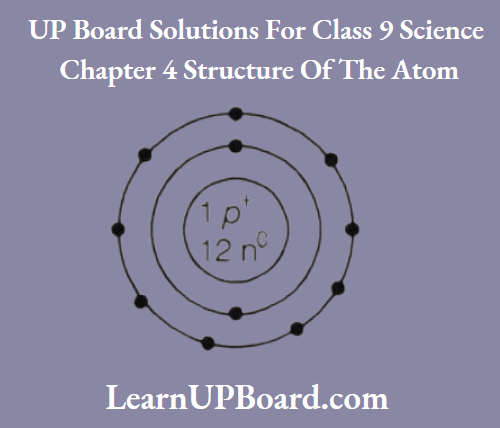
- What is wrong with this structure of an atom? Draw a correct representation of this atom.
- Write the chemical formula of the chloride of this element.
Answer:
1. The element A is Na and has three shells K, L, and M but here only 2 shells are given.
Further, Z-shell cannot have more than 8 electrons but here 9 electrons are given.
The correct structure is
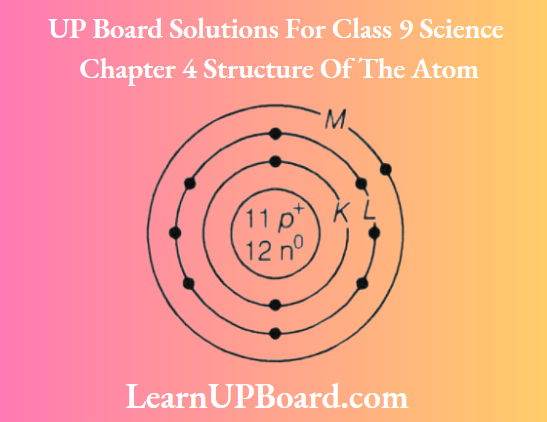
2. As Na has 1 valence electron, thus it has a valency of+1 and chlorine has a valency of -1. Hence, the formula of its chloride is ACl, i.e. NaCl.
Question 7. Write the electronic configuration and valency of the following.
- Chlorine
- Silicon
Answer:
- Chlorine (17Cl): \(\begin{aligned}
& K L M \\
& 2,8,7
\end{aligned}\) ⇒> Valency = 1 - Silicon (14Si) \(\begin{aligned}
& K L M \\
& 2,8,4
\end{aligned}\) 4 ⇒ Valency = 4
Question 8. Write the electronic configurations for the following elements and deduce their valencies.
- Magnesium
- Neon
- Sulphur
Answer:
Magnesium (12) = \(\begin{aligned}
& K L M \\
& 2,8,2
\end{aligned}\)
⇒ Valency = 2
2. Neon (10) = \(\begin{array}{cc}
K & L \\
2, & 8
\end{array}\)
⇒ Valency = 0
3. Sulphur (1 6) = \(\begin{aligned}
& K L M \\
& 2,8,6
\end{aligned}\)
⇒ Valency =8-6=2
Question 9. Find out the valency of atoms represented by the following figures.
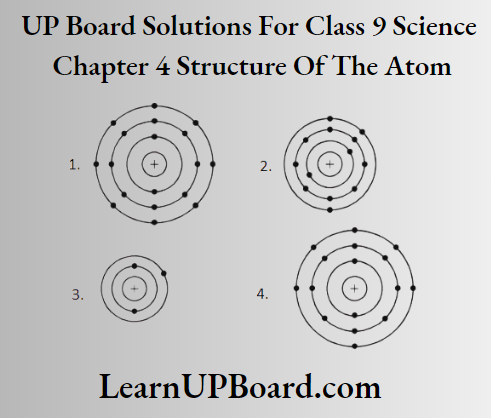
Answer:
Valency = 0 [∵ number of valence electrons = 8]
Valency = 3 [∵ number of valence electrons =3]
Valency = 1 [∵ number of valence electron = 1]
Valency = 2 [∵ number of valence electrons = 6]
[valency =8-6 = 2]
Question 10. The following data represents the distribution of electrons, protons, and neutrons in atoms of four elements A, B, C, and D.
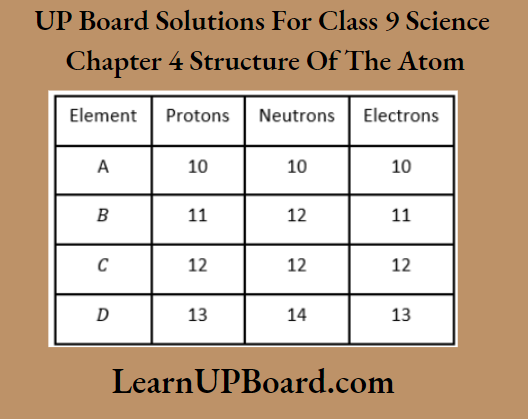
Solve the following questions.
- Write the electronic distribution of atoms of elements A and D.
- Element A is an inert gas. Why?
- What is the valency of element C?
Answer:
1. Electronic distribution of elements A and D.
⇒ \(\begin{aligned}
& \quad K L M \\
& A=2,8 \\
& D=2,8,3
\end{aligned}\)
2. The number of electrons in the outermost shell of element A is 8.
The outermost shell of this element is complete and the element does not need to gain or lose electrons to complete its outermost shell. Hence, A is an inert gas.
3. alency of element C (2, 8, 2) is 2.
Question 11. You are given the atom of an element 8X16. Find out the
- Number of protons, electrons, and neutrons in X.
- Valency of X.
- The chemical formula of the compound formed when X reacts with
- Hydrogen
- Carbon.
Answer:
1. Number of protons = 8
Number of electrons = 8
Number of neutrons =16-8=8
2. Valency = 8-6 = 2
∵ \(\left[\begin{array}{ll}
K & L \\
2, & 6
\end{array}\right]\)
3.
- H2X
- CX2
Question 12. An atom of an element has 5 electrons in L-shell.
- What is the atomic number of the element? State its valency.
- Identify the element and write its name.
Answer:
- Since there are 5 electrons in the Z-shell, so there must be 2 electrons in the X-shell. Thus, the element has a total of 7 electrons, which is equal to the number of protons, hence the atomic number of the element is 7.
- As Z-shell is the outermost shell and it contains 5 electrons. Therefore, it has 5 valence electrons. Thus, its valency is 3 [8 -5 = 3].
- An element having atomic number 7 is nitrogen (N).
Question 13.
- Answer the following questions.
- Name the scientist who discovered protons.
- What is the charge and mass of a proton?
- Where is a proton located in an atom?
- An atom of an element has a mass number of 28 u and its atomic number is
Answer:
1.
- Protons were discovered by E. Goldstein.
- The charge on a proton is + 1.6×10-19C and its mass is 1.67×10-27kg.
- The proton is located in the nucleus of an atom.
2. Number of neutrons
= Mass number – atomic number =28-14 = 14
The element is silicon (14Si28).
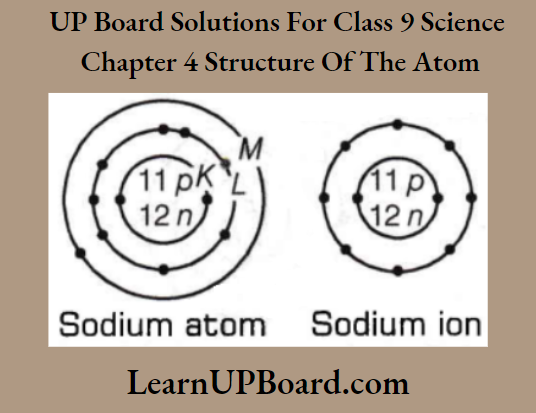
Question 14. Show diagrammatically the electron distributions in a sodium atom and a sodium ion and also give their atomic number.
Answer:
The atomic number of sodium (Z) = 11
The mass number of sodium = 23
∴ Number of protons in the nucleus = 11
Number of neutrons in the nucleus = 23 -11=12
Number of electrons =11
∴Electronic configuration of Na-atom = \(\begin{array}{ccc}
K & L & M \\
2, & 8, & 1
\end{array}\)
Na+ ion is formed from sodium atom by loss of an electron (present in the outermost shell). Hence, its electronic configuration is \(\begin{array}{cc}
K & L \\
2, & 8 .
\end{array}\). However, several protons and neutrons remain the same.
Question 15. An atom of an element has two electrons in the outermost M-shell. State
- Electronic configuration
- Atomic number
- Number of protons
- Valency of this element
Answer:
1. An atom of an element has two electrons in M-shell. Therefore, it’s electronic configuration = \(\begin{array}{ccc}
K & L & M \\
2, & 8, & 1
\end{array}\)
2. Atomic number = 2 + 8 + 2 = 12
3. Number of protons = 12
4. 2, because there are 2 valence electrons, which it can lose easily to achieve stable outermost electronic configuration.
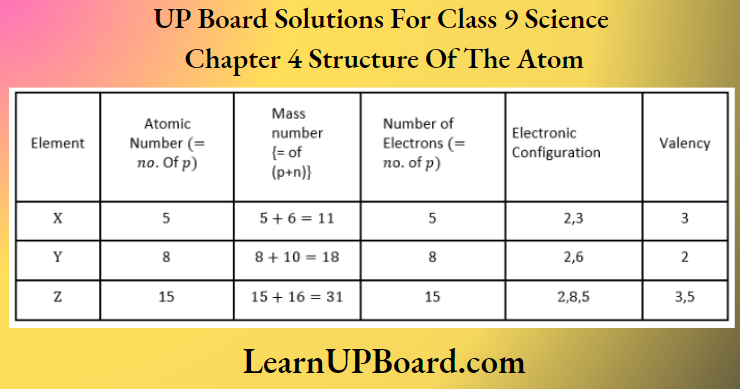
Question 16. What information do you get from the figure about the atomic number, mass number, and valency of atoms X, Y, and Z? Give your answer in a tabular form.
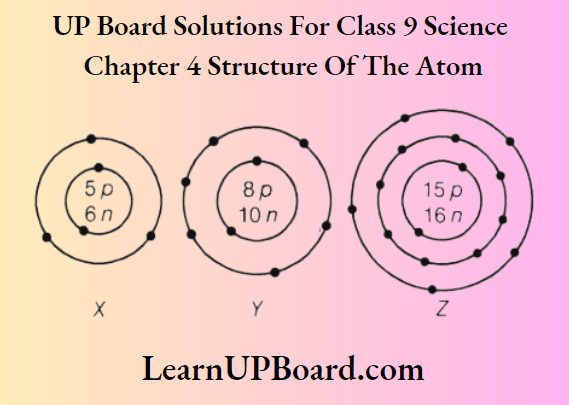
Answer:
The tabular form is as below:
Question 17. Complete the table based on the information available in the symbols given below.
⇒ \((1) { }_{17}^{35} \mathrm{Cl}
(2) { }_{35}^{81} \mathrm{Br}\)
Answer:
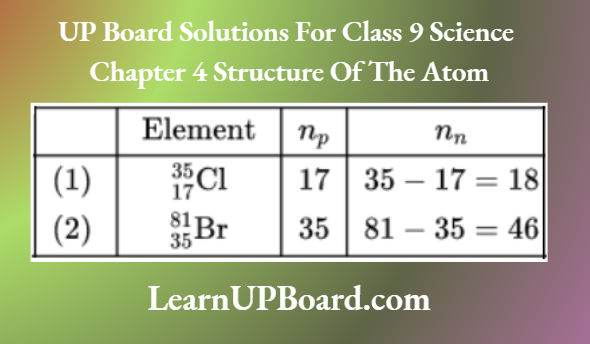
- The atomic number is written on the lower left side of the symbol of the element whereas the mass number is written on the upper left side of the symbol of the element.
- Number of protons (np) = Atomic number of atoms and number of neutrons (nn) = Mass number — atomic number.
Question 18. Complete the following table.
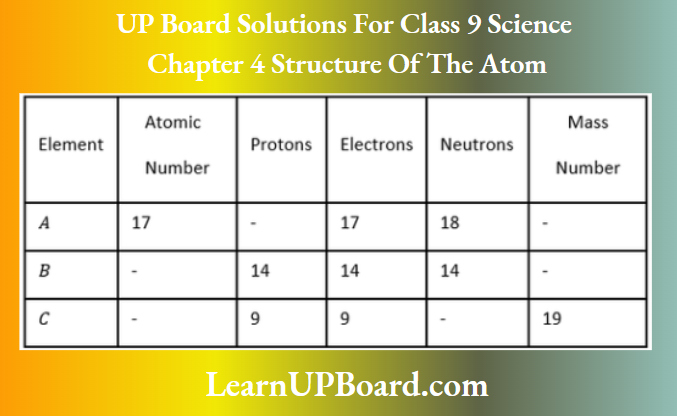
Answer:
A: Atomic number = Number of protons =17; Mass number = Number of protons + number of neutrons
= 17 + 18=35
B: Atomic number = Number of protons =14; Mass number = Number of protons + number of neutrons
= 14 + 14 = 28
C: Atomic number = Number of protons = 9; Number of neutrons = Mass number – number of protons
=19—9=10
Question 19. In the following table, the mass numbers and the atomic numbers of certain elements are given.
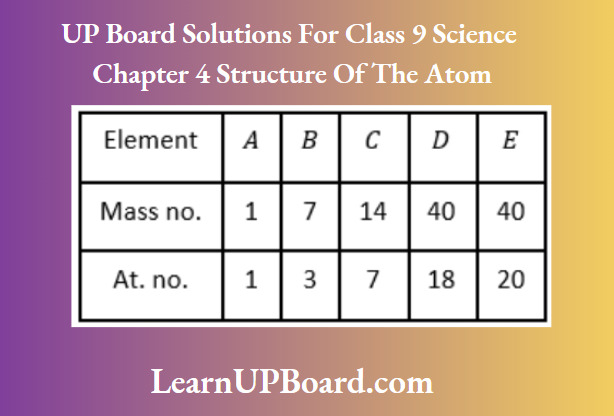
- What would be the valency of element C listed in the above table?
- Which two sub-atomic particles are equal in number in a neutral atom?
Answer:
1. The electronic configuration of C is 2(X), 5(L).
Hence, its valency is three because it gains three electrons to attain a stable electronic configuration.
[or valency can be found as, 8 – 5 = 3]
2. For a neutral atom,
Number of electrons = Number of protons Thus, electrons and protons are equal in number in a neutral atom.
Question 20. Two elements are represented as 17X35 and 12Y24.
- Which of these elements will lose and gain electrons? What is the number of electrons an atom loses, gains, or shares called?
- Write the electronic configurations of X and Y.
Answer:
1. Y will lose (2) electrons and X will gain (1) electron.
The number of electrons lost, gained, or shared by an atom is called its valency.
2. Electronic configuration of X (atomic number = 17) = 2, 8, 7
Electronic configuration of Y (atomic number = 12) = 2, 8, 2
Question 21. A metal (mass number = 40) having the same number of protons and neutrons, combines with two chlorine atoms. Identify the element with which the electronic configuration of this metal matches in the combined state.
Answer:
Given
A metal (mass number = 40) having the same number of protons and neutrons, combines with two chlorine atoms.
1: As mass number = Number of protons + number of neutrons
⇒ 40 = 2 x number of protons
⇒ Number of protons = 20
Hence, the metal is calcium (Ca).
Since it combines with 2 Cl-atoms (total valency = 2)
Hence, it will acquire 2 positive charges, after losing 2 electrons.
Electronic configuration of Ca2+ ion = \(\begin{array}{ccc}
K & L & M \\
2, & 8, & 8
\end{array}\)
This electronic configuration matches that of argon (Ar).
Question 22. In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
Answer:
Given
In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons.
The given statement is incorrect.
According to this statement: p > n > e
But actually, several protons can never be greater than the number of neutrons (except protium). Several neutrons can be equal to or greater than the number of protons because a mass number is equal to double the atomic number or greater than double the atomic number.
Of course, several neutrons can be greater than the number of electrons because several electrons are equal to several protons in the neutral atom.
Question 23. An ion M3- contains 10 electrons and 7 neutrons. What is the atomic number and mass number of the element M? Name the element.
The number of protons in M3- ion =10-3 = 7
∴ The atomic number is 7.
Mass number = Number of protons + number of neutrons
= 7 + 7
=14
As the atomic number is 7.
∴ The element is nitrogen.
Question 24. Sulfur dioxide (SO2) is a colorless pungent-smelling gas and is a major air pollutant.
- Write the electronic configuration of its constituent elements ‘sulfur and oxygen’ (Given, 16S32,8O16). Write the valency of sulfur and oxygen.
- Are sulphur and oxygen isotopes of the same element? Explain your answer.
Answer:
1. Electronic configuration of sulphur (S) : \(\begin{aligned}
& K L M \\
& 2,8,6
\end{aligned}\)
Electronic configuration of oxygen (O): 2, 6 Valency of both sulphur and oxygen is 2, as they tend to gain two electrons to achieve stable outer shell electronic configuration.
2. Isotopes are elements having the same atomic number but different mass numbers. Therefore, they are not isotopes as their atomic numbers are different.
Question 25. Study the data given below and answer the questions which follow:
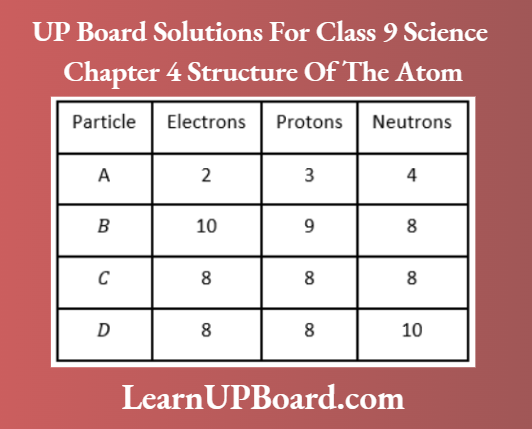
- Write the mass number and atomic number of particles A, B, C, and D.
- Which particles represent a pair of isotopes? Explain.
Answer:
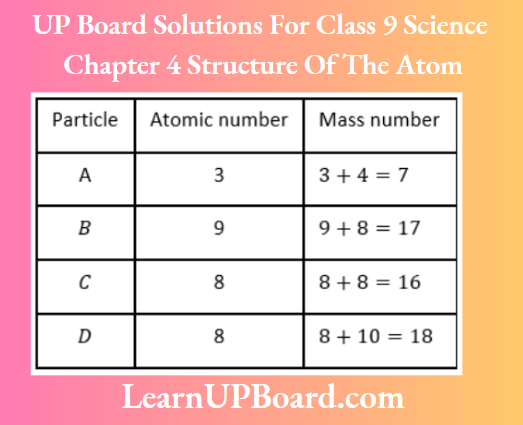
Particles C and D have the same number of protons, i.e. same atomic number but different mass numbers.
Question 26. The two isotopes of chlorine have mass numbers 35 and 37 and number of neutrons 18 and 20 respectively. Which one will have higher valency? Do they have the same physical or chemical properties?
Answer:
Given
The two isotopes of chlorine have mass numbers 35 and 37 and number of neutrons 18 and 20 respectively.
Number of protons in ,35Cl =35 -1 8 =17
⇒ Number of electrons = Number of protons =17
Similarly, the number of protons in 37Cl = 37 — 20 = 17
⇒ Number of electrons = Number of protons =17
Since both have the same number of electrons (i.e. 17). Hence, they will have the same valency (i.e. 1). Isotopes having the same number of electrons show the same chemical properties but may show some different physical properties.
Question 27.
- What is the number of electrons in CD ion (Cl– =17)?
- What is the electronic configuration of phosphorus (P = 15)?
Answer:
1. 17 +1 (gained) =18 electrons [because negative charge shows gain of electrons].
2. Atomic number of phosphorus =15 Electronic configuration = \(\begin{aligned}
& K L M \\
& 2,8,5
\end{aligned}\)
Question 28. An element M forms the compound MH3 when it reacts with hydrogen.
- Find the valency of element M.
- Write the valency of chlorine and sulfur.
Answer:
- The valency of M = 3 because it combines with three atoms of hydrogen.
- Valency of chlorine, Cl (2, 8, 7) = 8 – 7 = 1 Valency of sulfur, S (2, 8, 6) = 8-6 = 2
Question 29.
- Identify which of the following pairs are isotopes and which are isobars. Give reasons for your choice.
- \({ }^{58} A_{26},{ }^{58} B_{28},{ }^{79} X_{35},{ }^{80} Y_{35}\)
- Do isobars also have identical chemical properties to isotopes? State reason.
Answer:
- A and B are isobars as they are atoms of different elements having the same mass number. X and Y are isotopes as they are atoms of the same element (same atomic number) having different mass numbers.
- No, isobars do not have identical chemical properties because they have different atomic numbers as well as electronic configurations.
Question 30.
- Chlorine occurs in nature in two isotopic forms with masses 35 u and 37 u. The percentage of 35 Cl is 75%. Find the average atomic mass of a chlorine atom,
- Give any three applications of isotopes.
Answer:
1. \(\% \text { of }{ }^{37} \mathrm{Cl}=100-75=25 \%\)
Average atomic mass \(=\left(\frac{35 \times 75}{100}+\frac{37 \times 25}{100}\right)\)
⇒ \(\left(\frac{105}{4}+\frac{37}{4}\right)\)
⇒ \(\frac{142}{4}\)
= 35.50 u
2. Applications of isotopes
- An isotope of iodine is used in the treatment of goiter.
- An isotope of uranium is used as a fuel in nuclear reactors.
- An isotope of cobalt is used in the treatment of cancer.
Question 31. Based on the number of protons, neutrons, and electrons in the samples given below identify
- The cation.
- The pair of isobars, and the pair of isotopes.
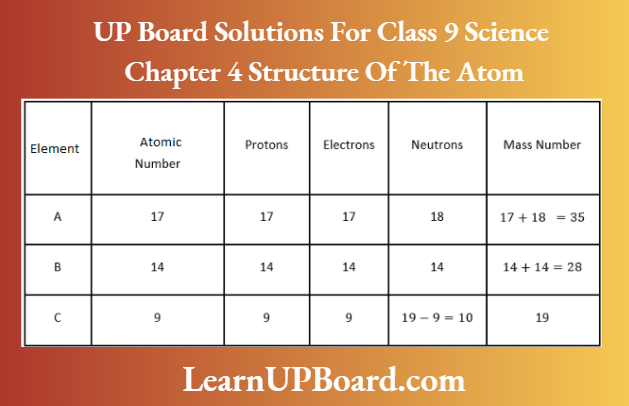
Answer:
- Sample A has more protons than the electrons. Hence, it is a cation.
- Sample B and C have the same mass number (Mass number = Number of protons + several neutrons =37) but different atomic numbers (i.e. 18 and 17 respectively). Hence, they are a pair of isobars. Samples C and D have the same atomic number but different mass numbers. Hence, they are a pair of isotopes.
Question 32. Elements from A to F have the distribution of electrons, protons, and neutrons in the following way.
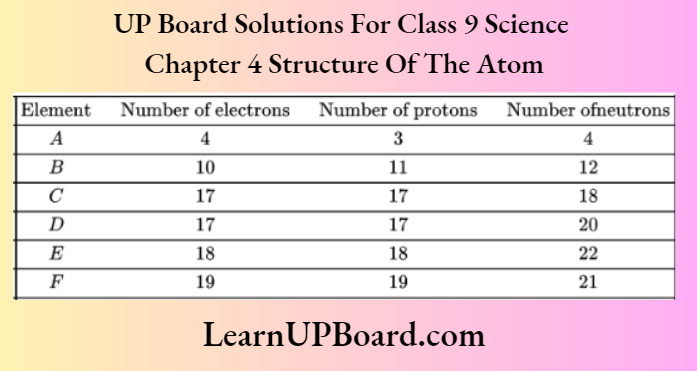
From the table, find
- A pair of ionic gas
- An atom of noble configuration
- A pair of isobars
- A pair of isotopes
Answer:
- A and B, because several electrons and protons are different for these elements.
- E, because it has filled outer shell electronic configuration \(\begin{aligned}
& K L M \\
& 2,8,8 .
\end{aligned}\). - E and E because these have the same mass number (number of protons + number of neutrons) but different atomic numbers.
- C and A because these have the same atomic number (number of protons) but different mass numbers.
Question 33. In the gold foil experiment of Geiger and Marsden, which paved the way for Rutherford’s model of an atom, — 1.00% of the a-particles were found to deflect at angles greater than 50°. If one mole of a-particles were bombarded on the gold foil, compute the number of a-particles that would deflect at angles less than 50°.
Answer:
From mole concept
1 mole =6.022 × 1023 particles (mole concept was discussed in the previous chapter)
Number of a-particles deflected at angles greater than 50° = 1% (Given)
∴ Several a-particles deflected at the angles less than 50° = 100-1=99%.
∴ The actual number of a-particles deflected at the angles
less than 50° = \(\frac{99}{100} \times 6.022 \times 10^{23}\)
= 5.96 × 1023 100
Hence, the number of a-particles deflect at an angle less than 500 ≈ 0.06 x 1023
Question 34. If the mass of a proton is 1.67 × 1027 kg and if the electron is found to be 1840 times lighter than hydrogen ion. Then find the charge-to-mass ratio of cathode rays.
[Given, a charge on 1 electron = 1.6×10-19C]
Answer:
Mass of H+ Mass of proton \(=\frac{\text { Mass of } \mathrm{H}^{+}}{1840}=\frac{\text { Mass of proton }}{1840}\)
⇒ \(\frac{1.67 \times 10^{-27} \mathrm{~kg}}{1840}
⇒ [latex]9.1 \times 10^{-31} \mathrm{~kg}\)
Since cathode rays are composed of electrons only. Hence, e/m ratio of cathode rays = e/m ratio of electron
⇒ \(\frac{1.6 \times 10^{-19} \mathrm{C}}{9.1 \times 10^{-31} \mathrm{~kg}}
⇒ [latex]1.76 \times 10^{11} \mathrm{C} \mathrm{kg}^{-1}\)
Question 35. If Z = 3, what would be the valency of the element? Also, name the element. Sol Electronic configuration of an element,
Answer:
Electronic configuration of elements,
∴ \((Z=3)=\begin{array}{cc} K & L \\ 2, & 1 \end{array}\)
Valency of the element = Number of valence electrons
= 1
Name of the element = Lithium (Li)
Question 36. The composition of the nuclei of two atomic species X and Y are given as under.
\(\begin{array}{lcc}
& X & Y \\
\text { Protons } & 6 & 6 \\
\text { Neutrons } & 6 & 8
\end{array}\)
Give the mass numbers of X and Y What is the relation between the two species?
Answer:
Mass number = Number of protons + number of neutrons Mass number of
X = 6 + 6=12
Mass number of F = 6 + 8 =14
To find the relation between X and Y
The atomic number of X = Number of protons = 6
The atomic number of Y = Number of protons = 6
It can be that the atomic numbers of both the elements X and F are the same (6) but their mass number are different, so, X and Y are isotopes of the same element (carbon). Hence, X and Fare U6C respectively.
Question 37. For the following statements, write T for True and F for False.
- J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
- A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
- The mass of an electron is about \(\frac{1}{2000}\) times that of a proton.
- An isotope of iodine is used for making tincture of iodine, which is used as a medicine.
Answer:
- (F)Because in J.J. Thomsons’ model, the nucleus was not present.
- (F)A Neutron is a fundamental particle (a subatomic particle) of the atom of an element, thus cannot be made by combining an electron and a proton. It is neutral, as it carries no charge.
- (T) Mass of electron is \(\frac{1}{1840}\) times, which is 1840 nearly about \(\frac{1}{2000}\) times that of proton.
- (T) Tincture of iodine is made by dissolving an isotope of iodine in alcohol (1-131).
Question 38. Rutherford’s a-particle scattering experiment was responsible for the discovery of
- Atomic nucleus
- Electron
- Proton
- Neutron
Answer: 1. Atomic nucleus
Based on his experiment, Rutherford predicted that the center part of the atom is positively charged and is responsible for the mass of the atom. He called these protons an atomic nucleus.
Question 39. Isotopes of an element have
- The same physical properties
- Different chemical properties
- Different numbers of neutrons
- Different atomic numbers
Answer: 3. Different number of neutrons
Isotopes have different physical properties, similar chemical properties, same atomic number but different mass numbers. Hence, isotopes of an element have different numbers of neutrons.
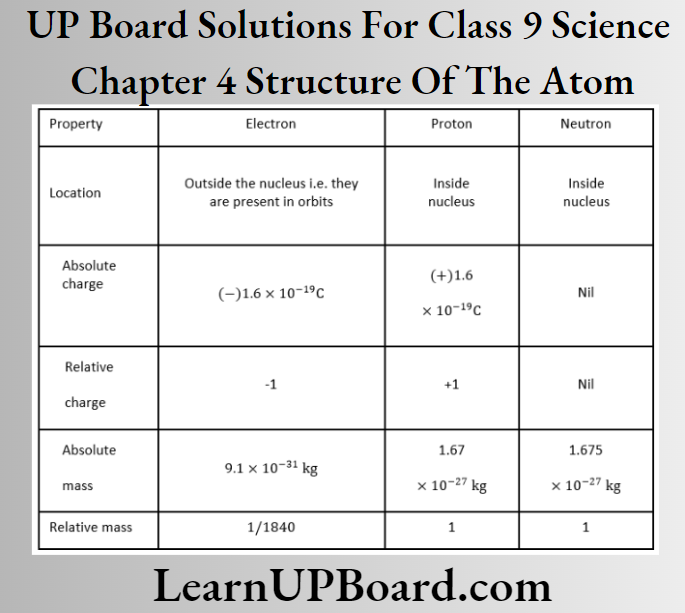
Question 40. Compare the properties of electrons, protons, and neutrons.
Answer: The properties of electrons, protons, and neutrons are as follows:
Question 41. What are the limitations Of J.J. Thomson’s model of the atom?
Answer:
Limitations of J.J. Thomson’s model of the atom are :
- It could not explain the experimental results of other scientists such as Rutherford, as there is no nucleus in the atomic model proposed by Thomson.
- It could not explain the stability of an atom, i.e. how positive and negative charges could remain so close together.
Question 42. What are the limitations of Rutherford’s model of the atom?
Answer:
Limitations of Rutherford’s model of the atom are:
- Rutherford’s model could not explain why a moving charge (electrons) does not lose energy and fall into the nucleus.
- It could not explain the stability of an atom when charged electrons are moving under the attractive force of a positively charged nucleus.
- The model could not explain how the electrons are distributed in the extranuclear portion of an atom.
Question 43. Describe Bohr’s model of the atom.
Answer:
The main postulates of Bohr’s model of an atom are as follows:
- Electrons revolve around the nucleus in special orbits called discrete orbits (energy levels).
- While revolving in discrete orbits, the electrons do not radiate energy.
- These orbits are represented by the letters, K, L, My Nor the numbers n = 1, 2, 3, 4.
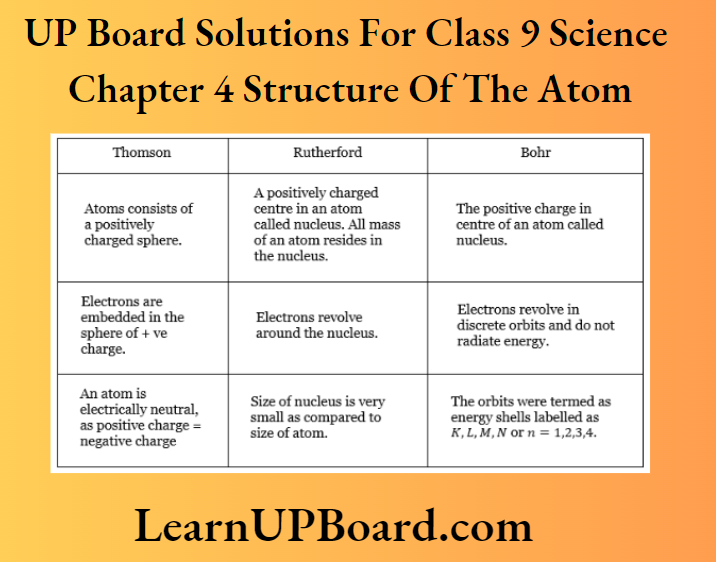
Question 44. Compare all the proposed models of an atom given in the chapter.
Answer:
Question 45. How will you find the valency of chlorine, sulphur, and magnesium?
Answer:
Magnesium is a metal while chlorine and sulphur are non-metals.
Valency of a metal = Number of valence electrons (i.e. number of electrons present in outermost shell)
Valency of a non-metal = 8 — number of valence electrons
- 12Mg = 2, 8, 2; Valency of magnesium = 2
- 16S = 2, 8, 6; Valency of sulphur = 8 – 6 = 2
- 17Cl = 2, 8, 7; Valency of chlorine = 8 – 7=1
Question 46. Helium atom has an atomic mass of 4u and two protons in its nucleus. How many neutrons does it have?
Answer:
Given
Helium atom has an atomic mass of 4u and two protons in its nucleus.
Atomic mass of helium = 4 u
Number of protons = 2
Let the number of neutrons = x
Number of protons + number of neutrons
=Atomic mass
2 + x = 4
⇒ x = 4 – 2
= 2
Question 47. What do you think would be the observation if the a-particle scattering experiment is carried out using a foil of a metal other than gold?
Or Why did Rutherford select a gold foil in his a-rays scattering experiment?
Answer:
If the α-particle scattering experiment is carried out using a foil of any other metal by Rutherford, there would be no change in observations. Since other metals are not so malleable. So such a thin foil is difficult to obtain.
- If we use a thick foil, more a-particles would bounce back and no idea about the location of positive mass in the atom would be available with such a correctness.
- This means as the thickness of the foil increases, the possibility of correctness for the experiment decreases. So, the use of gold in this case is preferred.
UP Board Solutions For Class 9 Science Chapter 4 Structure Of The Atom Very Short Answer Type Questions
Question 1. Electron attributes negative charge, and protons attribute positive charge. An atom has both but why there is no charge?
Answer:
A neutral atom has an equal number of protons and electrons. Hence, it has an equal amount of negative and positive charges which cancel out the effect of each other. Thus, the net electrical charge on an atom is zero.
Question 2. Which scientist concluded that the size of a nucleus is very small as compared to the size of an atom?
Answer: Ernest Rutherford.
Question 3. When a-particles are bombarded on a gold sheet, only a few of them get deflected, whereas most of them go straight undeflected. Give reasons.
Answer:
This occurs because the volume occupied by the nucleus is very small as compared to that of the atom, i.e. most of the part of an atom is empty. Therefore, most of the a-particles go undeflected.
Question 4. One electron is present in the outermost shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outermost shell?
Answer:
An element X is metal because one electron is present in the outermost shell, i.e. 1 valence electron. When this valence electron is removed from the outermost shell, a cation (positive ion) will be formed with a charge of +1.
Question 5. In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting a requisite number of electrons, then what would be the charge on the ion so formed?
Answer:
Element X has 6 electrons in the outermost shell. To acquire noble gas configuration, element X requires 2 electrons. Therefore, when it gains electrons it acquires a negative charge i.e. -2. The charge on the anion (X2-), so formed is -2.
Question 6. Which sub-atomic particle is not present in a hydrogen atom?
Answer: Neutron.
Question 7. An atom of an element is represented as 9X192. How many electrons and neutrons are present in this atom?
Answer:
Atomic number = Number of electrons = 9
Number of neutrons = Mass number – number of protons =19 -9=10
Question 8. What is the atomic number of the atom of an element X, which has 2 shells, K and L having 2 and 6 electrons respectively?
Answer:
K= 2, L = 6
Atomic number = Number of protons = Number of electrons in neutral atom Atomic number = 2 + 6=8
Question 9. What is the difference between Na and Na+ in terms of a number of electrons?
Answer:
Number of electrons in Na = 11
Number of electrons in Na+ =11-1=10
[∵ In case of positive ion, number of electrons = Number of protons – total positive charge]
Question 10. What happens to an element Z if its atom gains 3 electrons?
Answer:
If an atom gains electrons, the number of electrons gained is represented by a negative charge. So, Z will convert into Z3- after gaining 3 electrons.
Question 11. Write the mass number of neon and argon from the data given below.
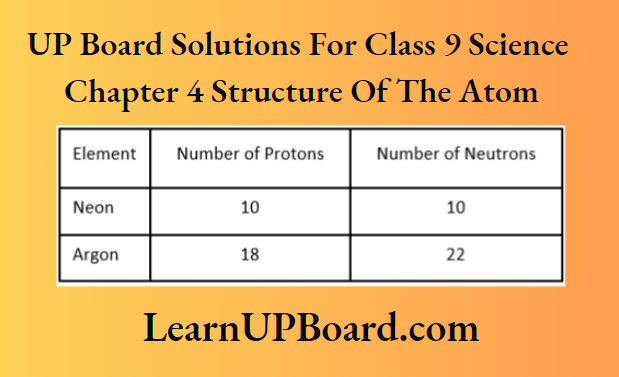
Answer:
Mass number = Number of protons + number of neutrons
For neon, mass number =10 + 10 = 20
For argon, mass number = 18 + 22 = 40
Question 12. Uranium-235 has 92 protons in its atom. Calculate the number of neutrons in this atom.
Answer:
Number of neutrons
= Mass number – number of protons
= 235 -92 = 143
Question 13. Calculate the number of neutrons present in the nucleus of an element X which is represented as 15X31.
Answer:
Atomic number, Z of 15X31=15
The mass number, A of 15X31=31
Number of neutrons = A-Z = 31 -15=16
Question 14. Is it possible for the atom of an element to have 1 electron, 1 proton, and no neutron? If so, name the element.
Answer:
Yes, protium (1H1) is an isotope of hydrogen that has 1 electron, 1 proton and no neutron.
Question 15. Which isotope of carbon is isotopic to X|C and is used in radiocarbon dating to determine the age of old samples of living organisms?
Answer: Carbon-14, 6C14
Question 16. Identify the pair of isotopes from the following:
⇒ \({ }_8^{16} X,{ }_7^{16} X,{ }_8^{17} X\)
Answer:
⇒ \({ }_8^{16} X \text { and }{ }_8^{17} X\) is isotope as they have the same atomic number, but different mass numbers.
Question 17. The atomic number of calcium and argon are 20 and 18 respectively but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Answer:
Given
The atomic number of calcium and argon are 20 and 18 respectively but the mass number of both these elements is 40.
The mass number of calcium =40, i.e. 20C40.
The mass number of argon =40, i.e. 18Ar40.
A pair of elements having the same mass number but different atomic numbers is called isobars.
Question 18. If X– contains 18 electrons, what is its atomic number?
Answer:
For X–, Atomic number = Number of protons = Number of electrons-negative charge= 18-1
= 17
Question 19. What is the n/p ratio of \(\begin{array}{r}
222 \\
8 8
\end{array}\) Ra?
Answer:
Number of neutrons, n – 222 – 88 =134
Number of protons, p = 88
∴ \(\frac{n}{p} \text { ratio }=\frac{134}{88}\)
= 1.52
Question 20. Which of the two would be chemically more reactive: element A with atomic number 18 or element D with atomic number 16 and why?
Answer:
Electronic configuration of 18A -2, 8, 8
It would be chemically inert due to its complete octet. Electronic configuration of 16D =2, 8, 6
To complete its octet, it will gain 2 electrons, therefore it will be more reactive.
Question 21. Which isotope of hydrogen contains the same number of electrons, protons, and neutrons?
Answer:
Deuterium (1D2),
Number of electrons (1),
Number of proton (1),
Number of neutrons (2 -1 = 1)
Question 22. What are canal rays?
Answer:
Canal rays
Canal rays or anode rays are the positively charged rays that are seen moving from the anode towards the cathode in a specially designed discharge tube when a high voltage is applied across the electrodes.
Question 23. If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
An electron is a negatively charged particle, whereas a proton is a positively charged particle and the magnitude of their charges is equal. Therefore, an atom containing one electron and one proton will not carry any charge. Thus, it will be a neutral atom.
Question 24. Based on Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer:
According to the Thomson model, an atom consists of a positively charged sphere, and the electrons are embedded in it. The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
Question 25. Based on Rutherford’s model of an atom, which sub-atomic particle is present in the nucleus of an atom?
Answer:
According to Rutherford’s model of an atom nucleus is positively charged, therefore, protons are present inside the nucleus.
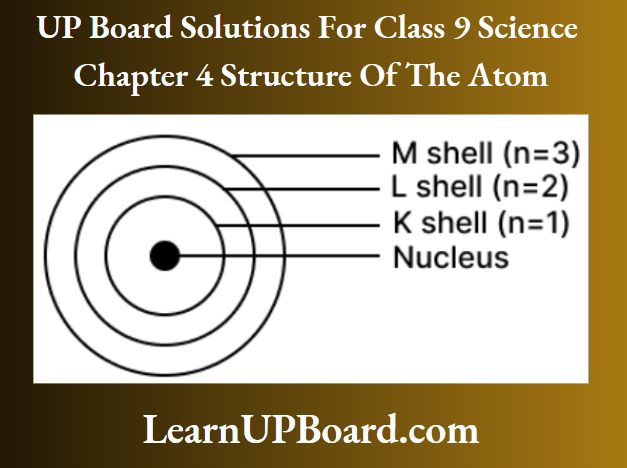
Question 26. Sketch Bohr’s model of an atom with three shells.
Answer:
Three shells or orbits are presented by the letters K, Z, M (or the numbers, n = 1, 2, 3) M-shell (n = 3)
Question 27. Name the three sub-atomic particles in an atom.
Answer:
The sub-atomic particles present in an atom are electrons, protons, and neutrons.
Question 28. If K and L-shells of an atom are full then what would be the total number of electrons in the atom?
Answer:
The maximum number of electrons that can occupy K and Z-shells of an atom are 2 and 8 respectively. Therefore, if the K and Z-shells of an atom are filled, then the total number of electrons in the atom would be (2 + 8) =10 electrons.
Question 29. If the number of electrons in an atom is 8 and the number of protons is also 8, then
- What is the atomic number of the atom?
- What is the charge on the atom?
Answer:
Atomic number = Number of protons = 8
The charge on the atom is zero because several electrons and several protons are equal.
Question 30. With the help of the table given on NCERT page 92, find out the mass number of oxygen and sulfur atoms.
Answer:
Mass number = Number of protons + number of neutrons
∴ Mass number of oxygen =8 + 8 = 16
and mass number of sulphur = 16 + 16=32
Question 31. For the symbols, H, D, and T tabulate three sub-atomic particles found in each of them.
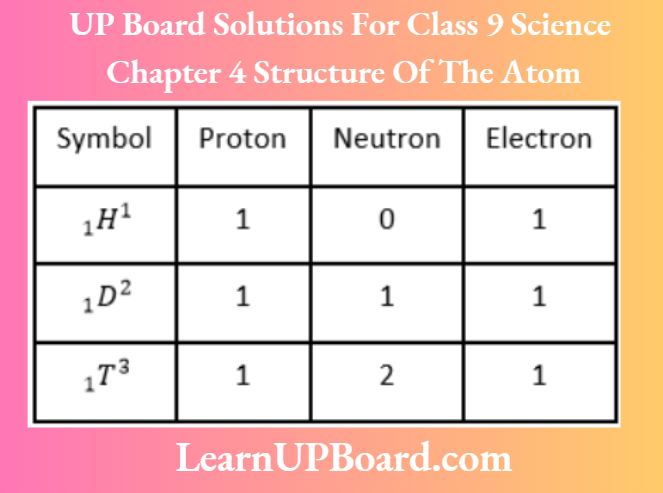
Answer: