NEET Chemistry For Equilibrium Multiple Choice Questions
Question 1. In liquid-gas equilibrium, the pressure of vapours above the liquid is constant at
- Constant temperature
- Low temperature
- High temperature
- None of these.
Answer: 1. Constant temperature
Vapour pressure is directly related to temperature. The greater is the temperature, the greater will be the vapour pressure. So to keep it constant, the temperature should be constant.
Question 2. \(3 \mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{O}_{3(g)}\) For the above reaction at 298 K, Kc is found to be 3.0 x 10-59. If the concentration of O2 at equilibrium is 0.040 M then the concentration of O3 in M is
- \(4.38 \times 10^{-32}\)
- \(1.9 \times 10^{-63}\)
- \(2.4 \times 10^{31}\)
- \(1.2 \times 10^{21}\)
Answer: 1. \(4.38 \times 10^{-32}\)
\(3 \mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{O}_{3(g)}\) For the above reaction at 298 K, Kc is found to be 3.0 x 10-59. If the concentration of O2 at equilibrium is 0.040 M

∴ \(K_c=\frac{\left[\mathrm{O}_3\right]^2}{\left[\mathrm{O}_2\right]^3}=3 \times 10^{-59}\)
Given: \(\left[\mathrm{O}_2\right]=0.040 \mathrm{M}\)
⇒ \(K_c=\frac{\left[\mathrm{O}_3\right]^2}{(0.040)^3}=3 \times 10^{-59}\)
⇒ \({\left[\mathrm{O}_3\right]^2=1.92 \times 10^{-63}}\)
⇒ \({\left[\mathrm{O}_3\right]=4.38 \times 10^{-32} \mathrm{M}}\)
Read and Learn More NEET MCQs with Answers
Question 3. The equilibrium constants of the following are \(\mathrm{N}_2+3 \mathrm{H}_2 \rightleftharpoons 2 \mathrm{NH}_3 ; K_1\); \(\mathrm{~N}_2+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{NO} ; K_2\); \(\mathrm{H}_2+\frac{1}{2} \mathrm{O}_2 \rightleftharpoons \mathrm{H}_2 \mathrm{O} ; K_3\)
The equilibrium constant (K) of the reaction: \(2 \mathrm{NH}_3+\frac{5}{2} \mathrm{O}_2 \stackrel{\mathrm{K}}{\rightleftharpoons} 2 \mathrm{NO}+3 \mathrm{H}_2 \mathrm{O}\) will be
- \(K_2 K_3^3 / K_1\)
- \(K_2 K_3 / K_1\)
- \(K_2^3 K_3 / K_1\)
- \(K_1 K_3^3 / K_2\)
Asnwer: 1. \(K_2 K_3^3 / K_1\)
From the given equations, \(2 \mathrm{NH}_3 \rightleftharpoons \mathrm{N}_2+3 \mathrm{H}_2 ; \frac{1}{K_1}\)….(1)
⇒ \(\mathrm{~N}_2+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{NO} ; K_2\)…..(2)
⇒ \(3 \mathrm{H}_2+\frac{3}{2} \mathrm{O}_2 \rightleftharpoons 3 \mathrm{H}_2 \mathrm{O} ; K_3^3\)….(3)
By adding equations (1), (2) and (3), we get \(2 \mathrm{NH}_3+\frac{5}{2} \mathrm{O}_2 \stackrel{K}{\rightleftharpoons} 2 \mathrm{NO}+3 \mathrm{H}_2 \mathrm{O}, K=\frac{K_2 K_3^3}{K_1}\)
Question 4. If the equilibrium constant for \(\mathrm{N}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{NO}_{(\mathrm{g})}\) is K, the equilibrium constant for \(\frac{1}{2} \mathrm{~N}_{2(g)}+\frac{1}{2} \mathrm{O}_{2(g)} \rightleftharpoons \mathrm{NO}_{(g)}\) will be
- 1/2 K
- K
- K²
- K½
Answer: 4. K½
If the reaction is multiplied by 1/2, then the equilibrium constant, K’ = K1/2
Question 5. Given that the equilibrium constant for the reaction, \(2 \mathrm{SO}_{2(g)}+\mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{SO}_{3(g)}\) has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature; \(\mathrm{SO}_{3(g)} \rightleftharpoons \mathrm{SO}_{2(g)}+\frac{1}{2} \mathrm{O}_{2(g)}\)
- \(1.8 \times 10^{-3}\)
- \(3.6 \times 10^{-3}\)
- \(6.0 \times 10^{-2}\)
- \(1.3 \times 10^{-5}\)
Answer: 3. \(6.0 \times 10^{-2}\)
⇒ \(2 \mathrm{SO}_{2(g)}+\mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{SO}_{3(g)}, K=278\)…..(1)
By reversing the equation (1), we get \(2 \mathrm{SO}_{3(g)} \rightleftharpoons 2 \mathrm{SO}_{2(g)}+\mathrm{O}_{2(g)}\)…..(2)
Equilibrium constant for this reaction is, \(K^{\prime}=\frac{1}{K}=\frac{1}{278}\)
By dividing the equation (2) by 2, we get the desired equation, \(\mathrm{SO}_{3(g)} \rightleftharpoons \mathrm{SO}_{2(g)}+\frac{1}{2} \mathrm{O}_{2(g)}\)….(3)
Equilibrium constant for this reaction, \(K^{\prime \prime}=\sqrt{K^{\prime}}=\sqrt{\frac{1}{K}}=\sqrt{\frac{1}{278}}=0.0599=0.06 \text { or } 6 \times 10^{-2}\)
Question 6. Given the reaction between 2 gases represented by A2 and B2 to give the compound AB(g). \(A_{2(g)}+B_{2(g)} \rightleftharpoons 2 A B_{(g)}\) At equilibrium, the concentration of \(A_2=3.0 \times 10^{-3} \mathrm{M}\), of \(B_2=4.2 \times 10^{-3} \mathrm{M}\), of \(A B=2.8 \times 10^{-3} \mathrm{M}\)
If the reaction takes place in a sealed vessel at 527°C, then the value of Kc will be
- 2.0
- 1.9
- 0.62
- 4.5
Answer: 3. 0.62
⇒ \(A_{2(g)}+B_{2(g)} \rightleftharpoons 2 A B_{(g)}\)
∴ \(K_c=\frac{[A B]^2}{\left[A_2\right]\left[B_2\right]}=\frac{\left(2.8 \times 10^{-3}\right)^2}{\left(3.0 \times 10^{-3}\right)\left(4.2 \times 10^{-3}\right)}=\frac{2.8 \times 2.8}{3.0 \times 4.2}=0.62\)
Question 7. For the reaction, \(\mathrm{N}_{2(g)}+\mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{NO}_{(g)}\), the equilibrium constant is K1. The equilibrium constant is K2 for the reaction, \(2 \mathrm{NO}_{(\mathrm{g})}+\mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{NO}_{2(\mathrm{~g})}\) What is K for the reaction, \(\mathrm{NO}_{2(g)} \rightleftharpoons \frac{1}{2} \mathrm{~N}_{2(g)}+\mathrm{O}_{2(g)} \text {? }\)
- \(\frac{1}{2 K_1 K_2}\)
- \(\frac{1}{4 K_1 K_2}\)
- \(\left[\frac{1}{K_1 K_2}\right]^{1 / 2}\)
- \(\frac{1}{K_1 K_2}\)
Answer: 3. \(\left[\frac{1}{K_1 K_2}\right]^{1 / 2}\)
⇒ \(\mathrm{N}_2+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{NO} ; K_1\)
⇒ \(2 \mathrm{NO}+\mathrm{O}_2 \rightleftharpoons 2 \mathrm{NO}_2 ; K_2\)
⇒ \(\mathrm{NO}_2 \rightleftharpoons \frac{1}{2} \mathrm{~N}_2+\mathrm{O}_2 ; K\)
∴ \(K_1=\frac{[\mathrm{NO}]^2}{\left[\mathrm{~N}_2\right]\left[\mathrm{O}_2\right]} ; K_2=\frac{\left[\mathrm{NO}_2\right]^2}{\left[\mathrm{NO}^2\left[\mathrm{O}_2\right]\right.}\)
K = \(\frac{\left[\mathrm{N}_2\right]^{1 / 2}\left[\mathrm{O}_2\right]}{\left[\mathrm{NO}_2\right]}=\sqrt{\frac{\left[\mathrm{N}_2\right]\left[\mathrm{O}_2\right] \times[\mathrm{NO}]^2\left[\mathrm{O}_2\right]}{\left[\mathrm{NO}^2 \times\left[\mathrm{NO}_2\right]^2\right.}} \Rightarrow K=\sqrt{\frac{1}{K_1 K_2}}\)
Question 8. The dissociation constants for acetic acid and HCN at 25°C are 1.5 x 10-5 and 4.5 x 10-10 respectively. The equilibrium constant for the equilibrium, \(\mathrm{CN}^{-}+\mathrm{CH}_3 \mathrm{COOH} \rightleftharpoons \mathrm{HCN}+\mathrm{CH}_3 \mathrm{COO}^{-}\) would be
- \(3.0 \times 10^{-5}\)
- \(3.0 \times 10^{-4}\)
- \(3.0 \times 10^4\)
- \(3.0 \times 10^5\)
Answer: 3. \(3.0 \times 10^4\)
Given, \(\mathrm{CH}_3 \mathrm{COOH} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}^{+}\)
⇒ \(K_1=\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]\left[\mathrm{H}^{+}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]}=1.5 \times 10^{-5}\)
HCN \(\rightleftharpoons \mathrm{H}^{+}+\mathrm{CN}^{-}\)
⇒ \(K_2=\frac{\left[\mathrm{CN}^{-}\right]\left[\mathrm{H}^{+}\right]}{[\mathrm{HCN}]}=4.5 \times 10^{-10}\)
⇒ \(\mathrm{CN}^{-}+\mathrm{CH}_3 \mathrm{COOH} \rightleftharpoons \mathrm{HCN}+\mathrm{CH}_3 \mathrm{COO}^{-}\)
K = \(\frac{\left[\mathrm{HCN}^{-}\right]\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]}{\left[\mathrm{CN}^{-}\right]\left[\mathrm{CH}_3 \mathrm{COOH}\right]}\)
K = \(\frac{K_1}{K_2}=\frac{1.5 \times 10^{-5}}{4.5 \times 10^{-10}}=0.3 \times 10^5 \text { or } K=3 \times 10^4\)
Question 9. The value of equilibrium constant of the reaction, \(\mathrm{HI}_{(\mathrm{g})} \rightleftharpoons \frac{1}{2} \mathrm{H}_{2(\mathrm{~g})}+\frac{1}{2} \mathrm{I}_{2(\mathrm{~g})}\) is 8.0. The equilibrium constant of the reaction \(\mathrm{H}_{2(g)}+\mathrm{I}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{HI}_{(\mathrm{g})}\) will be
- 16
- 1/8
- 1/16
- 1/64
Answer: 4. 1/64
⇒ \(\mathrm{HI}_{(\mathrm{g})} \rightleftharpoons 1 / 2 \mathrm{H}_{2(g)}+1 / 2 \mathrm{I}_{2(g)}\)
i.e. \(\mathrm{K}=\frac{\left[\mathrm{H}_2\right]^{1 / 2}\left[\mathrm{I}_2\right]^{1 / 2}}{[\mathrm{HI}]}=8\)
⇒ \(\mathrm{H}_{2(\mathrm{~g})}+\mathrm{I}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{HI}_{(g)}\)
K’ \(=\frac{[\mathrm{HI}]^2}{\left[\mathrm{H}_2\right]\left[\mathrm{I}_2\right]}=\left(\frac{1}{8}\right)^2 \Rightarrow K^{\prime}=\frac{1}{64}\)
Question 10. Equilibrium constants K1 and K2 for the following equilibrium: \(\mathrm{NO}_{(g)}+\frac{1}{2} \mathrm{O}_{2(g)} \stackrel{K_1}{\rightleftharpoons} \mathrm{NO}_{2(g)}\) and \(2 \mathrm{NO}_{2(\mathrm{~g})} \stackrel{\mathrm{K}_2}{\rightleftharpoons} 2 \mathrm{NO}_{(\mathrm{g})}+\mathrm{O}_{2(\mathrm{~g})}\) are related as
- \(K_2=1 / K_1^2\)
- \(K_2=K_1^2\)
- \(K_2=1 / K_1\)
- \(K_2=K_1 / 2\)
Answer: 1. \(K_2=1 / K_1^2\)
⇒ \(K_1=\frac{p_{\mathrm{NO}_2}}{p_{\mathrm{NO}} \cdot\left(p_{\mathrm{O}_2}\right)^{1 / 2}}\)…..(1)
⇒ \(K_2=\frac{\left(p_{\mathrm{NO}}\right)^2 \cdot p_{\mathrm{O}_2}}{\left(p_{\mathrm{NO}_2}\right)^2}\)….(2)
Taking the square root on both sides in Equation 2,
⇒ \( \sqrt{K_2}=\frac{p_{\mathrm{NO}} \cdot\left(p_{\mathrm{O}_2}\right)^{1 / 2}}{p_{\mathrm{NO}_2}} \Rightarrow \sqrt{K_2}=\frac{1}{K_1} \Rightarrow K_2=\frac{1}{K_1^2}\)
Question 11. If K1 and K2 are the respective equilibrium constants for the two reactions, \(\mathrm{XeF}_{6(g)}+\mathrm{H}_2 \mathrm{O}_{(g)} \rightarrow \mathrm{XeOF}_{4(g)}+2 \mathrm{HF}_{(g)}\);
\(\mathrm{XeO}_{4(g)}+\mathrm{XeF}_{6(g)} \rightarrow \mathrm{XeOF}_{4(g)}+\mathrm{XeO}_3 \mathrm{~F}_{2(g)^{\prime}}\) the equilibrium constant of the reaction, \(\mathrm{XeO}_{4(g)}+2 \mathrm{HF}_{(g)} \rightarrow \mathrm{XeO}_3 \mathrm{~F}_{2(g)}+\mathrm{H}_2 \mathrm{O}_{(g)} \text {, }\) will be
- \(K_1 / K_2\)
- \(K_1 \cdot K_2\)
- \(K_1 /\left(K_2\right)^2\)
- \(K_2 / K_1\)
Answer: 4. \(K_2 / K_1\)
Given, \(\mathrm{XeF}_6+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{XeOF}_4+2 \mathrm{HF}, K_{\text {eq }}=K_1\)
⇒ \(\mathrm{XeOF}_4+2 \mathrm{HF} \rightleftharpoons \mathrm{XeF}_6+\mathrm{H}_2 \mathrm{O}, K_{\text {eq }}=1 / K_1\)….(1) and
⇒ \(\mathrm{XeO}_4+\mathrm{XeF}_6 \rightleftharpoons \mathrm{XeOF}_4+\mathrm{XeO}_3 \mathrm{~F}_2, K_{\text {eq }}=K\)….(2)
The reaction, \(\mathrm{XeO}_4+2 \mathrm{HF} \rightleftharpoons \mathrm{XeO}_3 \mathrm{~F}_2+\mathrm{H}_2 \mathrm{O}\), can be obtained by adding equation (1) and equation (2).
So, the equilibrium constant for the above reaction can be obtained by multiplying the equilibrium constants of equation (1) and equation (2).
Hence, the value is \(\frac{K_2}{K_1}\)
Question 12. The equilibrium constant for the reaction \(\mathrm{N}_2+3 \mathrm{H}_2 \rightleftharpoons 2 \mathrm{NH}_3\) is K, then the equilibrium constant for the equilibrium \(2 \mathrm{NH}_3 \rightleftharpoons \mathrm{N}_2+3 \mathrm{H}_2\) is
- \(\sqrt{K}\)
- \(\sqrt{\frac{1}{K}}\)
- \(\frac{1}{K}\)
- \(\frac{1}{K^2}\)
Answer: 3. \(\sqrt{K}\)
The equilibrium constant for the reverse reaction will be 1/K.
Question 13. K1 and K2 are equilibrium constants for reactions (1) and (2) respectively. \(\mathrm{N}_{2(g)}+\mathrm{O}_{2(g)} \rightleftharpoons \mathrm{NO}_{(g)}\)…..(1); \(\mathrm{NO}_{(g)} \rightleftharpoons \frac{1}{2} \mathrm{~N}_{2(g)}+\frac{1}{2} \mathrm{O}_{2(g)}\)
- \(K_1=\left(\frac{1}{K_2}\right)^2\)
- \(K_1=K_2{ }^2\)
- \(K_1=\frac{1}{K_2}\)
- \(K_1=\left(K_2\right)^0\)
Answer: 1. \(K_1=\left(\frac{1}{K_2}\right)^2\)
Reaction (2) is the reversible reaction of (1) and is half of the reaction (1). Thus, rate constant can be given as \(K_2=\sqrt{\frac{1}{K_1}} \text { or } K_1=\left[\frac{1}{K_2}\right]^2\)
Question 14. The reaction, \(2 A_{(g)}+B_{(g)} \rightleftharpoons 3 C_{(g)}+D_{(g)}\) begins with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M. The value for the equilibrium constant for this reaction is given by the expression
- [(0.75)³ (0.25)] ÷ [(1.00)² (1.00)]
- [(0.75)³ (0.25)] ÷ [(0.50)² (0.75)]
- [(0.75)³ (0.25)] ÷ [(0.50)² (0.25)]
- [(0.75)³ (0.25)] ÷ [(0.75)² (0.25)]
Answer: 2. [(0.75)³ (0.25)] + [(0.50)² (0.75)]
The reaction, \(2 A_{(g)}+B_{(g)} \rightleftharpoons 3 C_{(g)}+D_{(g)}\) begins with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M.
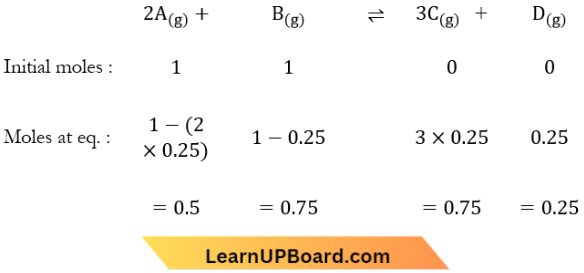
Equilibrium constant, \(K=\frac{[C]^3[D]}{[A]^2[B]}\)
∴ K= \(\frac{(0.75)^3(0.25)}{(0.5)^2(0.75)}\)
Question 15. The dissociation equilibrium of a gas AB2 can be represented as \(2 A B_{2(g)} \rightleftharpoons 2 A B_{(g)}+B_{2(g)}\) The degree of dissociation is x and is small compared to 1. The expression relating the degree of dissociation (x) with equilibrium constant Kp and total pressure P is
- \(\left(2 K_{/} / P\right)^{1 / 2}\)
- \(\left(K_p / P\right)\)
- \(\left(2 K_p / P\right)\)
- \(\left(2 K_p / P\right)^{1 / 3}\)
Answer: 4. \(\left(2 K_p / P\right)^{1 / 3}\)
The dissociation equilibrium of a gas AB2 can be represented as \(2 A B_{2(g)} \rightleftharpoons 2 A B_{(g)}+B_{2(g)}\) The degree of dissociation is x and is small compared to 1.
Amount of moles at equilibrium = 2(1 – x) + 2x + x = 2 + x
⇒ \(K_p=\frac{\left[p_{A B}\right]^2\left[p_{B_2}\right]}{\left[p_{A B_2}\right]^2}\)
⇒ \(K_p=\frac{\left(\frac{2 x}{2+x} \times P\right)^2 \times\left(\frac{x}{2+x} \times P\right)}{\left(\frac{2(1-x)}{2+x} \times P\right)^2}=\frac{\frac{4 x^3}{2+x} \times P}{4(1-x)^2}\)
⇒ \(K_p=\frac{4 x^3 \times P}{2} \times \frac{1}{4}\) (because \(1-x \approx 1\) and \(2+x \approx 2\))
x = \(\left(\frac{8 K_p}{4 P}\right)^{1 / 3} \Rightarrow x=\left(\frac{2 K_p}{P}\right)^{1 / 3}\)
Question 16. The values of \(K_{p_1}\) and \(K_{p_2}\) for the reactions,
Y \(\rightleftharpoons\) Z ……(1)
A \(\rightleftharpoons\) 2B……….(2)
are in the ratio 9: 1. If the degree of dissociation of X and A be equal, then total pressure at equilibrium (1) and (2) are in the ratio
- 36:1
- 1:1
- 3: 1
- 1: 9
Answer: 1. 36:1
X \(\rightleftharpoons\) +Z…(1)
A \(\rightleftharpoons 2 B\) ……(2)
X \(\rightleftharpoons\) Y+Z

Total no. of moles at equilibrium =1-α+2α =1+α
Similarly,
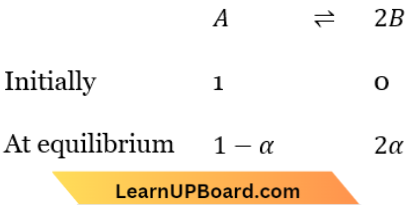
Total Number of moles at equilibrium = 1-α+2α=1+α
∴ \(K_{p_1}=\frac{p_Y \times p_Z}{p_X}=\frac{\frac{\alpha}{1+\alpha} \times P_1 \times \frac{\alpha}{1+\alpha} \times P_1}{\frac{1-\alpha}{1+\alpha} \times P_1}=\frac{\alpha^2 P_1}{(1+\alpha)(1-\alpha)}\)
∴ \(K_{p_2}=\frac{\left(p_B\right)^2}{p_A}=\frac{\left(\frac{2 \alpha}{1+\alpha} \times P_2\right)^2}{\frac{1-\alpha}{1+\alpha} \times P_2}=\frac{(2 \alpha)^2 P_2}{(1+\alpha)(1-\alpha)}\)
Now \(\frac{K_{p_1}}{K_{p_2}}=\frac{P_1}{4 P_2} \Rightarrow \frac{K_{p_1}}{K_{p_2}}=\frac{9}{1}=\frac{P_1}{4 P_2} \Rightarrow \frac{P_1}{P_2}=\frac{36}{1}=36: 1\)
Question 17. A 20-litre container at 400 K contains CO2(g) at pressure 0.4 atm and an excess of SrO (neglecting the volume of solid S2O). The volume of the container is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when the pressure of CO2 attains its maximum value, will be (Given that: \(\mathrm{SrCO}_{3(s)} \rightleftharpoons \mathrm{SrO}_{(s)}+\mathrm{CO}_{2(g)}\), Kp = 1.6 atm)
- 10 litre
- 4 litre
- 2 litre
- 5 litre
Answer: 4. 5 litre
20-litre container at 400 K contains CO2(g) at pressure 0.4 atm and an excess of SrO (neglecting the volume of solid S2O). The volume of the container is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when the pressure of CO2 attains its maximum value
⇒ \(\mathrm{SrCO}_{3(s)} \rightleftharpoons \mathrm{SrO}_{(s)}+\mathrm{CO}_{2(g)} ; K_p=1.6 \mathrm{~atm}\)
⇒ \(K_p=\frac{p_{\mathrm{CO}_2} \times p_{\mathrm{SrO}}}{p_{\mathrm{SrCO}_3}} \Rightarrow 1.6=p_{\mathrm{CO}_2}\) (because \(p_{\mathrm{SrO}}=p_{\mathrm{SrCO}_3}=1\))
∴ Maximum pressure of CO2 = 1.6 atm
Let the maximum volume of the container when the pressure of CO2 is 1.6 atm be V L
During the process, PV = constant
∴ 0.4 x 20 = 1.6 X V
⇒ V = \(\frac{0.4 \times 20}{1.6}=5 \mathrm{~L}\)
Question 18. In which of the following equilibriums Kc and Kp are not equal?
- \(2 \mathrm{NO}_{(g)} \rightleftharpoons \mathrm{N}_{2(g)}+\mathrm{O}_{2(g)}\)
- \(\mathrm{SO}_{2(g)}+\mathrm{NO}_{2(g)} \rightleftharpoons \mathrm{SO}_{3(g)}+\mathrm{NO}_{(g)}\)
- \(\mathrm{H}_{2(g)}+\mathrm{I}_{2(g)} \rightleftharpoons 2 \mathrm{HI}_{(g)}\)
- \(2 \mathrm{C}_{(s)}+\mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{CO}_{2(\mathrm{~g})}\)
Answer: 4. \(2 \mathrm{C}_{(s)}+\mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{CO}_{2(\mathrm{~g})}\)
Kc and Kp are related by the equation, \(K_p=K_c(R T)^{\Delta n_g}\)
where \({\Delta n_g}\) = difference in the no. of moles of products and reactants in the gaseous state.
for \(2 \mathrm{C}_{(s)}+\mathrm{O}_{2(g)} \rightleftharpoons 2 \mathrm{CO}_{2(g)}\)
∴ \(\Delta n_g=2-1=1 \neq 0\)
Question 19. If the concentration of OH- ions in the reaction \(\mathrm{Fe}(\mathrm{OH})_{3(s)} \rightleftharpoons \mathrm{Fe}_{(a q)}^{3+}+3 \mathrm{OH}_{(a q)}^{-}\) is decreased by 1/4 times, then equilibrium concentration of Fe3+ will increase by
- 64 times
- 4 times
- 8 times
- 16 times.
Answer: 1. 64 times
If the concentration of OH- ions in the reaction \(\mathrm{Fe}(\mathrm{OH})_{3(s)} \rightleftharpoons \mathrm{Fe}_{(a q)}^{3+}+3 \mathrm{OH}_{(a q)}^{-}\) is decreased by 1/4 times
Fe\((\mathrm{OH})_{3(s)} \rightleftharpoons \mathrm{Fe}_{(a q)}^{3+}+3 \mathrm{OH}_{(a q)}^{-}\)
K = \(\frac{\left[\mathrm{Fe}^{3+}\right]\left[\mathrm{OH}^{-}\right]^3}{\left[\mathrm{Fe}(\mathrm{OH})_3\right]}\)
K = \(\left[\mathrm{Fe}^{3+}\right]\left[\mathrm{OH}^{-}\right]^3\) (activity of solid is taken unity)
The concentration of OH– ion in the reaction is decreased by 1/4 times then the equilibrium concentration of Fe3+ will be increased by 64 times in order to keep the value of K constant.
Question 20. Equilibrium constant Kp for the following reaction \(\mathrm{MgCO}_{3(s)} \rightleftharpoons \mathrm{MgO}_{(s)}+\mathrm{CO}_{2(g)}\)
- \(K_p=p_{\mathrm{CO}_2}\)
- \(K_p=p_{\mathrm{CO}_2} \times \frac{p_{\mathrm{CO}_2} \times p_{\mathrm{MgO}}}{p_{\mathrm{MgCO}_3}}\)
- \(K_p=\frac{p_{\mathrm{CO}_2}+p_{\mathrm{MgO}}}{p_{\mathrm{MgCO}_3}}\)
- \(K_p=\frac{p_{\mathrm{MgCO}_3}}{p_{\mathrm{CO}_2} \times p_{\mathrm{MgO}}}\)
Answer: 1. \(K_p=p_{\mathrm{CO}_2}\)
Kp = PCO2.
Solids do not exert pressure, so their partial pressure is taken as unity.
Question 21. If the value of the equilibrium constant for a particular reaction is 1.6 x 1012, then at equilibrium the system will contain
- Mostly products
- Similar amounts of reactants and products
- All reactants
- Mostly reactants.
Answer: 1. Mostly products
The value of K is high which means the reaction proceeds almost to completion i.e., the system will contain mostly products.
Question 22. In the Haber process, 30 litres of dihydrogen and 30 litres of dinitrogen were taken for the reaction which yielded only 50% of the expected product. What will be the composition of a gaseous mixture under the aforesaid condition in the end?
- 20 litres of ammonia, 20 litres of nitrogen, 20 litres of hydrogen
- 10 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
- 20 litres of ammonia, 10 litres of nitrogen, and 30 litres of hydrogen
- 20 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
Answer: 2. 10 litres ammonia, 25 litres nitrogen, 15 litres hydrogen
In the Haber process, 30 litres of dihydrogen and 30 litres of dinitrogen were taken for the reaction which yielded only 50% of the expected product.
⇒ \(\begin{array}{ccc}
3 \mathrm{H}_2 & +\mathrm{N}_2 & \rightarrow 2 \mathrm{NH}_3 \\
3 & 1 & 2 \\
3 / 2 & 1 / 2 & 1 \\
10 \times \frac{3}{2} & 10 \times \frac{1}{2} & 10 \times 1 \\
15 & 5 & 10
\end{array}\)
The composition of a gaseous mixture under the aforesaid condition in the end will be
H2 =30-15=15 litres
N2 = 30 – 5 =25 litres; NH3 = l0 litres
Question 23. The reaction quotient (Q) for the reaction \(\mathrm{N}_{2(g)}+3 \mathrm{H}_{2(g)} \rightleftharpoons 2 \mathrm{NH}_{3(g)}\) is given by Q = \(\frac{\left[\mathrm{NH}_3\right]^2}{\left[\mathrm{~N}_2\right]\left[\mathrm{H}_2\right]^3}\). The reaction will proceed from right to left if
- Q = Kc
- Q < Kc
- Q>Kc
- Q = 0
where Kc is the equilibrium constant.
Answer: 3. Q>Kc
The reaction quotient (Q) for the reaction \(\mathrm{N}_{2(g)}+3 \mathrm{H}_{2(g)} \rightleftharpoons 2 \mathrm{NH}_{3(g)}\) is given by Q = \(\frac{\left[\mathrm{NH}_3\right]^2}{\left[\mathrm{~N}_2\right]\left[\mathrm{H}_2\right]^3}\).
⇒ \(\mathrm{N}_{2(\mathrm{~g})}+3 \mathrm{H}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{NH}_{3(\mathrm{~g})}\)
⇒ \(K_c=\frac{\left[\mathrm{NH}_3\right]^2}{\left[\mathrm{~N}_2\right]\left[\mathrm{H}_2\right]^3} ; \Delta n_{(\mathrm{g})}=2-4=-2\)
Thus, the reaction will go from right to left when Q > Kc
Question 24. The equilibrium concentrations of the species in the reaction A + B \(\rightleftharpoons\) C + D are 2, 3, 10 and 6 mol L-1, respectively at 300 K. ΔG° for the reaction is (R = 2 cal/mol K)
- -1381.80 cal
- -13.73 cal
- 1372.60 cal
- -137.26 cal
Answer: 1. -1381.80 cal
A + \(B \rightleftharpoons C+D\)
K = \(\frac{[C][D]}{[A][B]}=\frac{10 \times 6}{2 \times 3}=10\)
Δ\(G^{\circ}\)=-R T ln K
Δ\(G^{\circ}\)=-2.303 R T log K
Δ\(G^{\circ}=-2.303 \times 2 \times 300 \times \log 10\)
= \(-2.303 \times 2 \times 300=-1381.80 \mathrm{cal}\)
Question 25. Hydrolysis of sucrose is given by the following reaction : Sucrose + H2O \(\rightleftharpoons\) Glucose + Fructose If the equilibrium constant (KC) is 2 x 1013 at 300 K, the value of ΔrG° at the same temperature will be
- -8.314 J mol-1 K-1 x 300 K x ln(2 x 1013)
- 8.314 J mol-1 K-1 x 300 K x ln(2 x 1013)
- 8.314 J mol-1 K-1 x 300 K x ln(3 x 1013)
- -8.314 J mol-1 K-1 x 300 K x ln(4 x 1013)
Answer: 1. -8.314 J mol-1 K-1 x 300 K x ln(2 x 1013)
ΔG = ΔG° + RT In Q
At equilibrium, ΔG = 0 and Q = KC.
∴ 0 = ΔG°+ RT In KC.
⇒ ΔG° = -RT ln KC
= -8.314 J mol-1 K-1 x 300 K x ln(2 x 1013)
Question 26. Which of the following statements is correct for a reversible process in a state of equilibrium?
- ΔG° = -2.30 RT log K
- ΔG° = 2.30 RT log K
- ΔG = -2.30 RT log K
- ΔG = 2.30 RT log K
Answer: 1. ΔG° = -2.30 RT log K
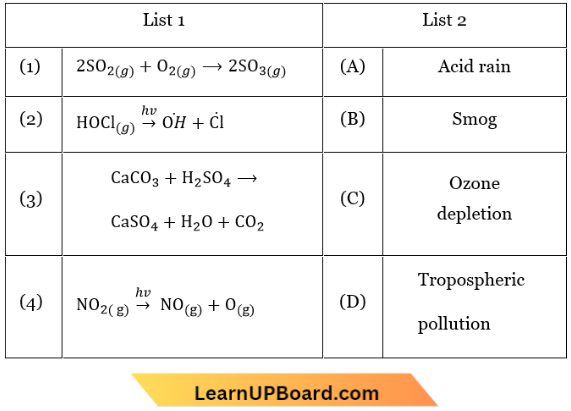
Question 27. Match List 1 (Equations) with List 2 (Type of Processes) and select the correct option.
- 1-(A), 2-(B), 3-(C), 4-(D)
- 1 -(C), 2-(D), 3-(B), 4-(A)
- 1-(D), 2-(A), 3-(B), 4-(C)
- 1-(B), 2-(A), 3-(D), 4-(C)
Answer: 3. 1-(D), 2-(A), 3-(B), 4-(C)
When Kp>Q, rate of forward reaction > rate of backward reaction.
∴ Reaction is spontaneous.
When ΔG° < RT ln Q, ΔG° is positive, the reverse reaction is feasible, thus reaction is non-spontaneous.
When Kp = Q, rate of forward reaction = rate of backward reaction.
∴ The reaction is in equilibrium.
When TΔS > ΔH, ΔG will be negative only when ΔH = +ve.
∴ The reaction is spontaneous and endothermic.
Question 28. Which one of the following conditions will favour the maximum formation of the product in the reaction \(A_{2(g)}+B_{2(g)} \rightleftharpoons X_{2(g)}, \Delta_r H=-X \mathrm{~kJ}?\)
- Low temperature and high pressure
- Low temperature and low pressure
- High temperature and high pressure
- High temperature and low pressure
Answer: 1. Low temperature and high pressure
On increasing the pressure and decreasing the temperature, equilibrium will shift in the forward direction.
Question 29. For the reversible reaction, \(\mathrm{N}_{2(\mathrm{~g})}+3 \mathrm{H}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{NH}_{3(\mathrm{~g})}+\text { heat }\) The equilibrium shifts in forward direction
- By increasing the concentration of NH3(g)
- By decreasing the pressure
- By decreasing the concentrations of N2(g) and H2(g)
- By increasing pressure and decreasing temperature.
Answer: 4. By increasing pressure and decreasing temperature.
As the forward reaction is exothermic and leads to a lowering of pressure (produces a lesser number of gaseous moles) hence, according to Le Chatelier’s principle, at high pressure and low temperature, the given reversible reaction will shift in the forward direction to form more product.
Question 30. For a given exothermic reaction, Kp and K’p are the equilibrium constants at temperatures T1 and T2, respectively. Assuming that the heat of the reaction is constant in the temperature range between T1 and T2 it is readily observed that
- \(K_p>K_p^{\prime}\)
- \(K_p<K_p^{\prime}\)
- \(K_p=K_p^{\prime}\)
- \(K_p=\frac{1}{K_p^{\prime}}\)
Answer: 1. \(K_p>K_p^{\prime}\)
log\(\frac{K_p^{\prime}}{K_p}=-\frac{\Delta H}{2.303 R}\left[\frac{1}{T_2}-\frac{1}{T_1}\right]\)
For an exothermic reaction, ΔH = -ve i.e., heal is evolved. The temperature T2 is higher than T1
Thus, \(\left(\frac{1}{T_2}-\frac{1}{T_1}\right)\) is negative.
so, \(\log K_p^{\prime}-\log K_p=- \text { ve } \quad \text { or } \quad \log K_p>\log K_p^{\prime}\)
or \(K_p>K_p^{\prime}\)
Question 31. KMnO4 can be prepared from K2MnO4 as per the region, \(3 \mathrm{MnO}_4^{2-}+2 \mathrm{H}_2 \mathrm{O} \rightleftharpoons 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+4 \mathrm{OH}^{-}\) The reaction can go to completion by removing OH– ions by adding
- CO2
- SO2
- HCl
- KOH
Answer: 1. CO2
HCI and SO2 are reducing agents, So, they can reduce MnO4–.
CO2 is neither an oxidising nor a reducing agent, it will provide only an acidic medium. It can shift the reaction in the forward direction and the reaction can go to completion.
Question 32. The value of ΔH for the reaction \(X_{2(g)}+4 Y_{2(g)} \rightleftharpoons 2 X Y_{4(g)}\) Formation of XY4(g) will be favoured at
- High temperature and high pressure
- Low pressure and low temperature
- High temperature and low pressure
- High pressure and low temperature.
Answer: High pressure and low temperature.
⇒ \(X_{2(g)}+4 Y_{2(g)} \rightleftharpoons 2 X Y_{4(g)}\)
Δng = -ve and ΔH = -ve
The reaction is favoured in the forward direction at low temperature and high pressure.
Question 33. For the reaction \(\mathrm{CH}_{4(\mathrm{~g})}+2 \mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons \mathrm{CO}_{2(\mathrm{~g})}+2 \mathrm{H}_2 \mathrm{O}_{(l)}\), ΔHr = -170.8 kJ mol-1. Which of the following statements is not true?
- The reaction is exothermic.
- At equilibrium, the concentrations of CO2(g) and H2O(l) are not equal.
- The equilibrium constant for the reaction is given by \(K_p=\frac{\left[\mathrm{CO}_2\right]}{\left[\mathrm{CH}_4\right]\left[\mathrm{O}_2\right]}\)
- Addition of CH4(g) or O2(g) at equilibrium mm will cause a shift to the right.
Answer: 3. The equilibrium constant for the reaction is given by \(K_p=\frac{\left[\mathrm{CO}_2\right]}{\left[\mathrm{CH}_4\right]\left[\mathrm{O}_2\right]}\)
For the reaction \(\mathrm{CH}_{4(\mathrm{~g})}+2 \mathrm{O}_{2(\mathrm{~g})} \rightleftharpoons \mathrm{CO}_{2(\mathrm{~g})}+2 \mathrm{H}_2 \mathrm{O}_{(l)}\), ΔHr = -170.8 kJ mol-1.
⇒ \(\mathrm{CH}_{4(g)}+2 \mathrm{O}_{2(g)} \rightleftharpoons \mathrm{CO}_{2(g)}+2 \mathrm{H}_2 \mathrm{O}_{(l)}\)
⇒ \(K_p=\frac{p_{\mathrm{CO}_2}}{p_{\mathrm{CH}_4} \cdot p_{\mathrm{O}_2}^2}\)
Question 34. Reaction \(\mathrm{BaO}_{2(s)} \rightleftharpoons \mathrm{BaO}_{(s)}+\mathrm{O}_{2(g)} ; \Delta H=+\mathrm{ve}\) In equilibrium condition, pressure of O2 depends on
- Increase mass of BaO2
- Increase the mass of BaO
- Increase the temperature on equilibrium
- Increase the mass of BaO2 and BaO.
Answer: 3. Increase the temperature on equilibrium
The pressure of O2 does not depend on concentration terms of other reactants (because both are in solid-state), Since this is an endothermic reaction if the temperature is raised, dissociation of BaO2 would occur, and more O2 is produced at equilibrium, the pressure of O2 increases.
Question 35. For any reversible reaction, if we increase the concentration of the reactants, then the effect on the equilibrium constant
- Depends on the amount of concentration
- Unchanged
- Decrease
- Increase.
Answer: 2. Unchanged
For a reaction, \(A+B \rightleftharpoons C+D\),
⇒ \(K_{\mathrm{eq}}\)=\(\frac{[C][D]}{[A][B]}\)
Increase in conc. of reactants will proceed the equilibrium in the forward direction giving more products so that the equilibrium constant value remains constant and independent of concentration.
Question 36. According to Le Chatelier’s principle, adding heat to a solid and liquid in equilibrium will cause the
- Temperature to increase
- Temperature to decrease
- Amount of liquid to decrease
- Amount of solid to decrease.
Answer: 4. Amount of solid to decrease.
When solid and liquid are in equilibrium, the increase in temperature results in an increase in the volume of liquid or a decrease in the amount of solid
Solid \(\rightleftharpoons\) Liquid
With the increase in temperature equilibrium shifts in the forward direction.
Question 37. Which one of the following information can be obtained on the basis of the Le Chatelier principle?
- Dissociation constant of a weak acid
- Entropy change in a reaction
- The equilibrium constant of a chemical reaction
- Shift in equilibrium position on changing the value of a constraint
Answer: 4. Shift in equilibrium position on changing the value of a constraint
According to Le Chatelier’s principle, if an equilibrium is subjected to a change in concentration, pressure temperature, etc. equilibrium shifts in such a way so as to undo the effect of a change imposed.
Question 38. Aqueous solution which of the following compounds is the best conductor of electric current?
- Hydrochloric acid, HCl
- Ammonia, NH3
- Fructose, C6H12O6
- Acetic acid, C2H4O2
Answer: 1. Hydrochloric acid, HCl
HCl is a strong acid and dissociates completely into in aqueous solution.
Question 39. Aqueous solution of acetic acid contains
- \(\mathrm{CH}_3 \mathrm{COO}^{-}\) and \(\mathrm{H}^{+}\)
- \(\mathrm{CH}_3 \mathrm{COO}^{-}, \mathrm{H}_3 \mathrm{O}^{+}\) and \(\mathrm{CH}_3 \mathrm{COOH}\)
- \(\mathrm{CH}_3 \mathrm{COO}^{-}, \mathrm{H}_3 \mathrm{O}^{+}\) and \(\mathrm{H}^{+}\)
- \(\mathrm{CH}_3 \mathrm{COOH}, \mathrm{CH}_3 \mathrm{COO}^{-}\) and \(\mathrm{H}^{+}\)
Answer: 2. \(\mathrm{CH}_3 \mathrm{COO}^{-}, \mathrm{H}_3 \mathrm{O}^{+}\) and \(\mathrm{CH}_3 \mathrm{COOH}\)
∴ \(\mathrm{CH}_3 \mathrm{COOH}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}_3 \mathrm{O}^{+}\)
As acetic acid is a weak acid so, it also contains some undissociated CH3COOH along with CH3COO– and H3O+ ions.
Question 40. Amongst the given options which of the following molecules/ion acts as a Lewis acid?
- BF3
- OH–
- NH3
- H2O
Answer: 1. BF3
Among the given molecules/ions, BF3 acts as a Lewis acid as it is an electron-deficient species, it can accept a lone pair of electrons.
Question 41. The conjugate base for Bronsted acids H2O and HF are
- \(\mathrm{H}_3 \mathrm{O}^{+}\) and \(\mathrm{H}_2 \mathrm{~F}^{+}\) respectively
- \(\mathrm{OH}^{-}\) and \(\mathrm{H}_2 \mathrm{~F}^{+}\), respectively
- \(\mathrm{H}_3 \mathrm{O}^{+}\) and \(\mathrm{F}^{-}\), respectively
- \(\mathrm{OH}^{-}\) and \(\mathrm{F}^{-}\), respectively.
Answer: 4. \(\mathrm{OH}^{-}\) and \(\mathrm{F}^{-}\), respectively.
⇒ \(\begin{array}{cc}
\text { Bronsted acid } & \text { Conjugate base } \\
\mathrm{H}_2 \mathrm{O} & \mathrm{OH}^{-} \\
\mathrm{HF} & \mathrm{F}^{-}
\end{array}\)
Question 42. Which of the following cannot act both as Bronsted acid and as Bronsted base?
- \(\mathrm{HCO}_3^{-}\)
- \(\mathrm{NH}_3\)
- \(\mathrm{HCl}\)
- \(\mathrm{HSO}_4^{-}\)
Answer: 3. \(\mathrm{HCl}\)
HCI cannot accept H+ ions and, therefore cannot act as Bronsted Base.
Question 43. Which of the following fluoro-compounds is most likely to behave as a Lewis base?
- BF3
- PF3
- CF4
- SiF4
Answer: 2. PF3
BF3 → Lewis acid (incomplete octet)
PF3 → Lewis base (presence of lone pair on P atom)
CF4 → Complete octet
SiF4 → Lewis acid (empty d-orbital in Si-atom)
Question 44. Which of these is least likely to act as a Lewis base?
- BF3
- PF3
- CO
- F–
Answer: 1. BF3
BF3 is Lewis acid (e– pair acceptor).
Question 45. Which is the strongest acid in the following?
- HClO4
- H2SO3
- H2SO4
- HClO3
Answer: 1. HClO4
⇒ \(\stackrel{+7}{\mathrm{HClO}}_4\) with the highest oxidation number and its conjugate base is resonance stabilised, hence it is the most acidic. CI is more electronegative than S.
Question 46. Which one of the following molecular hydrides acts as a Lewis acid?
- NH3
- H2O
- B2H6
- CH4
Answer: 3. B2H6
Compounds that are electron deficient act as Lewis acids. Out of the given hydrides, B2H6 satisfies this condition and is, therefore, a Lewis acid.
Question 47. Which of the following molecules acts as a Lewis acid?
- (CH3)2O
- (CH3)3P
- (CH3)3N
- (CH3)3B
Answer: 4. (CH3)3B
Lewis acids are electron-deficient compounds since (CH3)3 B is electron-deficient (due to an incomplete octet of B); it acts as a Lewis acid.
Question 48. Which one of the following statements is not true?
- Among halide ions, iodide is the most powerful reducing agent.
- Fluorine is the only halogen that does not show a variable oxidation state.
- HOCl is a stronger acid than HOBr.
- HF is a stronger acid than HCl
Answer: 4. HF is a stronger acid than HCl
Due to the strong hydrogen-fluorine bond, proton is not given off easily and hence, HF is the weakest acid.
Question 49. Which one of the following compounds is not a protonic acid?
- B(OH)3
- PO(OH)3
- SO(OH)2
- SO2(OH)2
Asnwer: 1. B(OH)3
B(OH)3 in an aqueous medium coordinates a molecule of water to form the hydrated species 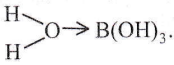
In this species, B3+ ion, because of its small size, has high polarizing power thereby pulling the sigma electron charge of the coordinated O atom towards itself. The coordinated oxygen, in turn, pulls the sigma electron charge of the OH bond of the attached water molecule towards itself. This facilitates the removal of H+ ions from the O – H bond.

Thus, the solution of B(OH)3 in water acts as a weak acid, and it is not a protonic acid.
Question 50. In \(\mathrm{HS}^{-}, \mathrm{I}^{-}, R-\mathrm{NH}_2, \mathrm{NH}_3\) order of proton accepting tendency will be
- \(\mathrm{I}^{-}>\mathrm{NH}_3>R-\mathrm{NH}_2>\mathrm{HS}^{-}\)
- \(\mathrm{NH}_3>\mathrm{R}-\mathrm{NH}_2>\mathrm{HS}^{-}>\mathrm{I}^{-}\)
- \(\mathrm{R}-\mathrm{NH}_2>\mathrm{NH}_3>\mathrm{HS}^{-}>\mathrm{I}^{-}\)
- \(\mathrm{HS}^{-}>\mathrm{R}-\mathrm{NH}_2>\mathrm{NH}_3>\mathrm{I}^{-}\)
Answer: 3. \(\mathrm{R}-\mathrm{NH}_2>\mathrm{NH}_3>\mathrm{HS}^{-}>\mathrm{I}^{-}\)
Proton accepting tendency is known as the strength of basicity.
In \(R-\ddot{\mathrm{N}}_2\), N has a lone pair of electrons which intensifies due to electron releasing R-group and increases the tendency to donate a lone pair of electrons to H+.
Secondly, as the size of the ion increases, there is less attraction for H+ to form a bond with the H-atom and are is basic. Thus the order of proton accepting tendency: \(\mathrm{R}-\mathrm{NH}_2>\mathrm{NH}_3>\mathrm{HS}^{-}>\mathrm{I}^{-}\)
Question 51. The conjugate acid of NH–2 is
- \(\mathrm{NH}_4 \mathrm{OH}\)
- \(\mathrm{NH}_2^{-}\)
- \(\mathrm{NH}_4^{+}\)
- \(\mathrm{NH}_3\)
Answer: 4. \(\mathrm{NH}_3\)
NH–2 + H+ → NH3 (conjugate acid)
Substance + H+ → conjugate acid
Substance – H+ → conjugate base
Question 52. Which compound is electron deficient?
- \(\mathrm{BeCl}_2\)
- \(\mathrm{BCl}_3\)
- \(\mathrm{CCl}_4\)
- \(\mathrm{PCl}_5^3\)
Answer: 2. \(\mathrm{BCl}_3\)
In BCl3 the central atom ‘B’ is sp² hybridised and contains only ‘six’-electrons in its valence shell. Therefore, it is electron deficient.
Question 53. The strongest conjugate base is
- \(\mathrm{SO}_4^{2-}\)
- \(\mathrm{Cl}^{-}\)
- \(\mathrm{NO}_3^{-}\)
- \(\mathrm{CH}_3 \mathrm{COO}^{-}\)
Answer: 4. \(\mathrm{CH}_3 \mathrm{COO}^{-}\)
⇒ \(\underset{\text { Weak acid }}{\mathrm{CH}_3 \mathrm{COOH}} \rightleftharpoons \underset{\text { Strong conjugate base }}{\mathrm{CH}_3 \mathrm{COO}^{-}}+\mathrm{H}^{+}\)
As CH3COOH is the weakest acid, its conjugate base (CH3COO–) is the strongest base, H2SO4, HCl, and HNO3 are strong acids, so their conjugate bases are weak.
Question 54. Which of the following is not a Lewis acid?
- \(\mathrm{SiF}_4\)
- \(\mathrm{C}_2 \mathrm{H}_4\)
- \(\mathrm{BF}_3\)
- \(\mathrm{FeCl}_3\)
Answer: 2. \(\mathrm{C}_2 \mathrm{H}_4\)
In BF3 and FeCI3 molecules, the central atoms have incomplete octets and in SiF4 the central atom has empty d-orbitals. Hence, according to Lewis’s concept, these are Lewis acids
Question 55. Repeated use of which one of the following fertilizers would increase the acidity of the soil?
- Ammonium sulphate
- Superphosphate of lime
- Urea
- Potassium nitrate
Answer: 1. Ammonium sulphate
Ammonium sulphate is a salt of strong acid (H2SO4 and weak base (NH4OH). Therefore, repeated use of ammonium sulphate would increase the concentration of sulphuric acid, while ammonia from NH4OH is used up by the plant. Hence, the acidity of the soil will increase.
Question 56. The pKb of dimethylamine and pKa of acetic acid are 3.27 and 4.77 respectively at T(K). The correct option for the pH of dimethylammonium acetate solution is
- 6.25
- 8.50
- 5.50
- 7.75
Answer: 4. 7.75
The pKb of dimethylamine and pKa of acetic acid are 3.27 and 4.77 respectively at T(K).
For a salt of weak acid and weak base
pH = \(7+\frac{1}{2}\left(\mathrm{p}_a-\mathrm{p} K_b\right)\)
Given, \(\mathrm{p} K_a=4.77, \mathrm{p}_b=3.27\)
pH = \(7+\frac{1}{2}(4.77-3.27)=7.75\)
Question 57. Find out the solubility of Ni(OH)2 in 0.1 M NaOH. Given that the ionic product of Ni(OH)2 is 2 x 10-15.
- \(2 \times 10^{-13} \mathrm{M}\)
- \(2 \times 10^{-8} \mathrm{M}\)
- \(1 \times 10^{-13} \mathrm{M}\)
- \(1 \times 10^8 \mathrm{M}\)
Answer: 1. \(2 \times 10^{-13} \mathrm{M}\)
⇒ \(\mathrm{Ni}(\mathrm{OH})_2 \rightleftharpoons \mathrm{Ni}_s^{2+}+\underset{2 s}{2 \mathrm{OH}^{-}}\) where s is the solubility of \(\mathrm{Ni}(\mathrm{OH})_2\)
⇒ \(\underset{0.1 \mathrm{M}}{\mathrm{NaOH}} \rightleftharpoons \underset{0.1 \mathrm{M}}{\mathrm{Na}^{+}}+\underset{0.1 \mathrm{M}}{\mathrm{OH}^{-}}\)
⇒ \(\left[\mathrm{OH}^{-}\right]=2 s+0.1\) approx 0.1(because 2 s<<<0.1)
Ionic product of \(\mathrm{Ni}(\mathrm{OH})_2=\left[\mathrm{Ni}^{2+}\right]\left[\mathrm{OH}^{-}\right]^2\) \(2 \times 10^{-15}=s(0.1)^2\)
s = \(\frac{2 \times 10^{-15}}{0.1 \times 0.1}=2 \times 10^{-13} \mathrm{M}\)
Question 58. The pH of 0.01 M NaOH(aq) solution will be
- 7.01
- 2
- 12
- 9
Answer: 3. 12
⇒ \(\underset{0.01 M}{\mathrm{NaOH}} \rightleftharpoons \mathrm{Na}^{+}+ \underset{0.1 M}{\mathrm{OH}^{-}}\)
∴ [OH–] = 0.01 M
∴ pOH = -log [OH–] = -log(0.01) = 2
∴ pH = 14 – pOH = 14-2 = 12
Question 59. The following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations:
- \(60 \mathrm{~mL} \frac{\mathrm{M}}{10} \mathrm{HCl}+40 \mathrm{~mL} \frac{\mathrm{M}}{10} \mathrm{NaOH}\)
- \(55 \mathrm{~mL} \frac{\mathrm{M}}{10} \mathrm{HCl}+45 \mathrm{~mL} \frac{\mathrm{M}}{10} \mathrm{NaOH}\)
- \(75 \mathrm{~mL} \frac{\mathrm{M}}{5} \mathrm{HCl}+25 \mathrm{~mL} \frac{\mathrm{M}}{5} \mathrm{NaOH}\)
- \(100 \mathrm{~mL} \frac{\mathrm{M}}{10} \mathrm{HCl}+100 \mathrm{~mL} \frac{\mathrm{M}}{10} \mathrm{NaOH}\)
pH of which one of them will be equal to 1?
- B
- A
- D
- C
Answer: 4. C
pH = 1, so [H+] = 10-1
For the acid-base mixture: N1V1 – N2V2 = N3V3.
(For NaOH and HCl, Normaiity = Molarity)
- \(M_1\left(\mathrm{H}^{+}\right)=\frac{60 \times \frac{1}{10}-40 \times \frac{1}{10}}{100}=2 \times 10^{-2} \mathrm{M}\) i.e. \(\mathrm{pH}=1.698 \approx 1.7\)
- \(M_2\left(\mathrm{H}^{+}\right)=\frac{55 \times \frac{1}{10}-45 \times \frac{1}{10}}{100}=\frac{1}{100}=10^{-2} \mathrm{M}\) i.e. \(\mathrm{pH}=2\)
- \(M_3\left(\mathrm{H}^{+}\right)=\frac{75 \times \frac{1}{5}-25 \times \frac{1}{5}}{100}=10^{-1} \mathrm{M}\) i.e. \(\mathrm{pH}=1\)
- \(M_4\left(\mathrm{H}^{+}\right)=\frac{100 \times \frac{1}{10}-100 \times \frac{1}{10}}{200}=0\) i.e. \(\mathrm{pH}=7\)
Question 60. The percentage of pyridine (C5H5N) that forms pyridinium ion (C5H5N+H) in a 0.10 M aqueous pyridine solution (Kb for C5H5N = 1.7 x 10-9) is
- 0.0060%
- 0.013%
- 0.77%
- 1.6%
Answer: 2. 0.013%
⇒ \(\underset{0.10\mathrm{M}}{\mathrm{C}_5 \mathrm{H}_5 \mathrm{~N}}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{C}_5 \mathrm{H}_5 \stackrel{+}{\mathrm{N}} \mathrm{H}+\mathrm{OH}^{-}\)
α \(=\sqrt{\frac{K_b}{C}}=\sqrt{\frac{1.7 \times 10^{-9}}{0.10}}=1.30 \times 10^{-4}\)
∴ Percentage of pyridine that forms pyridinium ion = 1.30 x 10-4 x 100 = 0.013%
Question 61. What is the pH of the resulting solution when equal volumes of 0.1 M NaOH and 0.01 M HCl are mixed?
- 2.0
- 7.0
- 1.04
- 12.65
Answer: 4. 12.65
One mole of NaOH is completely neutralised by one mole of HCl
Hence, 0.01 mole of NaOH will be completely neutralised by 0.01 mole of HCl.
NaOH left unneutralised = 0.1 – 0.01 = 0.09 mol
As equal volumes of two solutions are mixed, \([\mathrm{OH}]^{-}=\frac{0.09}{2}=0.045 \mathrm{M}\)
⇒ pOH =2 -log(0.04s) = 1.35 ∴ pH = 14 – 1.35 = 12.65
Question 62. Which of the following salts will give the highest pH in water?
- KCI
- NaCl
- Na2CO3
- CuSO4
Answer: 3. Na2CO3
Na2CO3 which is a salt of NaOH (strong base) and H2CO3 (weak acid) will produce a basic solution with pH greater than 7.
Question 63. Accumulation of lactic acid (HC3H5O3), a monobasic acid in tissues leads to pain and a feeling of fatigue. In a 0.10 M aqueous solution, lactic acid is 3.7% dissociated. The value of dissociation constant, Ka, for this acid, will be
- \(1.4 \times 10^{-5}\)
- \(1.4 \times 10^{-4}\)
- \(3.7 \times 10^{-4}\)
- \(2.8 \times 10^{-4}\)
Answer: 2. \(1.4 \times 10^{-4}\)
Accumulation of lactic acid (HC3H5O3), a monobasic acid in tissues leads to pain and a feeling of fatigue. In a 0.10 M aqueous solution, lactic acid is 3.7% dissociated.
Degree of dissociation, \(\alpha=\frac{3.7}{100}=0.037\)
According to Ostwald’s formula, \(K_a=\alpha^2 C=(0.037)^2 \times 0.10=1.369 \times 10^{-4} \approx 1.4 \times 10^{-4}\)
Question 64. At 100°C the Kw of water is 55 times its value at 25°C. What will be the pH of the neutral solution? (log 55 = 1.74)
- 7.00
- 7.87
- 5.13
- 6.13
Answer: 4. 6.13
We know that, at \(25^{\circ} \mathrm{C}, K_w=1 \times 10^{-14}\)
At \(100^{\circ} \mathrm{C}, K_w=55 \times 10^{-14}\)
(because \(K_w=\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]\))
⇒ \(K_w=\left[\mathrm{H}^{+}\right]^2\)
⇒ \(\mathrm{H}^{+}=\sqrt{K_w}\)
⇒ \(\mathrm{H}^{+}=\sqrt{55 \times 10^{-14}} \Rightarrow \mathrm{pH}=-\log \left[\mathrm{H}^{+}\right]\)
pH = \(-\log \left[\sqrt{55 \times 10^{-14}}\right]\)
= \(\frac{1}{2}\left[-\log \left(55 \times 10^{-14}\right)\right]=\frac{1}{2}[-\log 55+14 \log 10]\)
= \(\frac{1}{2}[-1.74+14]=\frac{1}{2}[12.26]=6.13\)
Question 65. Equimolar solutions of the following substances were prepared separately. Which one of these will record the highest pH value?
- BaCl2
- AlCl3
- LiCl
- BeCl2
Answer: 1. BaCl2
BaCl2 is made up of Ba(OH)2 and HCl.
AlCl3 is made up of Al(OH)3 and HCL
LiCl is made up of LiOH and HCl.
BeCI2 is made up of Be(OH)2 and HCl.
Ba(OH)2 is the strongest base among the given options and thus has the maximum pH
Question 66. What is [H+] in mol/L of a solution that is 0.20 M in CH3COONa and 0.10 M in CH3COOH? (Ka for CH3COOH = 1.8 X 10-5)
- \(3.5 \times 10^{-4}\)
- \(1.1 \times 10^{-5}\)
- \(1.8 \times 10^{-5}\)
- \(9.0 \times 10^{-6}\)
Answer: 4. \(9.0 \times 10^{-6}\)
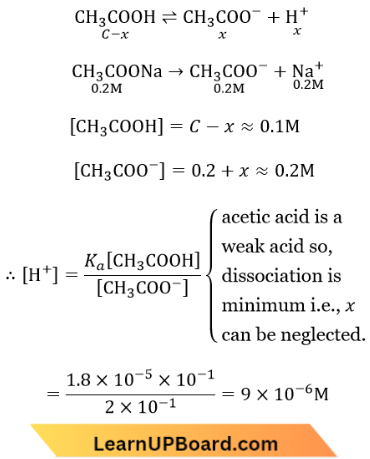
Question 67. The ionization constant of ammonium hydroxide is 1.77 x 10-5 at 298 K. Hydrolysis constant of ammonium chloride is
- \(6.50 \times 10^{-12}\)
- \(5.65 \times 10^{-13}\)
- \(5.65 \times 10^{-12}\)
- \(5.65 \times 10^{-10}\)
Answer: 4. \(5.65 \times 10^{-10}\)
NH4CI is a salt of strong acid and weak base, so the hydrolysis constant is \(K_h=\frac{K_w}{K_b}\)
Given, \(K_b\left(\mathrm{NH}_4 \mathrm{OH}\right)=1.77 \times 10^{-5}\)
∴ \(K_w=10^{-14}\)
∴ \(K_h=\frac{10^{-14}}{1.77 \times 10^{-5}}=0.565 \times 10^{-9} \text { or } K_h=5.65 \times 10^{-10}\)
Question 68. What is the [OH–] in the final solution prepared by mixing 20.0 mL of 0.050 M HCl with 30.0 mL of 0.10 M Ba(OH)2?
- 0.40 M
- 0.0050 M
- 0.12 M
- 0.10 M
Answer: 4. 0.10 M
Millimoles of H+ produced = 20 x 0.05 = 1
Millimoles of OH– produced = 30 x 0.1 x 2 = 6
(Each Ba(OH)2 gives 2OH–.)
∴ Millimoles of OH– remaining in solution = 6 – 1 = 5
Total volume of solution = 20 + 30 = 50 mL
∴ \(\left[\mathrm{OH}^{-}\right]=\frac{5}{50}=0.1 \mathrm{M}\)
Question 69. Equal volumes of three acid solutions of pH 3, 4 and 5 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
- \(3.7 \times 10^{-3} \mathrm{M}\)
- \(1.11 \times 10^{-3} \mathrm{M}\)
- \(1.11 \times 10^{-4} \mathrm{M}\)
- \(3.7 \times 10^{-4} \mathrm{M}\)
Answer: 4. \(3.7 \times 10^{-4} \mathrm{M}\)
pH = \(-\log \left[\mathrm{H}^{+}\right]\)
or \(\left[\mathrm{H}^{+}\right]=10^{-\mathrm{pH}} ;\left[\mathrm{H}^{+}\right]\) of solution 1 = \(10^{-3}\)
∴ \(\left[\mathrm{H}^{+}\right]\) of soln. \(2=10^{-4} ;\left[\mathrm{H}^{+}\right]\) of solution. \(3=10^{-5}\)
Total concentration of \(\left[\mathrm{H}^{+}\right]=10^{-3}\left(1+1 \times 10^{-1}+1 \times 10^{-2}\right)\)
⇒ \(10^{-3}\left(\frac{1}{1}+\frac{1}{10}+\frac{1}{100}\right) \Rightarrow 10^{-3}\left(\frac{100+10+1}{100}\right)\)
⇒ \(10^{-3}\left(\frac{111}{100}\right)=1.11 \times 10^{-3}\)
So, \(\mathrm{H}^{+}\) ion concentration in mixture of equal volumes of these acid solution = \(\frac{1.11 \times 10^{-3}}{3}=3.7 \times 10^{-4} \mathrm{M}\)
Question 70. A weak acid, HA, has a Ka of 1.00 x 10-5. If 0.100 mol of this acid is dissolved in one litre of water, the percentage of acid dissociated at equilibrium is closest to
- 1.00%
- 99.9%
- 0.100%
- 99.0%
Answer: 1. 1.00%
A weak acid, HA, has a Ka of 1.00 x 10-5. If 0.100 mol of this acid is dissolved in one litre of water,
For a weak acid, the degree of dissociation,
α \(=\sqrt{\frac{K_a}{C}}=\sqrt{\frac{1 \times 10^{-5}}{0.1}}=10^{-2}\) i.e. 1.00%
Question 71. Calculate the pOH of a solution at 25°C that contains 1 x 10-10 M of hydronium ions, i.e. \(\mathrm{H}_3 \mathrm{O}^{+}\).
- 4.000
- 9.000
- 1.000
- 7.000
Answer: 1. 4.000
Given, [H3O+] = 1 x 10-10 or, pH = 10
Now at 25°C, pH + POH = PKw = 14
or, pOH = 14-PH = 14-10=4
Question 72. The hydrogen ion concentration of a 10-8 M HCl aqueous solution at 298 K (Kw = 10-14) is
- \(1.0 \times 10^{-8} \mathrm{M}\)
- \(1.0 \times 10^{-6} \mathrm{M}\)
- \(1.0525 \times 10^{-7} \mathrm{M}\)
- \(9.525 \times 10^{-8} \mathrm{M}\)
Answer: 3. \(1.0525 \times 10^{-7} \mathrm{M}\)
10-8 M HCl = 10-8 MH+
Also from water, [H+] = 10-7
Total [H+] = 10-7 + 0.10 x 10-7 = 1.1 x 10-7 M
Question 73. At 25°C, the dissociation constant of a base, BOH, is 1.0 x 10-12. The concentration of hydroxyl ions in 0.01 M aqueous solution of the base would be
- \(1.0 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}\)
- \(1.0 \times 10^{-6} \mathrm{~mol} \mathrm{~L}^{-1}\)
- \(2.0 \times 10^{-6} \mathrm{~mol} \mathrm{~L}^{-1}\)
- \(1.0 \times 10^{-7} \mathrm{~mol} \mathrm{~L}^{-1}\)
Answer: 4. \(1.0 \times 10^{-7} \mathrm{~mol} \mathrm{~L}^{-1}\)
C = \(0.01 \mathrm{M}\)
⇒ \(K_b=1 \times 10^{-12} \text { at } 25^{\circ} \mathrm{C}\)
⇒ \(\begin{array}{lcccc}
& \mathrm{BOH} & & B^{+}+\mathrm{OH}^{-} \\
\text {Initially } & \mathrm{C} & & 0 & 0 \\
\text { At eq. } & \mathrm{C}-\mathrm{C} \alpha & & \mathrm{C} \alpha & \mathrm{C} \alpha
\end{array}\)
⇒ \({\left[\mathrm{OH}^{-}\right]=\mathrm{C} \alpha}\)
⇒ \({\left[\mathrm{OH}^{-}\right]=\sqrt{K_b \mathrm{C}}=\sqrt{1 \times 10^{-12} \times 10^{-2}}}\)
⇒ \({\left[\mathrm{OH}^{-}\right]=10^{-7} \mathrm{~mol} \mathrm{~L}^{-1}}\)
Question 74. Which has the highest pH?
- CH3COOK
- Na2CO3
- NH4Cl
- NaNO3
Answer: 2. Na2CO3
NH4OH is a weak base but HCl is a strong acid in solution, so the pH of NH4Cl solution is comparable. NaNO3 is a salt of a strong base and strong acid, so the pH of the solution will be 7.
Hydrolysis of potassium acetate (a salt of a weak acid and a strong alkali) gives a weakly alkaline solution since the acetate ion acts as a weak base.
⇒ \(\mathrm{CH}_3 \mathrm{COOK}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{CH}_3 \mathrm{COOH}+\mathrm{K}^{+}+\mathrm{OH}^{-}\)
The pH of this solution = 8.8.
Hydrolysis of sodium carbonate (a salt of strong alkali and a weak acid) gives an alkaline solution.
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3+2 \mathrm{H}_2 \mathrm{O} \rightarrow 2\left(\mathrm{Na}^{+}+\mathrm{OH}^{-}\right)+\mathrm{H}_2 \mathrm{CO}_3\)
The pH of this solution is > 10
Question 75. Ionisation constant of CH3COOH is 1.7 x 10-5 and concentration of H+ ions is 3.4 x 10-4. Then find out the initial concentration of CH3COOH molecules,
- \(3.4 \times 10^{-4}\)
- \(3.4 \times 10^{-3}\)
- \(6.8 \times 10^{-4}\)
- \(6.8 \times 10^{-3}\)
Answer: 4. \(6.8 \times 10^{-3}\)
⇒ \(\mathrm{CH}_3 \mathrm{COOH} \rightleftharpoons \mathrm{CH}_3 \mathrm{COO}^{-}+\mathrm{H}^{+}\)
⇒ \(K_a=\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]\left[\mathrm{H}^{+}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]}\)
⇒ \({\left[\mathrm{CH}_3 \mathrm{COOH}\right]=\frac{3.4 \times 10^{-4} \times 3.4 \times 10^{-4}}{1.7 \times 10^{-5}}=6.8 \times 10^{-3}}\)
Question 76. The correct relation between dissociation constants of a dibasic acid is
- \(K_{a_1}=K_{a_2}\)
- \(K_{a_1}>K_{a_2}\)
- \(K_{a_1}<K_{a_2}\)
- \(K_{a_1}=\frac{1}{K_{a_2}}\)
Answer: 2. \(K_{a_1}>K_{a_2}\)
- \(\mathrm{H}_2 A \stackrel{K_{a_1}}{\rightleftharpoons} \mathrm{HA}^{-}+\mathrm{H}^{+}\)
- \(\mathrm{HA}^{-} \stackrel{K_{a_2}}{\rightleftharpoons} A^{2-}+\mathrm{H}^{+}\)
In the 1st step, the H+ ion comes from the neutral molecule, while in the 2nd step, the H+ ion comes from negatively charged ions. The presence of the -ve charge makes the removal of the H+ ion difficult.
Thus, \(K_{a_1}>K_{a_2}\)
Question 77. Which statement is wrong about pH and H+?
- pH of neutral water is not zero.
- Adding 1 N solution of CH3COOH and 1 N solution of NaOH, the pH will be seven.
- [H+] concentrated and cold H2SO4.
- Mixing solution of CH3COOH and HCl, the pH will be less than 7.
Answer: 2. Adding 1 N solution of CH3COOH and 1 N solution of NaOH, the pH will be seven.
After mixing 1 N solution of CH3COOH (weak acid) and 1 N NaOH (strong base), the resulting solution will have free OH– ions. Thus, pH will be higher than 7.
Question 78. The concentration of [H+] and concentration of [OH–] of a 0.1 aqueous solution of 2% ionised weak acid is [ionic product of water = 1 x 10-14]
- \(2 \times 10^{-3} \mathrm{M}\) and \(5 \times 10^{-12} \mathrm{M}\)
- \(1 \times 10^{-3} \mathrm{M}\) and \(3 \times 10^{-11} \mathrm{M}\)
- \(0.02 \times 10^{-3} \mathrm{M}\) and \(5 \times 10^{-11} \mathrm{M}\)
- \(3 \times 10^{-2} \mathrm{M}\) and \(4 \times 10^{-13} \mathrm{M}\)
Answer: 1. \(2 \times 10^{-3} \mathrm{M}\) and \(5 \times 10^{-12} \mathrm{M}\)
[H+] = Cα = 0.1 x 0.02 = 2 x 10-3 M
(As degree of dissociation = 2% = 0.02)
Hence, \(\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{2 \times 10^{-3}}=5 \times 10^{-12} \mathrm{M}\)
Question 79. The hydride ion H– is a stronger base than its hydroxide ion OH–. Which of the following reactions will occur if sodium hydride (NaH) is dissolved in water?
- \(\mathrm{H}^{-}+\mathrm{H}_2 \mathrm{O} \rightarrow\) no reaction
- \(\mathrm{H}_{(a q)}^{-}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{H}_2 \mathrm{O}\)
- \(\mathrm{H}_{(a q)}^{-}+\mathrm{H}_2 \mathrm{O}_{(b)} \rightarrow \mathrm{OH}^{-}+\mathrm{H}_2\)
- None of these.
Answer: 3. \(\mathrm{H}_{(a q)}^{-}+\mathrm{H}_2 \mathrm{O}_{(b)} \rightarrow \mathrm{OH}^{-}+\mathrm{H}_2\)
⇒ \(\mathrm{NaH}+\mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{NaOH}+\mathrm{H}_2\)
or, \(\mathrm{H}_{(a q)}^{-}+\mathrm{H}_2 \mathrm{O}_{(l)} \rightarrow \mathrm{OH}^{-}+\mathrm{H}_2 \uparrow\)
Question 80. The ionic product of water at 25°C is 10-14. Its ionic product at 90°C will be,
- \(1 \times 10^{-14}\)
- \(1 \times 10^{-16}\)
- \(1 \times 10^{-20}\)
- \(1 \times 10^{-12}\)
Answer: 4. \(1 \times 10^{-12}\)
At high temperatures, the value of ionic products increases.
Question 81. If α is the dissociation constant, then the total number of moles for the reaction, 2HI → H2 + I2 will be
- 1
- 1 – α
- 2
- 2 – α
Answer: 3. 2

Total number of moles = 2(1 – α) + 2α = 2
Question 82. The pH value of N/10 NaOH solution is
- 12
- 13
- 10
- 11
Answer: 2. 13
Since NaOH is a strong base, therefore it completely ionises. Thus, the hydroxyl ion concentration is equal to that of the base itself. We know that the concentration of OH– is N/ 10. NaOH=0.1 =10-1
Therefore value of \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\frac{K_w}{\left[\mathrm{OH}^{-}\right]}=\frac{1 \times 10^{-14}}{10^{-1}}=1 \times 10^{-13}\)
pH = – log [H3O+] = – log [1 x 10-13] = 13
Question 83. The pH value of a 10 M solution of HCl is
- Equal to 1
- Equal to 2
- Less than 0
- Equal to 0
Answer: 3. Less than 0
Since HCl is a strong acid and it completely ionises, therefore H3O+ ions concentration is equal to that of the acid itself i.e., [H3O+] = [HCl] = 10 M.
Therefore, pH = – log [H3O+] = – log [10] = – 1
Question 84. At 80°C, distilled water has [H3O+] concentration equal to 1 x 10-6 mole/litre. The value of Kw at this temperature will be
- 1 x 10-12
- 1 x 10-15
- 1 x 10-6
- 1 x 10-9
Answer: 1. 1 x 10-12
⇒ \(\left[\mathrm{H}_3 \mathrm{O}^{+}\right]=\left[\mathrm{OH}^{-}\right]=1 \times 10^{-6}\) mole/litre
⇒\(K_w=\left[\mathrm{H}_3 \mathrm{O}^{+}\right]\left[\mathrm{OH}^{-}\right]=\left[1 \times 10^{-6}\right] \times\left[1 \times 10^{-6}\right]=1 \times 10^{-12}\)
Question 85. 0.1 M solution of which one of these substances will act basic?
- Sodium borate
- Ammonium chloride
- Calcium nitrate
- Sodium sulphate
Answer: 1. Sodium borate
Sodium borate is a salt formed from a strong base (NaOH) and weak acid (H3BO3). Hence, sodium borate will act as a basic solution.
Question 86. The compound whose water solution has the highest pH is
- NaCl
- NaHCO3
- Na2CO3
- NH4Cl
Answer: 3. Na2CO3
NH4CI and NaHCO3 are acidic in nature and NaCl is neutral. Only Na2CO3 is basic and thus, has the highest pH.
Question 87. The pH of the solution containing 50 mL each of 0.10 M sodium acetate and 0.01 M acetic acid is [Given: pKa of CH3COOH = 4.57]
- 5.57
- 3.57
- 4.57
- 2.57
Answer: 1. 5.57
It is an acidic buffer.
For acidic buffer, pH = \(\mathrm{p} K_a+\log \frac{[\text { Salt }]}{[\text { Acid }]}\)
= \(4.57+\log \frac{0.1}{0.01}=4.57+\log 10=4.57+1=5.57\)
Question 88. Which will make a basic buffer?
- 100 mL of 0.1 M HCl + 100 mL of 0.1 M NaOH
- 50 mL of 0.1 M NaOH + 25 mL of 0.1 M CH3COOH
- 100 mL of 0.1 M CH3COOH + 100 mL of 0.1M NaOH
- 100 mL of 0.1 M HCl + 200 mL of 0.1 M NH4OH
Answer: 4. 100 mL of 0.1 M HCl + 200 mL of 0.1 M NH4OH
Acid-base titration: \(\underset{10 \mathrm{mmol}}{\mathrm{HCl}}+\underset{20 \mathrm{mmol}}{\mathrm{NH}_4 \mathrm{OH}} \longrightarrow \mathrm{NH}_4 \mathrm{Cl}\)
∴ HCI is the limiting reagent.
Solution contains NH4OH (weak base) and NH4CI (sait of strong acid and weak base). Therefore, a basic buffer will be formed.
Question 89. Which one of the following pairs of solutions is not an acidic buffer?
- CH3COOH and CH3COONa
- H2CO3 and Na2CO3
- H3PO4 and Na3PO4
- HClO4 and NaClO4
Answer: 4. HClO4 and NaClO4
An acidic buffer is a mixture of a weak acid and its salt with a strong base. HCIO4 is a strong acid.
Question 90. The dissociation constant of a weak acid is 1 x 10-4. In order to prepare a buffer solution with a pH = 5, the [Salt]/[Acid] ratio should be
- 4:5
- 10:1
- 5: 4
- 1: 10
Answer: 2. 10:1
pH = \(\mathrm{p} K_a+\log \frac{[\text { Salt }]}{[\text { Acid }]}\)
5 = \(-\log K_a+\log \frac{[\text { Salt }]}{[\text { Acid }]}\) (because \(p K_a=-\log K_a\))
5 = \(-\log \left[1 \times 10^{-4}\right]+\log \frac{[\text { Salt }]}{[\text { Acid }]}\)
5 = \(4+\log \frac{[\text { Salt }]}{[\text { Acid }]}, 5-4=\log \frac{[\text { Salt }]}{[\text { Acid }]}\)
1 = \(\log \frac{[\text { Salt }]}{[\text { Acid }]}, \frac{[\text { Salt }]}{[\text { Acid }]}=10\) i.e. 10: 1
Question 91. Buffer solutions have constant acidity and alkalinity because
- These give unionised acid or base on reaction with added acid or alkali
- Acids and alkalies in these solutions are shielded from attack by other ions
- They have a large excess of H+ or OH– ions
- They have a fixed value of pH.
Answer: 1. These give unionised acid or base on reaction with added acid or alkali
Question 92. A buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of NH+4 is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 x 10-5, what is the pH of this solution? (log 2.7 = 0.43)
- 9.08
- 9.43
- 11.72
- 8.73
Answer: 2. 9.43
A buffer solution is prepared in which the concentration of NH3 is 0.30 M and the concentration of NH+4 is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 x 10-5
⇒ \(\left[\mathrm{NH}_3\right]=0.30 \mathrm{M}_3 K_b=1.8 \times 10^{-5}\)
⇒ \({\left[\mathrm{NH}_4^{+}\right]=0.20 \mathrm{M}}\)
⇒ \(\mathrm{pK}_b=-\log \left(1.8 \times 10^{-5}\right)=4.74\)
pOH = \(\mathrm{p} K_b+\log \frac{[\text { salt }]}{[\text { base }]}=4.74+\log \frac{0.2}{0.3}=4.56\)
pH = (14-4.56)=9.44
Question 93. In a buffer solution containing equal concentrations of B– and HB, the Kb for B– is 10-10. The pH of the buffer solution is
- 10
- 7
- 6
- 4
Answer: 4. 4
We know, pOH = \(p K_b+\log \frac{\left[B^{-}\right]}{[\mathrm{HB}]}\)
Since, [B–] = [HB] (given)
∴ pOH = pKb ⇒pOH = 10
∴ pH = 44- 10=4
Question 94. Which of the following pairs constitutes a buffer?
- HCl and KCl
- HNO2 and NaNO2
- NaOH and NaCl
- HNO3 and NH2NO3
Answer: 2. HNO2 and NaNO2
HNO2 (weak acid) and NaNO2 (salt of conjugate base) is an example of acidic buffer
Question 95. The rapid change of pH near the stoichiometric point of an acid-base titration is the basis of indicator detection. pH of the solution is related to the ratio of the concentrations of the conjugate acid (H In) and base (In–) forms of the indicator by the expression
- \(\log \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]}=\mathrm{p} K_{\text {In }}-\mathrm{pH}\)
- \(\log \frac{[\mathrm{HIn}]}{\left[\mathrm{In}^{-}\right]}=\mathrm{p} K_{\text {In }}-\mathrm{pH}\)
- \(\log \frac{[\mathrm{HIn}]}{\left[\mathrm{In}^{-}\right]}=\mathrm{pH}-\mathrm{p} K_{\text {In }}\)
- \(\log \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]}=\mathrm{pH}-\mathrm{p} K_{\text {In }}\)
Answer: 4. \(\log \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]}=\mathrm{pH}-\mathrm{p} K_{\text {In }}\)
Let us consider the formation of a salt of a weak acid and a strong base.
In\(^{-}+\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{HIn}+\mathrm{OH}^{-}\)
⇒ \(K_h=\frac{[\mathrm{HIn}]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{In}^{-}\right]}\)….(1)
Other equations present in the solution are
⇒ \(\mathrm{HIn} \rightleftharpoons \mathrm{H}^{+}+\mathrm{In}^{-}\)
⇒ \(\mathrm{H}_2 \mathrm{O} \rightleftharpoons \mathrm{H}^{+}+\mathrm{OH}^{-}\)
⇒ \(K_{\mathrm{In}}=\frac{\left[\mathrm{H}^{+}\right]\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]}\)….(2)
⇒ \(K_{\mathrm{w}}=\left[\mathrm{H}^{+}\right]\left[\mathrm{OH}^{-}\right]\)….(3)
From (2) and (3), \(\frac{K_w}{K_{\text {In }}}=\frac{[\mathrm{HIn}]\left[\mathrm{OH}^{-}\right]}{\left[\mathrm{In}^{-}\right]}=K_h\)……(4)
⇒ \({\left[\mathrm{OH}^{-}\right]=\frac{K_w}{K_{\text {In }}} \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]}}\)
⇒ \(\log \left[\mathrm{OH}^{-}\right]=\log K_w-\log K_{\text {In }}+\log \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]}\)
⇒ \(-\mathrm{pOH}=-\mathrm{p} K_w+\mathrm{p} K_{\text {In }}+\log \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]} \)
⇒ \(\mathrm{p} K_w-\mathrm{pOH}=\mathrm{p} K_{\text {In }}+\log \frac{\left[\mathrm{In}^{-}\right]}{[\mathrm{HIn}]} \text { or, } \mathrm{pH}=\mathrm{p} K_{\text {In }}+\log \frac{\left[\mathrm{In}^{-}\right]}{\left[\mathrm{HIn}^{-}\right]}\)
i.e. \(\log \frac{[\mathrm{In}]}{[\mathrm{HIn}]}=\mathrm{pH}-\mathrm{p} K_{\text {In }}\)
Question 96. The solution of 0.1 N NH4OH and 0.1 N NH4Cl has a pH of 9.25. Then find out pKb of NH4OH.
- 9.25
- 4.75
- 3.75
- 8.25
Answer: 2. 4.75
A solution of 0.1 N NH4OH and 0.1 N NH4CI is a buffer solution.
According to Henderson’s equation, the pH of a basic buffer
pH = \(14-\mathrm{p} K_b-\log \frac{[\text { Salt }]}{[\text { Base }]} \Rightarrow \mathrm{p} K_b=14-\mathrm{pH}-\log \frac{[\text { Salt }]}{[\text { Base }]}\)
⇒ \(\mathrm{p} K_b=14-9.25-\log \frac{0.1}{0.1}\)
⇒ \(\mathrm{p} K_b=14-9.25=4.75\)
∴ \(\mathrm{pK}_b \text { of } \mathrm{NH}_4 \mathrm{OH}=4.75\)
Question 97. A physician wishes to prepare a buffer solution at pH = 3.85 that efficiently resists changes in pH yet contains only a small concentration of the buffering agents. Which of the following weak acids together with its sodium salt would be best to use?
- 2, 5-Dihydroxybenzoic acid (pKa= 2.97)
- Acetoacetic acid (pKa = 3.58)
- m-Chlorobenzoic acid (pKa = 3.98)
- p-Chlorocinnamic acid (pKa = 4.41)
Answer: 2. Acetoacetic acid (pKa = 3.58)
A physician wishes to prepare a buffer solution at pH = 3.85 that efficiently resists changes in pH yet contains only a small concentration of the buffering agents.
pH of an acidic buffer solution is given by Henderson equation: pH = \(\mathrm{pH}=\mathrm{pK}_a+\log \frac{[\text { Salt }]}{[\text { Acid }]}\)
Its buffer capacity = pKa±1
Since a buffer solution is more effective in the pH range pKa±1 therefore, the weak acid having pKa = 3.58 together with its sodium salt is chosen. Acetoacetic acid is, therefore, a suitable weak acid.
Question 98. The pH value of blood does not appreciably change by a small addition of an acid or a base, because the blood
- Can be easily coagulated
- Contains iron as a part of the molecule
- Is a body fluid
- Contains serum protein which acts as a buffer.
Answer: 4. Contains serum protein which acts as a buffer.
The pH value of the blood is maintained constant by the buffer solution present in the blood itself. Buffer solutions resist the change in pH values.
Question 99. pH of a saturated solution of Ca(OH)2 is 9. The solubility product (Ksp) of Ca(OH)2 is
- 0.5 x 10-10
- 0.5 x 10-15
- 0.25 x 10-10
- 0.125 x 10-15
Answer: 2. 0.5 x 10-15
pH of the saturated solution of Ca(OH)2 = 9
∴ pOH of the saturated solution of Ca(OH)2 = 14 – 9 = 5
⇒ [OH–] = 10-5 (pH + pOH = 14)
⇒ \(\mathrm{Ca}(\mathrm{OH})_2 \rightleftharpoons \underset{s}{\mathrm{Ca}^{2+}}+\underset{2s}{2 \mathrm{OH}^{-}}\)
⇒ \(K_{\text {sp }}=\left[\mathrm{Ca}^{2+}\right]\left[\mathrm{OH}^{-}\right]^2=\left[1 / 2 \times 10^{-5}\right]\left[10^{-5}\right]^2\)
= \(0.5 \times 10^{-15}\)
Question 100. The molar solubility of CaF2 (Ksp = 5.3 x 10-11) in 0.1 M solution of NaF will be
- 5.3 x 10-11 mol L-1
- 5.3 x 10-8 mol L-1
- 5.3 x 10-9 mol L-1
- 5.3 x 10-10 mol L-1
Answer: 3. 5.3 x 10-9 mol L-1
⇒ \(\mathrm{CaF}_2 \longrightarrow \underset{s}{\mathrm{Ca}^{2+}}+\underset{2 s}{2 \mathrm{~F}^{-}}\)
⇒ \(\mathrm{NaF} \longrightarrow \underset{0.1 M}{\mathrm{Na}^{+}}+\underset{0.1 M}{\mathrm{~F}^{-}}\)
⇒ \({\left[\mathrm{Ca}^{2+}\right]=s,\left[\mathrm{~F}^{-}\right]=(2 s+0.1)=0.1 \mathrm{M}}\)
⇒ \(K_{s p}=\left[\mathrm{Ca}^{2+}\right]\left[\mathrm{F}^{-}\right]^2\)
⇒ \(5.3 \times 10^{-11}=(s)(0.1)^2\)
s = \(\frac{5.3 \times 10^{-11}}{(0.1)^2}=5.3 \times 10^{-9} \mathrm{~mol} \mathrm{~L}^{-1}\)
∴ Molar solubility is \(5.3 \times 10^{-9} \mathrm{~mol} \mathrm{~L}^{-1}\)
Question 101. The solubility of BaSO4 in water is 2.42 x 10-3 g L-1 at 298 K. The value of its solubility product (Ksp) will be (Given molar mass of BaSO4 = 233 g mol-1)
- 1.08 x 10-10 mol² L-2
- 1.08 x 10-12 mol² L-2
- 1.08 x 10-14 mol² L-2
- 1.08 x 10-8 mol² L-2
Answer: 1. 1.08 x 10-10 mol² L-2
Solubility of \(\mathrm{BaSO}_4\),
s = \(\frac{2.42 \times 10^{-3}}{233} \mathrm{~mol} \mathrm{~L}^{-1}=1.04 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}\)
⇒ \(\mathrm{BaSO}_4\) ionizes completely in the solution as
⇒ \(\mathrm{BaSO}_{4(s)} \rightleftharpoons \mathrm{Ba}_{(a q)}^{2+}+\mathrm{SO}_{4(a q)}^{2-}\)
⇒ \(K_{s p}=\left[\mathrm{Ba}^{2+}\right]\left[\mathrm{SO}_4^{2-}\right]=s^2\)
= \(\left(1.04 \times 10^{-5}\right)^2=1.08 \times 10^{-10} \mathrm{~mol}^2 \mathrm{~L}^{-2}\)
Question 102. The concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.2 x 10-4 mol L-1. The Solubility product of Ag2C2O4 is
- 2.66 x 10-12
- 4.5 x 10-11
- 5.3 x 10-12
- 2.42 x 10-8
Answer: 3. 5.3 x 10-12
Let solubility of Ag2C2O4 be s mol L-1
⇒ \(\underset{s}{\mathrm{Ag}_2 \mathrm{C}_2 \mathrm{O}_{4(s)}} \rightleftharpoons \underset{2 s}{2 \mathrm{Ag}_{(a q)}^{+}}+\underset{s}{\mathrm{C}_2 \mathrm{O}_{4(a q)}^{2-}}\)
Ksp = [Ag+]2 [C2O2-4]
Ksp = (2s)²(s)=4s³
Ksp = 4x (1.1x 10-4)3 ([Ag+] =2s=2.2x 10-4)
Ksp =5.3×10-12
Question 103. The solubility of AgCl(s) with solubility product 1.6 x 10-10 in 0.1 M NaCl solution would be
- 1.26 x 10-5 M
- 1.6x 10-9 M
- 1.6x 10-11 M
- Zero.
Answer: 2. 1.6x 10-9 M
Let solubility of AgCl in moles per litre.
⇒ \(\underset{s}{\mathrm{AgCl}_{(a q)}} \rightleftharpoons \underset{s}{\mathrm{Ag}_{(a q)}^{+}}+\underset{(s+0.1)}{\mathrm{Cl}_{(a q)}^{-}}\)
(0.1 M NaCl solution also provides 0.1 M Cl– ion).
Ksp = [Ag+] [CI–]; 1.6 x 10-10 = s(s + 0.1)
1.6 x 10-10 = s(0.1) (‘s<<<<0.1)
1.6 x 10-10 = s(0.1)
s = \(\frac{1.6 \times 10^{-10}}{0.1}=1.6 \times 10^{-9} \mathrm{M}\)
Question 104. MY and NY3 two nearly insoluble salts, have the same Ksp values of 6.2 x 10-13 at room temperature. Which statement would be true in regard to MY and NY3?
- The salts MY and NY3 are more soluble in 0.5 M KY than in pure water.
- The addition of the salt of KY to the solution of MY and NY3 will have no effect on their solubilities.
- The molar solubilities of MY and NY3 in water are identical.
- The molar solubility of MY in water is less than that of NY3
Answer: 4. The molar solubility of MY in water is less than that of NY3
For \(M Y: K_{s p}=s_1^2\)
⇒ \(s_1=\sqrt{K_{s p}}=\sqrt{6.2 \times 10^{-13}}=7.87 \times 10^{-7} \mathrm{~mol} \mathrm{~L}^{-1}\)
For \(N Y_3: K_{s p}=\left(s_2\right)\left(3 s_2\right)^3=27 s_2^4\)
⇒ \(s_2=\sqrt[4]{\frac{6.2 \times 10^{-13}}{27}}=3.89 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1}\)
Hence, the molar solubility of MY in water is less than that of NY3.
Question 105. The Ksp of Ag2CrO4, AgCl, AgBr and Agl are respectively, 1.1 x 10-12, 1.8 x 10-10, 5.0 x 10-13, 8.3 x 10-17. Which one of the following salts will precipitate last if AgNO3 solution is added to the solution containing equal moles of NaCl, NaBr, Nal and Na2CrO4?
- AgBr
- Ag2CrO4
- Agl
- AgCl
Answer: 2. Ag2CrO4
The Ksp of Ag2CrO4, AgCl, AgBr and Agl are respectively, 1.1 x 10-12, 1.8 x 10-10, 5.0 x 10-13, 8.3 x 10-17.
⇒ \(\begin{array}{lcc}
Salt & {1}{c}{K_{s p}} & Solubility \\
\mathrm{Ag}_2 \mathrm{CrO}_4 & 1.1 \times 10^{-12}=4 s^3 & s=\sqrt[3]{\frac{K_{s p}}{4}}=0.65 \times 10^{-4} \\
\mathrm{AgCl} & 1.8 \times 10^{-10}=s^2 & s=\sqrt{K_{s p}}=1.34 \times 10^{-5} \\
\mathrm{AgBr} & 5 \times 10^{-13}=s^2 & s=\sqrt{K_{s p}}=0.71 \times 10^{-6} \\
\mathrm{AgI} & 8.3 \times 10^{-17}=s^2 & s=\sqrt{K_{s p}}=0.9 \times 10^{-8}
\end{array}\)
The solubility of Ag2CrO4 is highest thus, it will be precipitated at last.
Question 106. Using the Gibbs’ energy change, ΔG° = +63.3 kJ, for the following reaction, \(\mathrm{Ag}_2 \mathrm{CO}_{3(s)} \rightleftharpoons 2 \mathrm{Ag}_{(a q)}^{+}+\mathrm{CO}_3^{2-}{ }_{(a q)}\) the Ksp of Ag2CO3(s) in water at 25 °C is
(R = 8.314 J K-1 mol-1)
- 3.2 x10-26
- 8.0 x 10-12
- 2.9 x10-3
- 7.9 x 10-2
Answer: 2. 8.0 x 10-12
ΔG° = -2.303 RT logKsp
63.3 x 10³ J = – 2.303 x 8.314 x 298 log Ksp
63.3 x 10³ J = -5705.84 logKsp
⇒ \(\log K_{s p}=-\frac{63.3 \times 10^3}{5705.84}=-11.09\)
⇒ \(K_{s p}={antilog}(-11.09)=8.128 \times 10^{-12}\)
Question 107. The values of K of CaCO3 and CaC2O4 are 4.7 x 10-9 and 1.3 x 10-9 respectively at 25°C. If the mixture of these two is washed with water, what is the concentration of Ca2+ ions in water?
- 5.831 x 10-5 M
- 6.856 x 10-5 M
- 3.606 x 10-5 M
- 7.746 x 10-5 M
Answer: 4. 7.746 x 10-5 M
⇒ \(\mathrm{CaCO}_3 \rightarrow \underset{x}{\mathrm{Ca}^{2+}}+\underset{x}{\mathrm{CO}_3^{2-}}\)
⇒ \(\mathrm{CaC}_2 \mathrm{O}_4 \rightarrow \underset{y}{\mathrm{Ca}^{2+}}+\underset{y}{\mathrm{C}_2 \mathrm{O}_4^{2-}}\)
Now, \(\left[\mathrm{Ca}^{2+}\right]=x+y\)
and \(x(x+y)=4.7 \times 10^{-9}\)…….(1)
y(x+y)=1.3\(\times 10^{-9}\)…..(2)
Dividing equations (1) and (2) we get \(\frac{x}{y}=3.6\)
∴ x = 3.6 y
Putting this value in equation (2), we get \(y(3.6 y+y)=1.3 \times 10^{-9}\)
On solving, we get \(y=1.68 \times 10^{-5}\)
and \(x=3.6 \times 1.68 \times 10^{-5}=6.05 \times 10^{-5}\)
∴ \(\left[\mathrm{Ca}^{2+}\right]=(x+y)=\left(1.68 \times 10^{-5}\right)+\left(6.05 \times 10^{-5}\right)\)
∴ \(\left[\mathrm{Ca}^{2+}\right]=7.73 \times 10^{-5} \mathrm{M}\)
Question 108. Identify the correct order of solubility in aqueous medium.
- Na2S > CuS > ZnS
- Na2S > ZnS > CuS
- CuS > ZnS > Na2S
- ZnS > Na2S > CuS
Answer: 2. Na2S > ZnS > CuS
Sodium sulphide is soluble in water. The solubility product (and hence solubility) of ZnS is larger than that of CuS
Question 109. pH of a saturated solution of Ba(OH)2 is 12. The value of the solubility product (K ) of Ba(OH)2 is
- 3.3 x 10-7
- 5.0 x 10-7
- 4.0 x 10-6
- 5.0 x 10-6
Answer: 2. 5.0 x 10-7
pH of solution =12
⇒ \({\left[\mathrm{H}^{+}\right]=10^{-12}}\)
⇒ \({\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{10^{-12}}=10^{-2}}\)
⇒ \(\mathrm{Ba}(\mathrm{OH})_2 \rightleftharpoons \mathrm{Ba}^{2+}+2 \mathrm{OH}^{-}\)
2s = \(10^{-2} \Rightarrow s=\frac{10^{-2}}{2}\)
⇒ \(K_{s p}=(s)(2 s)^2=4 s^3\)
= \(4 \times\left(\frac{10^{-2}}{2}\right)^3=\frac{4}{8} \times 10^{-6}=5 \times 10^{-7}\)
Question 110. In qualitative analysis, the metals of group 1 can be separated from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and Pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl– concentration is 0.10 M. What will the concentrations of Ag+ and Pb2+ be at equilibrium?
(\(K_{s p} \text { for } \mathrm{AgCl}=1.8 \times 10^{-10}, K_{s p} \text { for } \mathrm{PbCl}_2=1.7 \times 10^{-5}\))
- \(\left[\mathrm{Ag}^{+}\right]=1.8 \times 10^{-7} \mathrm{M},\left[\mathrm{Pb}^{2+}\right]=1.7 \times 10^{-6} \mathrm{M}\)
- \(\left[\mathrm{Ag}^{+}\right]=1.8 \times 10^{-11} \mathrm{M},\left[\mathrm{Pb}^{2+}\right]=8.5 \times 10^{-5} \mathrm{M}\)
- \(\left[\mathrm{Ag}^{+}\right]=1.8 \times 10^{-9} \mathrm{M},\left[\mathrm{Pb}^{2+}\right]=1.7 \times 10^{-3} \mathrm{M}\)
- \(\left[\mathrm{Ag}^{+}\right]=1.8 \times 10^{-11} \mathrm{M},\left[\mathrm{Pb}^{2+}\right]=1.7 \times 10^{-4} \mathrm{M}\)
Answer: 3. \(\left[\mathrm{Ag}^{+}\right]=1.8 \times 10^{-9} \mathrm{M},\left[\mathrm{Pb}^{2+}\right]=1.7 \times 10^{-3} \mathrm{M}\)
In qualitative analysis, the metals of group 1 can be separated from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and Pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl– concentration is 0.10 M.
⇒ \(K_{s p}[\mathrm{AgCl}]=\left[\mathrm{Ag}^{+}\right]\left[\mathrm{Cl}^{-}\right]\)
⇒ \({\left[\mathrm{Ag}^{+}\right]=\frac{1.8 \times 10^{-10}}{10^{-1}}=1.8 \times 10^{-9} \mathrm{M}}\)
⇒ \(K_{s p}\left[\mathrm{PbCl}_2\right]=\left[\mathrm{Pb}^{2+}\right]\left[\mathrm{Cl}^{-}\right]^2\)
⇒ \({\left[\mathrm{~Pb}^{2+}\right]=\frac{1.7 \times 10^{-5}}{10^{-1} \times 10^{-1}}=1.7 \times 10^{-3} \mathrm{M}}\)
Question 111. H2S gas when passed through a solution of cations containing HCl precipitates the cations of the second group of qualitative analysis but not those belonging to the fourth group. It is because
- The presence of HCl decreases the sulphide ion concentration
- The solubility product of group 2 sulphides is more than that of group 4 sulphides
- The presence of HCl increases the sulphide ion concentration
- Sulphides of group 4 cations are unstable in HCl.
Answer: 1. Presence of HCl decreases the sulphide ion concentration
H2S gas when passed through a solution of cations containing HCl precipitates the cations of the second group of qualitative analysis but not those belonging to the fourth group.
The cations of group 2 are precipitated as their sulphides.
The solubility product of sulphide of group 2 radicals is very low. Therefore, even with a low conc. of S2- ions, the ionic product exceeds the value of their solubility product and the radicals of group 2 get precipitated. The low. of S2- ions is obtained by passing H2S gas through the solution of the salt in the presence of oil. HCI suppresses the degree of ionisation of H2S by the common ion effect.
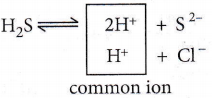
Note that the solubility product of group 4 radicals is quite high. It is necessary to suppress the conc. of S2- ions, otherwise radicals of group 4 will also get precipitated along with group 2 radicals.
Question 112. The solubility product of a sparingly soluble salt AX2 is 3.2 x 10-11. Its solubility (in moles/L) is
- 5.6 x 10-6
- 3.1 x 10-4
- 2 x 10-4
- 4 x 10-4
Answer: 3. 2 x 10-4
⇒ \(K_{s p}=3.2 \times 10^{-11}\)
⇒ \(A X_2 \rightleftharpoons \underset{s}{A^{2+}}+\underset{2 s}{2 X^{-}}\)
⇒ \(K_{s p}=s \times(2 s)^2=4 s^3 ; \text { i.e., } 3.2 \times 10^{-11}=4 s^3\)
or, \(s^3=0.8 \times 10^{-11}=8 \times 10^{-12}\)
s = \(2 \times 10^{-4}\)
Question 113. The solubility product of Agl at 25°C is 1.0 x 10-16 mol² L-2. The solubility of Agl in 10-4 N solution of KI at 25°C is approximately (in mol L-1)
- 1.0 x 10-16
- 1.0 x 10-12
- 10 x 10-10
- 1.0 x 10-8
Answer: 2. 1.0 x 10-12
⇒ \(AgI \rightleftharpoons \mathrm{Ag}^{+}+\mathrm{I}^{-}\)
⇒ \(\mathrm{KI} \rightleftharpoons \underset{10^{-4} \mathrm{M}}{\mathrm{K}^{+}}+\underset{10^{-4} \mathrm{M}}{\mathrm{I}^{-}}\)
(For \(KI, 1 \mathrm{~N}=1 \mathrm{M}\))
⇒ \({\left[\mathrm{I}^{-}\right]=s+10^{-4}}\)
⇒ \(K_{s p}=\left[\mathrm{Ag}^{+}\right]\left[\mathrm{I}^{-}\right]\)
⇒ \(1 \times 10^{-16}=s\left(s+10^{-4}\right) \Rightarrow 1 \times 10^{-16}=s^2+10^{-4} s\)
⇒ \(1 \times 10^{-16}=10^{-4} s\)
s = \(\frac{1 \times 10^{-16}}{10^{-4}}=1 \times 10^{-12} \mathrm{~mol} \mathrm{~L}^{-1}\) (because \(s^2<<<10^{-4} s\))
Question 114. The solubility of MX2 type electrolytes is 0.5 x 10-4 mol/lit., then find out Ksp of electrolytes.
- 5 x 10-12
- 25 x 10-10
- 1 x 10-13
- 5 x 10-13
Answer: 4. 5 x 10-13
Let s be the solubility of the electrolyte MX2.
⇒ \(\left[M^{2+}\right]=s,\left[X^{-}\right]=2 s\)
Solubility product, \(K_{s p}=s \times(2 s)^2=4 s^3;s=0.5 \times 10^{-4} \mathrm{~mol} /\) litre
∴ \(K_{s p}=4 \times\left(0.5 \times 10^{-4}\right)^3;K_{s p}=5 \times 10^{-13}\)
Question 115. The solubility of M2S salt is 3.5 x 10-6 then find out the solubility product.
- 1.7 x 10-6
- 1.7 x 10-16
- 1.7 x 10-18
- 1.7 x 10-12
Answer: 2. 1.7 x 10-16
For reaction, \(M_2 \mathrm{~S} \rightleftharpoons \underset{2 s}{2 M^{+}}+\underset{s}{\mathrm{~S}^{2-}}\)
Solubility \(=3.5 \times 10^{-6}\)
Solubility product, \(K_{s p}=\left[M^{+}\right]^2\left[\mathrm{~S}^{2-}\right]\)
= \((2 s)^2 s=4 s^3=4 \times\left(3.5 \times 10^{-6}\right)^3=1.7 \times 10^{-16}\)
Question 116. The solubility of a saturated solution of calcium fluoride is 2 x 10-4 moles per litre. Its solubility product is
- 22 x 10-11
- 14 x 10-4
- 2 x 10-2
- 32 x 10-12
Answer: 4. 32 x 10-12
For \(\mathrm{CaF}_2\), decomposition is as follows:
⇒ \(\mathrm{CaF}_2 \rightarrow \underset{s}{ } \mathrm{Ca}^{2+}+\underset{2 s}{2 \mathrm{~F}^{-}}\)
⇒ \(K_{s p}=\left[\mathrm{Ca}^{2+}\right]\left[\mathrm{F}^{-}\right]^2=s \times(2 s)^2\)
or \(K_{s p}=4 s^3 \Rightarrow K_{s p}=4 \times\left(2 \times 10^{-4}\right)^3 \Rightarrow K_{s p}=32 \times 10^{-12}\)
Question 117. The solubility products of CuS, Ag2S and HgS are 10-31, 10-44 and 10-54 respectively. The solubilities of these sulphides are in the order
- HgS > Ag2S > CuS
- CuS > Ag2S > HgS
- Ag2S > CuS > HgS
- Ag2S > HgS > CuS
Answer: 2. CuS > Ag2S > HgS
The greater the solubility product, the greater is the solubility.
Question 118. The solubility of AgCl will be minimal in
- 0.01 M CaCl2
- Pure water
- 0.001 M AgNO3
- 0.01M NaCl
Answer: 1.0.01 M CaCl2
There are a greater number of Cl– ions in CaCl2 compared to others. Hence, the solubility of AgCl will be minimal in 0.01M CaCl2 due to the common ion effect.
Question 119. Which one of the following is most soluble?
- \(\mathrm{Bi}_2 \mathrm{~S}_3\left(K_{s p}=1 \times 10^{-70}\right)\)
- \(\mathrm{Ag}_2 \mathrm{~S}\left(K_{s p}=6 \times 10^{-51}\right)\)
- \({CuS}\left(K_{s p}=8 \times 10^{-37}\right)\)
- \({MnS}\left(K_{s p}=7 \times 10^{-16}\right)\)
Answer: 4. \({MnS}\left(K_{s p}=7 \times 10^{-16}\right)\)
The higher the value of the solubility product, the greater the solubility.
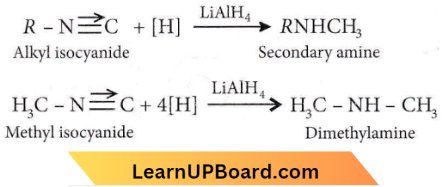
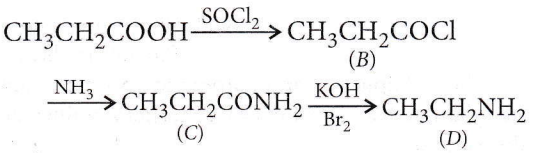

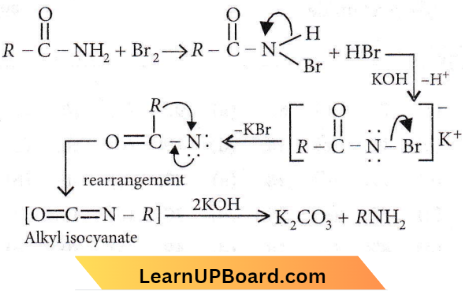

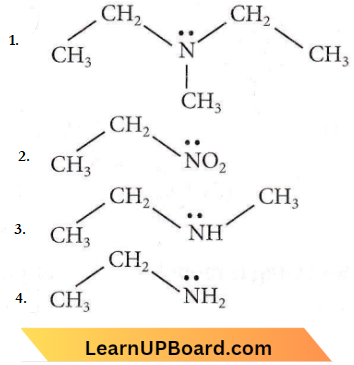
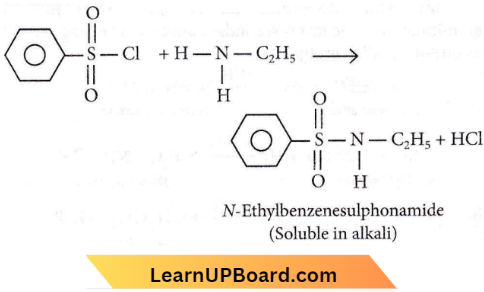
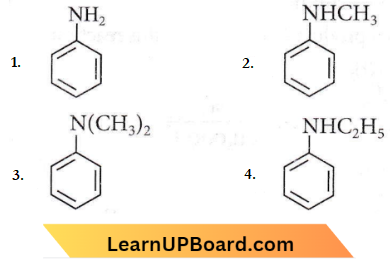
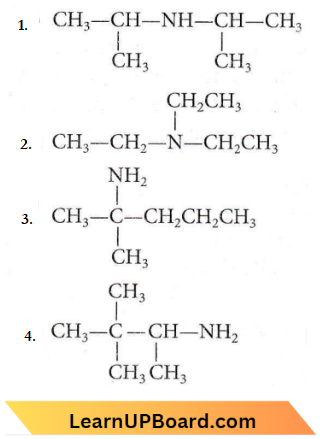
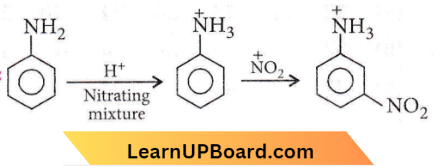
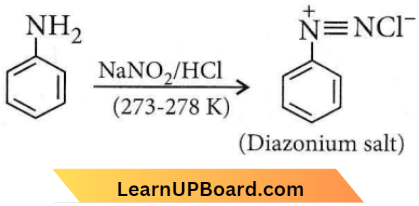
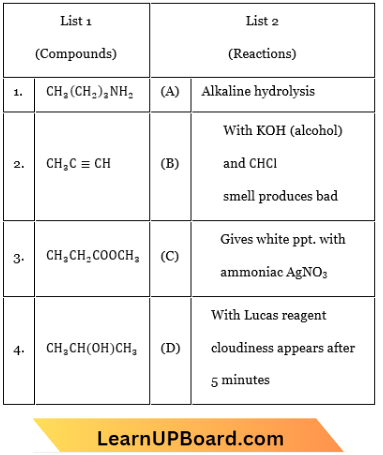
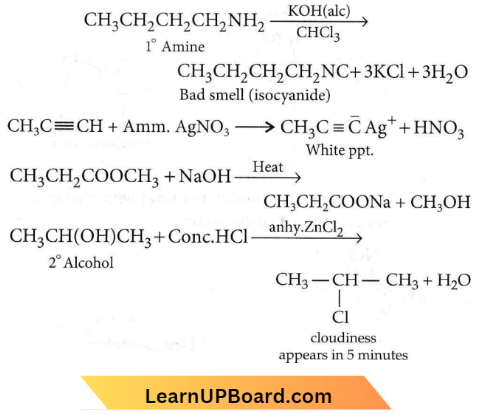
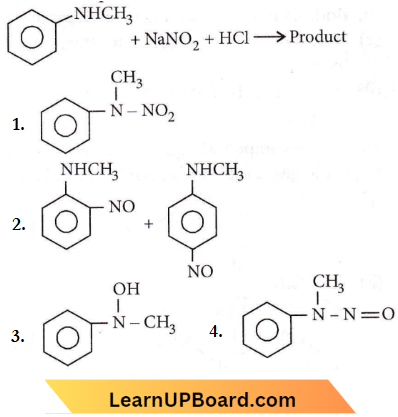
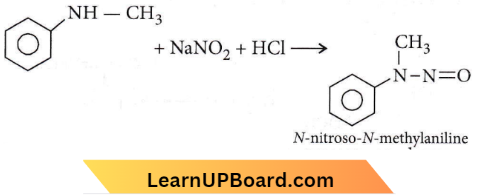
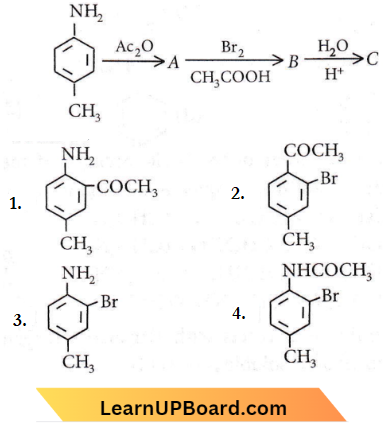
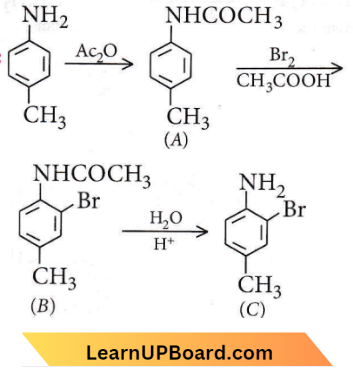
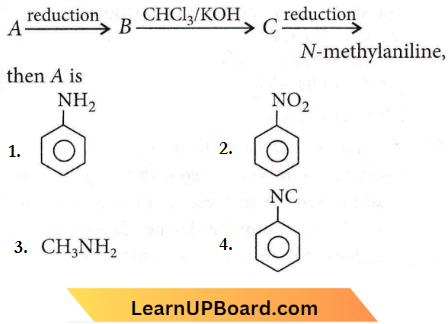
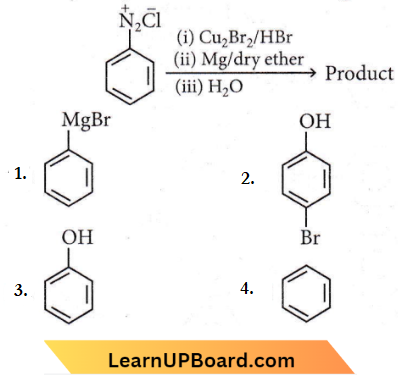
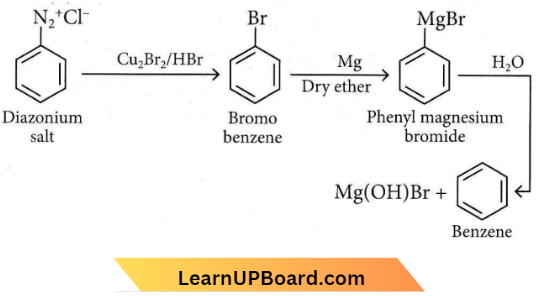
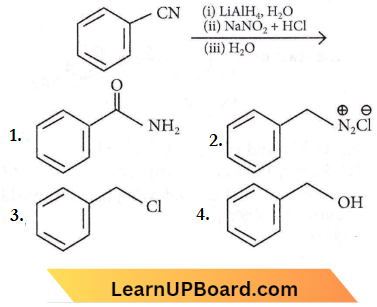
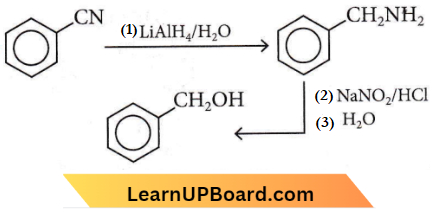
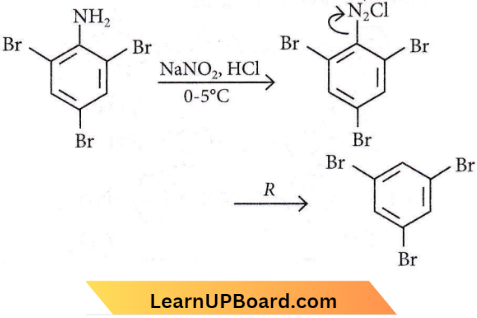
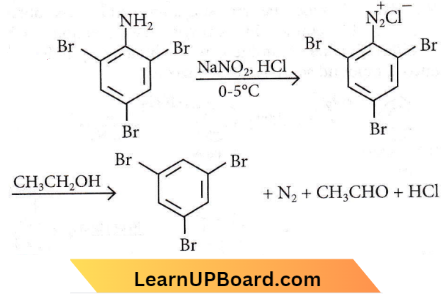
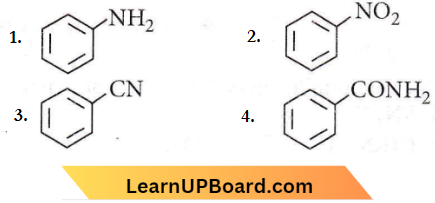
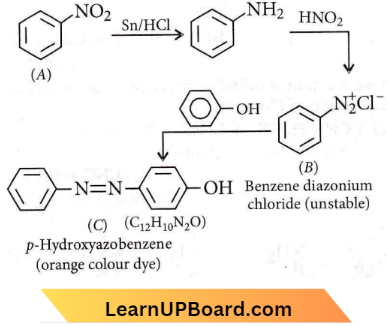
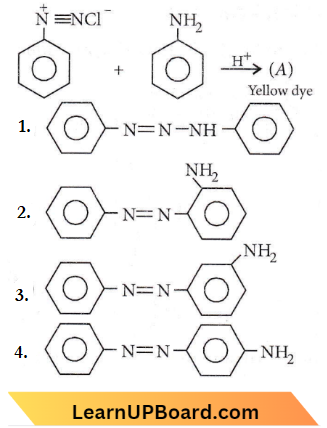
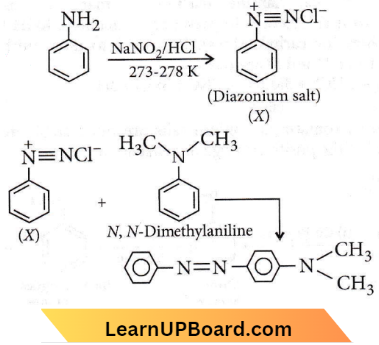
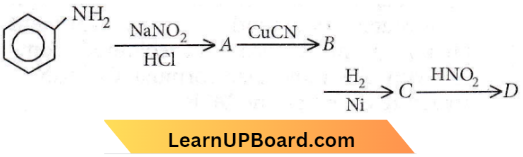
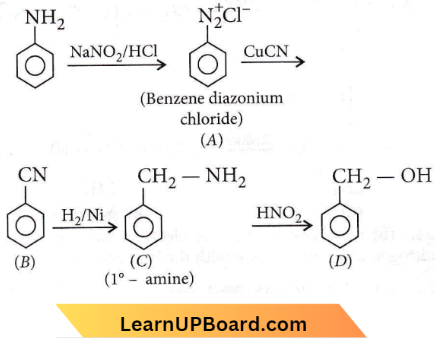
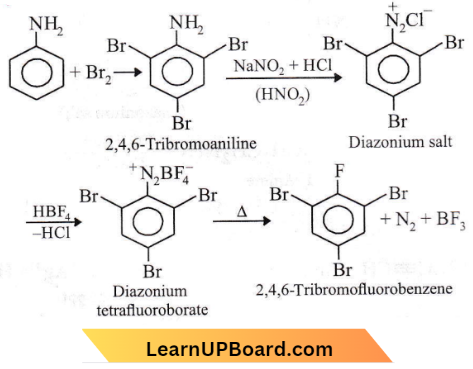
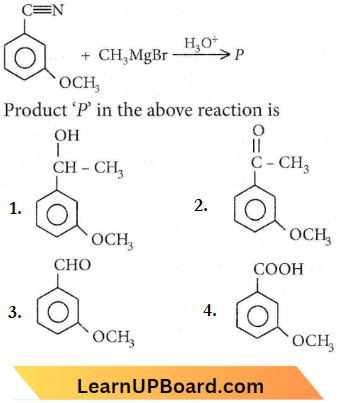
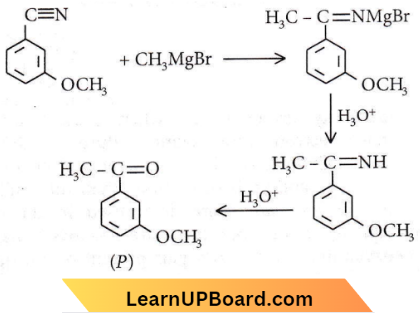
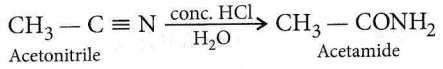

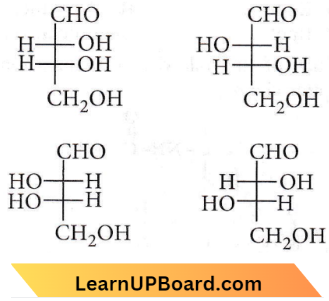
 (peptide bond)
(peptide bond) group.
group.
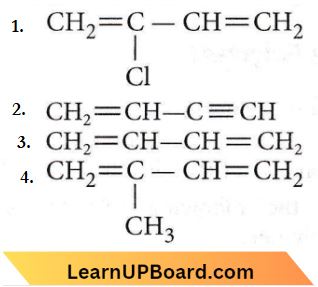
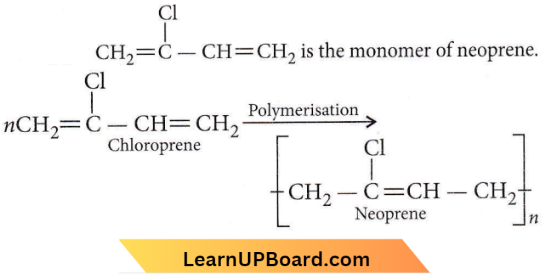
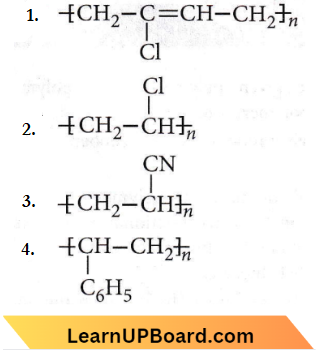
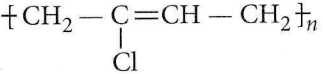
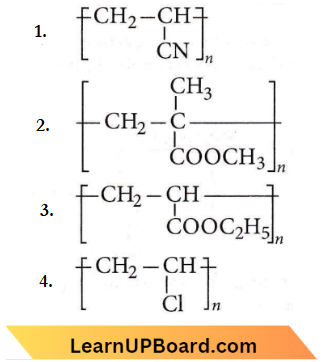
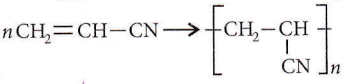
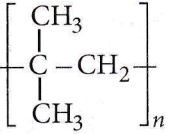
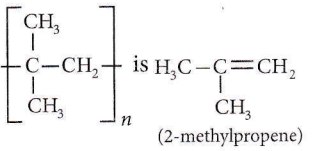
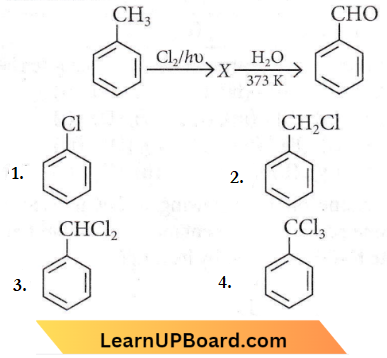
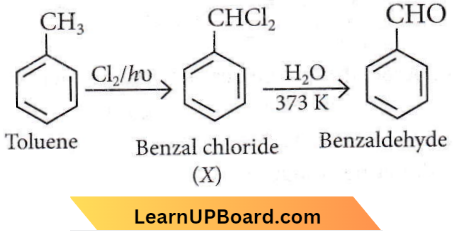
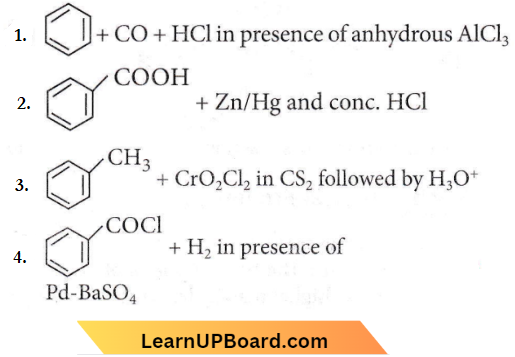
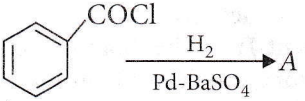
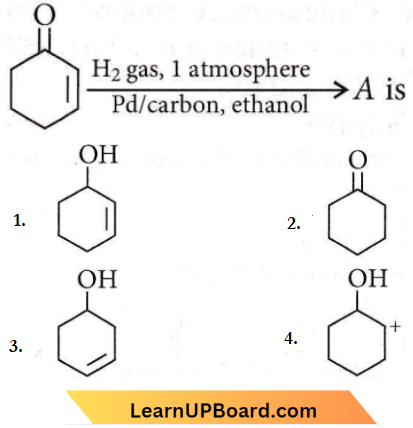
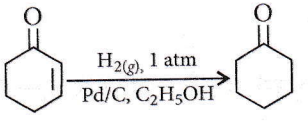
 the group will give a yellow precipitate of iodoform (CH3) when reacting with iodine and alkali.
the group will give a yellow precipitate of iodoform (CH3) when reacting with iodine and alkali.
 give positive iodoform hence, (1), (2) and (3) will give positive iodoform not (4).
give positive iodoform hence, (1), (2) and (3) will give positive iodoform not (4).
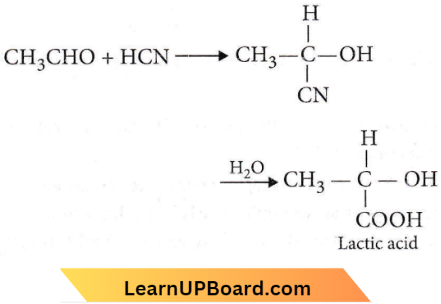
 are
are
 group show iodoform test.
group show iodoform test.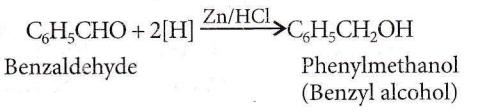
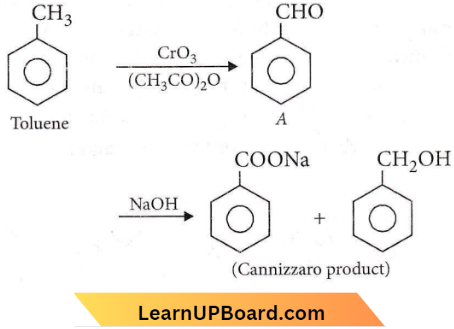
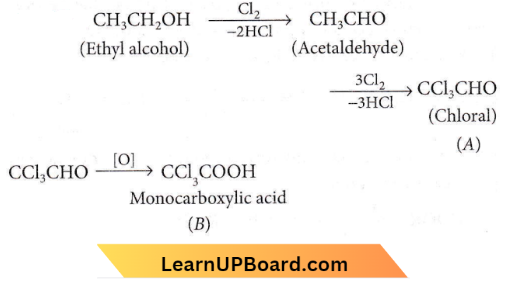
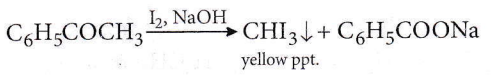
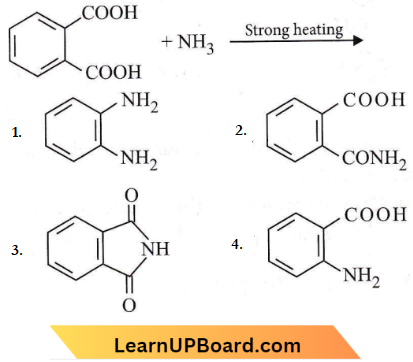
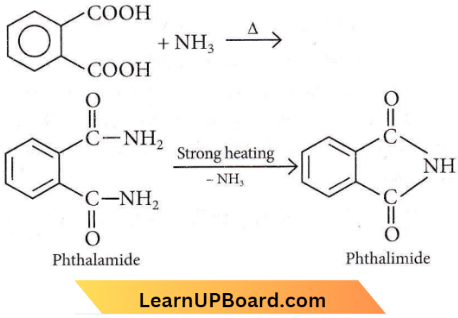
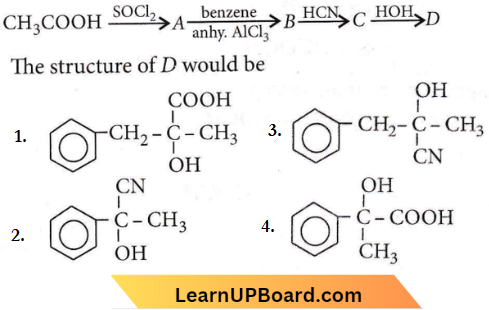
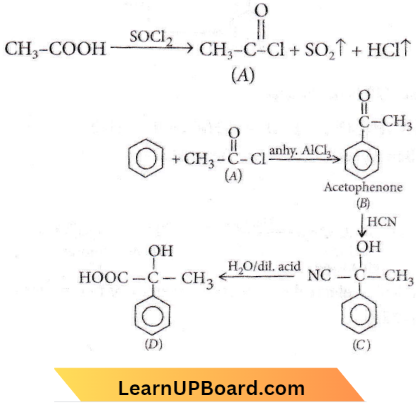
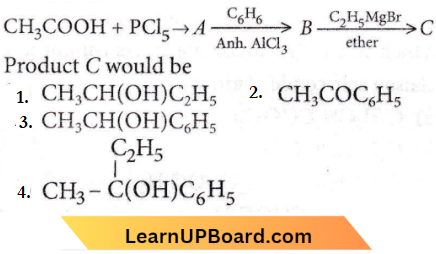
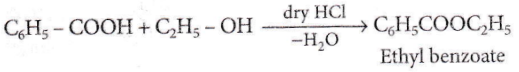
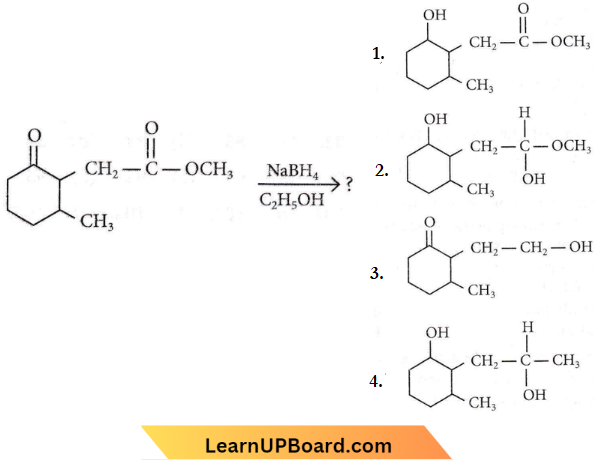
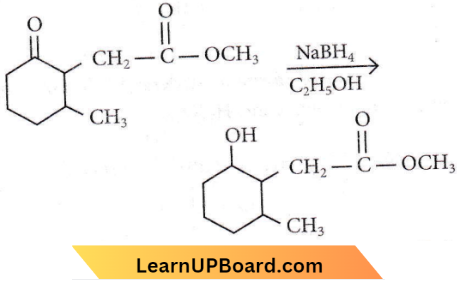
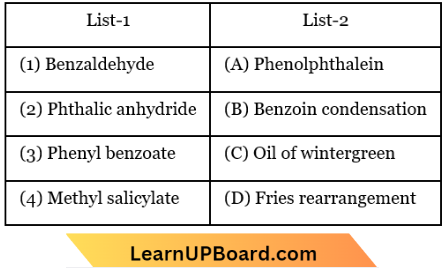
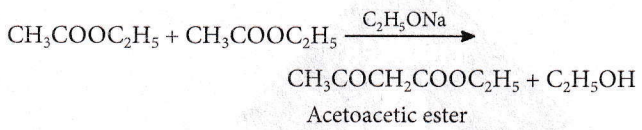
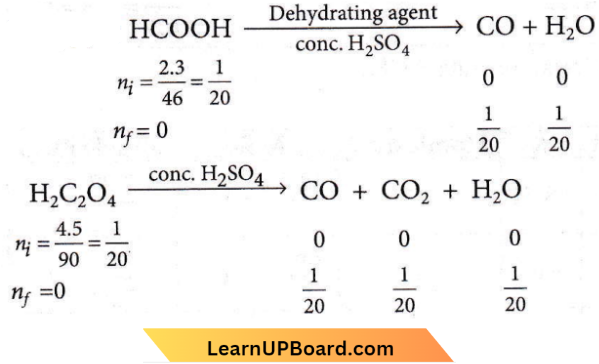
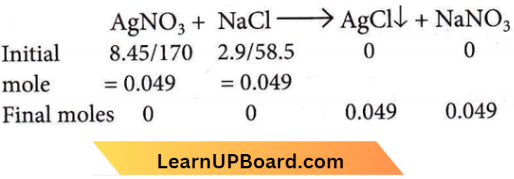
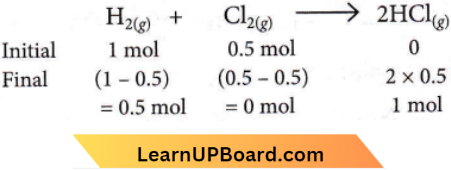

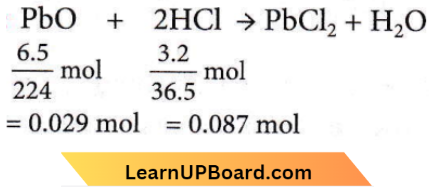
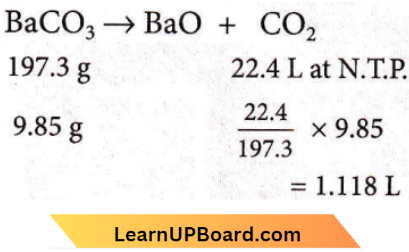

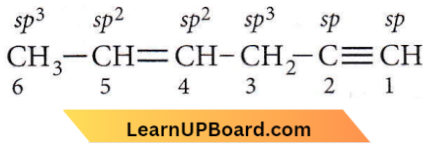
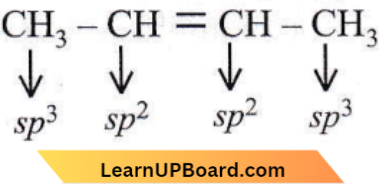
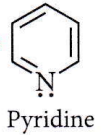
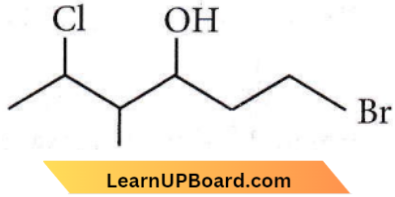
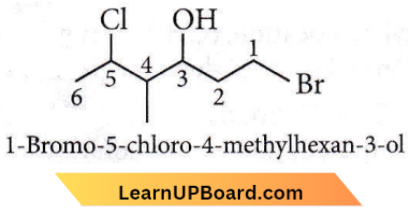
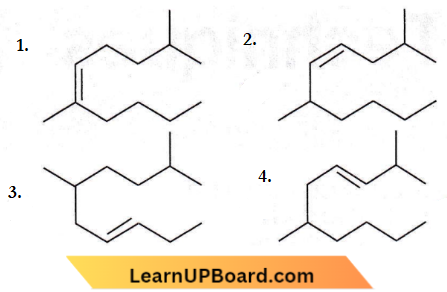
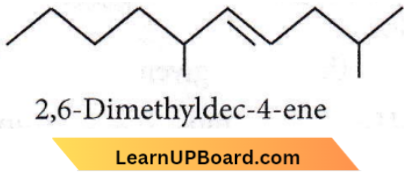

 group should get priority over methyl group.;
group should get priority over methyl group.;
 with methyl lithium gives which of the following species?
with methyl lithium gives which of the following species?
 is most stable due to hyperconjugation.
is most stable due to hyperconjugation. is aromatic because it has
is aromatic because it has








 is the true structure of H2O2
is the true structure of H2O2