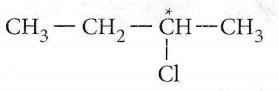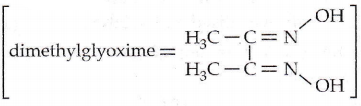NEET Chemistry For Coordination Compounds Multiple Choice Questions
Question 1. The correct order of the stoichiometries of AgCl formed when AgNO3 in excess is treated with the complexes: \(\mathrm{CoCl}_3 \cdot 6 \mathrm{NH}_3, \mathrm{CoCl}_3 .5 \mathrm{NH}_3, \mathrm{CoCl}_3 \cdot 4 \mathrm{NH}_3\) respectively is
- 3AgCl, 1AgCl, 2AgCl
- 3AgCl, 2AgCl, 1AgCl
- 2AgCl, 3AgCl, 2AgCl
- 1AgCl, 3AgCl, 2AgCl
Answer: 2. 3AgCl, 2AgCl, 1AgCl
⇒ \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3+3 \mathrm{AgNO}_3 \rightarrow 3 \mathrm{AgCl} \downarrow\) + \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]\left(\mathrm{NO}_3\right)_3\)
⇒ \({\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right] \mathrm{Cl}_2+2 \mathrm{AgNO}_3 \rightarrow 2 \mathrm{AgCl} \downarrow+\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right]\left(\mathrm{NO}_3\right)_2}\)
⇒ \({\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right] \mathrm{Cl}+\mathrm{AgNO}_3 \rightarrow \mathrm{AgCl} \downarrow+\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right] \mathrm{NO}_3}\)
Question 2. Cobalt(3) chloride forms several octahedral complexes with ammonia. Which of the following will not give a test for chloride ions with silver nitrate at 25 °C?
- \(\mathrm{CoCl}_3 \cdot 5 \mathrm{NH}_3\)
- \(\mathrm{CoCl}_3 \cdot 6 \mathrm{NH}_3\)
- \(\mathrm{CoCl}_3 \cdot 3 \mathrm{NH}_3\)
- \(\mathrm{CoCl}_3 \cdot 4 \mathrm{NH}_3\)
Answer: 3. \(\mathrm{CoCl}_3 \cdot 3 \mathrm{NH}_3\)
For octahedral complexes, the coordination number is 6.
Hence, \(\mathrm{CoCl}_3 \cdot 3 \mathrm{NH}_3 \text { i.e., }\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]\) will not ionise and will not give test for Cl– ion with silver nitrate.
Question 3. An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetra aquachromium(3) chloride. The number of moles of AgCl precipitated would be
- 0.003
- 0.01
- 0.001
- 0.002
Answer: 3. 0.001
An excess of AgNO3 is added to 100 mL of a 0.01 M solution of dichlorotetra aquachromium(3) chloride.
⇒ \(\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_4 \mathrm{Cl}_2\right] \mathrm{Cl}+\mathrm{AgNO}_3 \rightarrow\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_4 \mathrm{Cl}_2\right] \mathrm{NO}_3+\mathrm{AgCl}\)
No. of millimoles of solution = 100 mL x 0.01 M
=1 millimole
= 10-3 mole
So, a mole of AgCl = 0.001
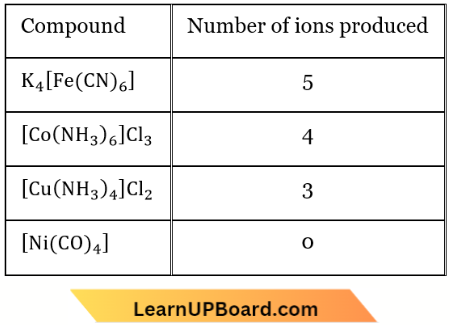
Question 4. Which of the following will exhibit maximum ionic conductivity?
- \(\mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3\)
- \(\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right] \mathrm{Cl}_2\)
- \(\left[\mathrm{Ni}(\mathrm{CO})_4\right]\)
Answer: 1. \(\mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]\)
Read and Learn More NEET MCQs with Answers
Ionic conductance increases with increasing the number of ions, produced after decomposition.
Question 5. A coordination complex compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three-mole ions in an aqueous solution. On reaching this solution with an excess of AgNO3 solution, we get two moles of AgCl precipitate. The ionic formula for this complex would be
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{NO}_2\right)\right] \mathrm{Cl}_2\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}\right]\left[\mathrm{Cl}\left(\mathrm{NO}_2\right)\right]\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{NO}_2\right) \mathrm{Cl}\right]\left[\left(\mathrm{NH}_3\right) \mathrm{Cl}\right]\)
- \(\left(\mathrm{Co}\left(\mathrm{NH}_3\right)_5\right]\left[\left(\mathrm{NO}_2\right)_2 \mathrm{Cl}_2\right]\)
Answer: 1. \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{NO}_2\right)\right] \mathrm{Cl}_2\)
A coordination complex compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three-mole ions in an aqueous solution. On reaching this solution with an excess of AgNO3 solution, we get two moles of AgCl precipitate.
As the complex gives two moles of AgCl ppt. with AgNO3 solution, the complex must have two ionisable Cl atoms. Hence, the probable complex, which gives three mole ions may be \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{NO}_2\right] \mathrm{Cl}_2\)
⇒ \({\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{NO}_2\right] \mathrm{Cl}_2 \rightarrow\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{NO}_2\right]^{2+}+2 \mathrm{Cl}^{-}}\)
Question 6. Which complex compound is most stable?
- \(\left[\mathrm{CoCl}_2(e n)_2\right] \mathrm{NO}_3\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]_2\left(\mathrm{SO}_4\right)_3\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{H}_2 \mathrm{O}\right) \mathrm{Br}\right]\left(\mathrm{NO}_3\right)_2\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3\left(\mathrm{NO}_3\right)_3\right]\)
Answer: 1. \(\left[\mathrm{CoCl}_2(e n)_2\right] \mathrm{NO}_3\)
Chelating ligands (for example, en) form more stable complexes than unidentate ligands.
Question 7. Ethylene diamine tetraacetate (EDTA) ion is
- Tridentate ligand with three “N” donor atoms
- Hexadentate ligand with four “O” and two “N” donor atoms
- Unidentate ligand
- Bidentate ligand with two “N” donor atoms.
Answer: 2. Hexadentate ligand with four “O” and two “N” donor atoms
Ethylenediaminetetraacetate ion (EDTA4-) is an important hexadentate ligand. It can bind through two nitrogen and four oxygen atoms to a central metal ion.
Question 8. The correct increasing order of trans-effect of the following species is
- \(\mathrm{NH}_3>\mathrm{CN}^{-}>\mathrm{Br}^{-}>\mathrm{C}_6 \mathrm{H}_5^{-}\)
- \(\mathrm{CN}^{-}>\mathrm{C}_6 \mathrm{H}_5^{-}>\mathrm{Br}^{-}>\mathrm{NH}_3\)
- \(\mathrm{Br}^{-}>\mathrm{CN}^{-}>\mathrm{NH}_3>\mathrm{C}_6 \mathrm{H}_5^{-}\)
- \(\mathrm{CN}^{-}>\mathrm{Br}^{-}>\mathrm{C}_6 \mathrm{H}_5^{-}>\mathrm{NH}_3\)
Answer: 2. \(\mathrm{CN}^{-}>\mathrm{C}_6 \mathrm{H}_5^{-}>\mathrm{Br}^{-}>\mathrm{NH}_3\)
The intensity of the trans-effect (as measured by the increase in the rate of substitution of the trans ligand) follows the sequence: \(\mathrm{CN}^{-}>\mathrm{C}_6 \mathrm{H}_5^{-}>\mathrm{Br}^{-}>\mathrm{NH}_3\)
Question 9. The sum of the coordination number and oxidation number of the metal M in the complex [M(en)2(C2O4)lCl (where en is ethylenediamine) is
- 6
- 7
- 8
- 9
Answer: 4. 9
[M(en)2(C2O4)]Cl:
Oxidation number of metal = +3
Coordination number of metal = 6
∴ Sum of oxidation number and coordination number = 3 + 6 = 9
Question 10. The anion of acetylacetone (acac) forms Co(acac)3 chelate with Co3+. The rings of the chelate are
- Five membered
- Four membered
- Six membered
- Three membered.
Answer: 3. Six membered
The ligand acetylacetone forms a six-membered chelate ring in the complex [Co(acac)3].
Question 11. Which of the following statements is true?
- Silicon exhibits 4 coordination numbers in its compound.
- The bond energy of F2 is less than Cl2
- Mn(3) oxidation state is more stable than Mn(2) in aqueous state.
- Elements of 15th gp show only +3 and +5 oxidation states.
Answer: 2. Bond energy of F2 is less than Cl2.
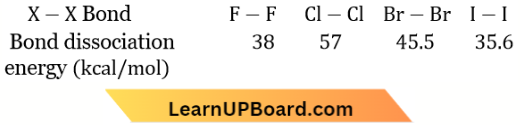
The bond energy of F2 is less than Cl2 due to interelectronic repulsions in small-sized F-atoms. Silicon exhibits coordination number 6. In an aqueous state, Mn(2) is more stable.
⇒ \(\mathrm{Mn} \rightleftharpoons \mathrm{Mn}^{2+}+2 e^{-}\)
The common oxidation states of t5th group elements are -3, +3 and +5
Question 12. Coordination number of Ni in [Ni(C2O4)3]4–is
- 3
- 6
- 4
- 2
Answer: 2. 6
(C2O42-) → bidentate ligand.
3 molecules attached from two sides with Ni make coordination number 6.
Question 13. The coordination number and oxidation state of Cr in K3[Cr(C2O4)3] are respectively
- 3 and + 3
- 3 and 0
- 6 and + 3
- 4 and + 2
Answer: 3. 6 and + 3
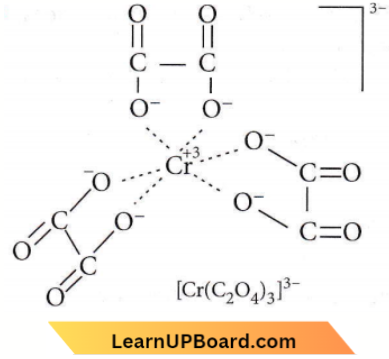
The number of atoms of the ligands that are directly bound to the central metal is known as the coordination number. It is six here.
Oxidation state : Let oxidation state of Cr be x + 3 (+1) + x+3(-2) =0+ 3 +x- 6= 0 = x=+3
Question 14. Which of the following ligands is expected to be bidentate?
- \(\mathrm{CH}_3 \mathrm{NH}_2\)
- \(\mathrm{CH}_3 \mathrm{C} \equiv \mathrm{N}\)
- \(\mathrm{Br}\)
- \(\mathrm{C}_2 \mathrm{O}_4{ }^{2-}\)
Asnwer: 4. \(\mathrm{C}_2 \mathrm{O}_4{ }^{2-}\)
When a ligand has two groups that are capable of bonding to the central atom, it is said to be bidentate. Thus, the only ligand, which is expected to be bidentate is (C2O42-) as
Question 15. Homoleptic complex from the following complexes is
- Pentaamminecarbonatocobalt(3) chloride
- Triamminetriaquachromium(3) chloride
- Potassium trioxalatoaluminate(3)
- Diamminechloridonitrito-N-platinum(2)
Answer: 3. Potassium trioxalatoaluminate(3)
Complexes in which a metal is bound to only one kind of ligand are known as homoleptic complexes. Potassium trioxalatoaluminate(3), K3[AlC2O4)3] is an example of a homoleptic complex.
Question 16. The IUPAC name of the complex [Ag(H2O)2][Ag(CN)2] is
- Dicyanidosilver(2) diaquaargentate(2)
- Diaquasilver (2) dicyanidoargentate(2)
- Dicyanidosilver(1) diaquaargentate(1)
- Diaquasilver (1) dicyanidoargentate(1).
Answer: 4. Diaquasilver (1) dicyanidoargentate(1).
Question 17. The name of complex ion, [Fe(CN)6]3- is
- Hexacyanitoferrate(3) ion
- Tricyanoferrate(3) ion
- Hexacyanidoferrate(3) ion
- Hexacyanoiron(3) ion.
Answer: 3. Hexacyanidoferrate(3) ion
Question 18. The correct IUPAC name for [CrF2(en)2]Cl is
- Chlorodifluoridoethylenediaminechromium (3) chloride
- Difluoridobis(ethylenediamine)chromium (3) chloride
- Difluorobis-(ethylenediamine)chromium (3) chloride
- Chlorodifluoridobis(ethylenediamine) chromium(3).
Answer: 2. Difluoridobis(ethylenediamine)chromium (3) chloride
Question 19. The hypothetical complex chlorodiaquatriammine cobalt(3) chloride can be represented as
- \(\left[\mathrm{CoCl}\left(\mathrm{NH}_3\right)_3\left(\mathrm{H}_2 \mathrm{O}\right)_2\right] \mathrm{Cl}_2\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3\left(\mathrm{H}_2 \mathrm{O}\right) \mathrm{Cl}_3\right]\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_2\right)_3\left(\mathrm{H}_2 \mathrm{O}\right)_2 \mathrm{Cl}\right]\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3\left(\mathrm{H}_2 \mathrm{O}\right)_3\right] \mathrm{Cl}_3\)
Answer: 1. \(\left[\mathrm{CoCl}\left(\mathrm{NH}_3\right)_3\left(\mathrm{H}_2 \mathrm{O}\right)_2\right] \mathrm{Cl}_2\)
Chlorodiaquatriamminecobalt(3) chloride can be represented as \(\left[\mathrm{CoCl}\left(\mathrm{NH}_3\right)_3\left(\mathrm{H}_2 \mathrm{O}\right)_2\right] \mathrm{Cl}_2\)
Question 20. IUPAC name of [Pt(NH3)3(Br)(NO2)Cl]Cl is
- Triamminebromochloronitroplatinum(4) chloride
- Triamminebromonitrochloroplatinum(4) chloride
- Triamminechlorobromonitroplatinum(4) chloride
- Triamminenitrochlorobromoplatinum(4) chloride.
Answer: 1. Triamminebromochloronitroplatinum(4) chloride
The ligands are named in the alphabetic order according to the latest IUPAC system. So, the name of \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_3 \mathrm{Br}\left(\mathrm{NO}_2\right) \mathrm{Cl}\right] \mathrm{Cl}\) is triamminebromochloronitroplatinum(4) chioride. (The oxidation no. of ‘Pt’ is +4)
Question 21. The formula of dichloro bis(urea)copper(2) is
- \(\left[\mathrm{Cu}\left\{\mathrm{O}=\mathrm{C}\left(\mathrm{NH}_2\right)_2\right\} \mathrm{Cl}\right] \mathrm{Cl}\)
- \(\left[\mathrm{CuCl}_2\right]\left\{\mathrm{O}=\mathrm{C}\left(\mathrm{NH}_2\right)_2\right\}\)
- \(\left[\mathrm{Cu}\left\{\mathrm{O}=\mathrm{C}\left(\mathrm{NH}_2\right)_2\right\} \mathrm{Cl}_2\right.\)
- \(\left[\mathrm{CuCl}_2\left\{\mathrm{O}=\mathrm{C}\left(\mathrm{NH}_2\right)_2\right\}_2\right]\)
Answer: 4. \(\left[\mathrm{CuCl}_2\left\{\mathrm{O}=\mathrm{C}\left(\mathrm{NH}_2\right)_2\right\}_2\right]\)
The formula of dichloro bis(urea)copper(2) is [CuCl2{(NH2)2Co}2]
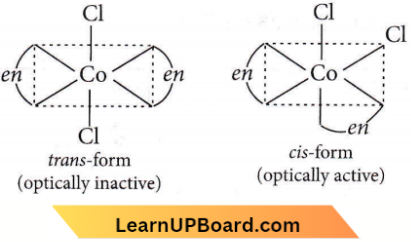
Question 22. The type of isomerism shown by the complex [CoCl2(en)2] is
- Geometrical isomerism
- Coordination isomerism
- Ionization isomerism
- Linkage isomerism.
Answer: 1. Geometrical isomerism
[CoCl2(en)2], exhibits geometrical isomerism, as the coordination number of Co is 6 and this compound has octahedral geometry.
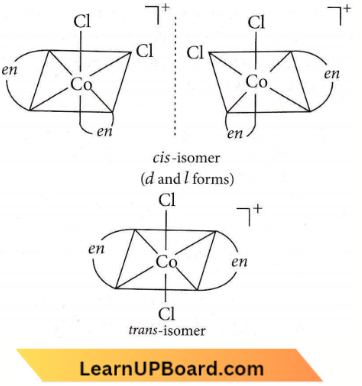
Question 23. Number of possible isomers for the complex [Co(en)2Cl2]Cl will be (en = ethylenediamine)
- 1
- 3
- 4
- 2
Answer: 2. 3
Possible isomers of [Co(en)2Cl2]Cl
Question 24. The complexes [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6] are the examples of which type of isomerism?
- Linkage isomerism
- Ionization isomerism
- Coordination isomerism
- Geometrical isomerism
Answer: 3. Coordination isomerism
Coordination isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in the complex example, \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Cr}(\mathrm{CN})_6\right]\) and \(\left.\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Co}(\mathrm{CN})_6\right]\)
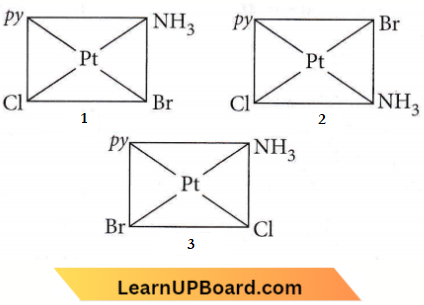
Question 25. The complex, [Pt(py)(NH2)BrCl] will have how many geometrical isomers?
- 3
- 4
- 0
- 2
Answer: 1. 3
⇒ \(\left[\mathrm{Pt}(p y)\left(\mathrm{NH}_3\right) \mathrm{BrCl}\right]\) can have three isomers.
Question 26. The existence of two different coloured complexes with the composition of [Co(NH3)4Cl2]+ is due to
- Linkage isomerism
- Geometrical isomerism
- Coordination isomerism
- Ionization isomerism.
Answer: 2. Geometrical isomerism
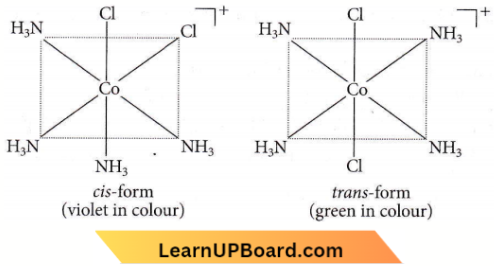
Question 27. Which one of the following complexes is not expected to exhibit isomerism?
- \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_4\left(\mathrm{H}_2 \mathrm{O}\right)_2\right]^{2+}\)
- \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]\)
- \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]\)
- \(\left[\mathrm{Ni}(e n)_3\right]^{2+}\)
Answer: 3. \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]\)
Compounds having tetrahedral geometry do not exhibit isomerism due to the presence of symmetry elements. Here, [Ni(NH3)2Cl2] has a tetrahedral geometry.
Question 28. Which of the following does not show optical isomerism?
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]^0\)
- \(\left[\mathrm{Co}(\text { en }) \mathrm{Cl}_2\left(\mathrm{NH}_3\right)_2\right]^{+}\)
- \(\left[\mathrm{Co}(e n)_3\right]^{3+}\)
- \(\left[\mathrm{Co}(e n)_2 \mathrm{Cl}_2\right]^{+}\)(en = ethylenediamine)
Answer: 1. \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]^0\)
Optical isomerism is shown by
- Complexes of the type \({\left[M(A A)_2 Y_2\right]}\), containing one symmetrical bidentate ligand i.e., \(\left[\mathrm{Co}(e n) \mathrm{Cl}_2\left(\mathrm{NH}_3\right)_2\right]^{+} \text {. }\)
- Complexes of the type \(\left[M(A A)_3\right]\), containing a symmetrical bidentate ligand i.e., \(\left[\mathrm{Co}(e n)_3\right]^{3+}\).
- Complexes of the type \(\left[M(A A)_2 X_2\right]\), i.e. \(\left[\mathrm{Co}(e n)_2 \mathrm{Cl}_2\right]^{+}\).
However, complexes of the type \(\left[M A_3 B_3\right]\) show geometrical isomerism, known as fac-mer isomerism.
∴ \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]\) exhibits fac-mer isomerism.
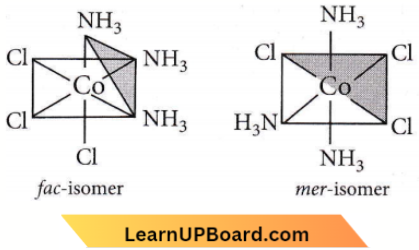
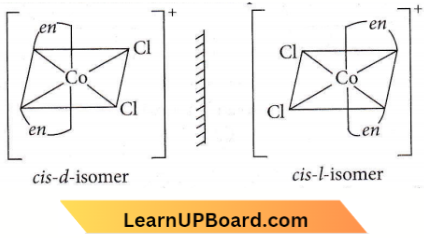
Question 29. Which of the following will give a pair of enantiomorphs?
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]\left[\mathrm{Co}(\mathrm{CN})_6\right]\)
- \(\left[\mathrm{Co}(\text { en })_2 \mathrm{Cl}_2\right] \mathrm{Cl}\)
- \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_4\right]\left[\mathrm{PtCl}_6\right]\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right] \mathrm{NO}_2\) (en \(=\mathrm{NH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NH}_2\))
Answer: 2. \(\left[\mathrm{Co}(\text { en })_2 \mathrm{Cl}_2\right] \mathrm{Cl}\)
Either a pair of crystals, molecules or compounds that are mirror images of each other but are not identical, and that rotate the plane of polarised light equally, but in opposite directions are called enantiomorphs.
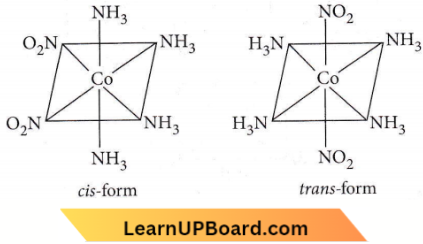
Question 30. [CO(NH3)4(NO2)2]Cl exhibits
- Linkage isomerism, geometrical isomerism and optical isomerism
- Linkage isomerism, ionization isomerism and optical isomerism
- Linkage isomerism, ionization isomerism and geometrical isomerism
- Ionization isomerism, geometrical isomerism and optical isomerism.
Answer: 3. Linkage isomerism, ionization isomerism and geometrical isomerism
Ionization isomerism arises when the coordination compounds give different ions in solution.
⇒ \({\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{NO}_2\right)_2\right] \mathrm{Cl} \rightleftharpoons\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{NO}_2\right)_2\right]^{+}+\mathrm{Cl}^{-}}\)
⇒ \({\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{NO}_2\right) \mathrm{Cl}\right] \mathrm{NO}_2 \rightleftharpoons\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{NO}_2\right) \mathrm{Cl}\right]^{+}+\mathrm{NO}_2^{-}}\)
Linkage isomerism occurs in complex compounds which contain ambidentate ligands like \(\mathrm{NO}_2^{-}, \mathrm{SCN}^{-}, \mathrm{CN}^{-}, \mathrm{S}_2 \mathrm{O}_3^{2-}\) and \(\mathrm{CO}\).
⇒ \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4\left(\mathrm{NO}_2\right)_2\right] \mathrm{Cl} \text { and }\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4(\mathrm{ONO})_2\right] \mathrm{Cl}\) are linkage isomers NO–2 is linked through N or through O. Octahedral complexes of the type Ma4b2 exhibit geometrical isomerism
Question 31. Which one of the following is expected to exhibit optical isomerism? (en = ethylenediamine)
- cis- \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]\)
- trans- \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}_2\right]\)
- cis- \(\left[\mathrm{Co}(\text { en })_2 \mathrm{Cl}_2\right]^{+}\)
- trans- \(\left[\mathrm{Co}(\text { en })_2 \mathrm{Cl}_2\right]^{+}\)
Answer: 3. cis- \(\left[\mathrm{Co}(\text { en })_2 \mathrm{Cl}_2\right]^{+}\)
Optical isomerism is not shown by square planar complexes.
Octahedral complexes of general formulae, \(\left[M a_2 b_2 c_2\right]^{n \pm},[M a b c d e f],\left[M(A A)_3\right]^{n \pm},\left[M(A A)_2 a_2\right]^{n \pm}\) (where AA = symmetrical bidentate ligand), \(\left[M(A A)_2 a b\right]^{n \pm} \text { and }\left[M(A B)_3\right]^{n \pm}\)
(where AB = unsymmetrical ligands) show optical isomerism does not show optical isomerism (superimposable mirror image). But cis-form shows optical isomerism.
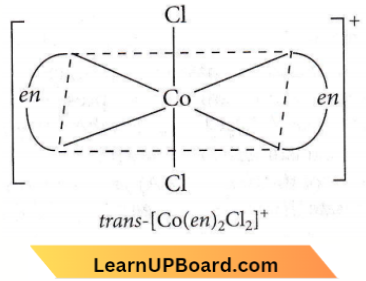
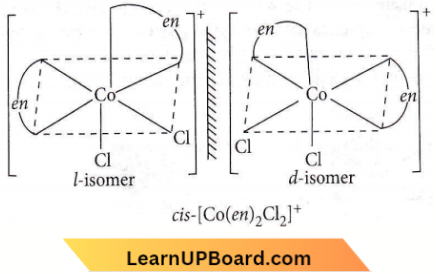
Question 32. Which of the following coordination compounds would exhibit optical isomerism?
- Pentaamminenitrocobalt(3) iodide
- Diamminedichloroplatinum(2)
- trans-Dicyanobis(ethylenediamine) chromium(3) chloride
- tris-(Ethylenediamine)cobalt(3) bromide
Answer: 4. tris-(Ethylenediamine)cobalt(3) bromide
⇒ \(\left[\mathrm{Co}(e n)_3\right]^{3+}\)
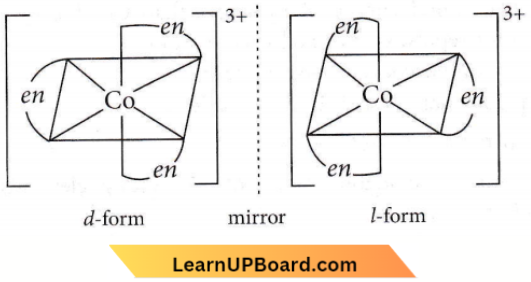
Question 33. Which of the following will give a maximum number of isomers?
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right]\)
- \(\left[\mathrm{Ni}(\text { en })\left(\mathrm{NH}_3\right)_4\right]^{2+}\)
- \(\left[\mathrm{Ni}\left(\mathrm{C}_2 \mathrm{O}_4\right)(e n)_2\right]^{2-}\)
- \(\left[\mathrm{Cr}(\mathrm{SCN})_2\left(\mathrm{NH}_3\right)_4\right]^{+}\)
Answer: 4. \(\left[\mathrm{Cr}(\mathrm{SCN})_2\left(\mathrm{NH}_3\right)_4\right]^{+}\)
[Cr(SCN)2(NH3)4]+ shows linkage, geometrical and optical isomerism.
Question 34. Which complex compound will give four isomers?
- \(\left[\mathrm{Fe}(e n)_3\right] \mathrm{Cl}_3\)
- \(\left[\mathrm{Co}(e n)_2 \mathrm{Cl}_2\right] \mathrm{Cl}\)
- \(\left[\mathrm{Fe}\left(\mathrm{PPh}_3\right)_3 \mathrm{NH}_3 \mathrm{ClBr}\right] \mathrm{Cl}\)
- \(\left[\mathrm{Co}\left(\mathrm{PPh}_3\right)_3 \mathrm{Cl}\right] \mathrm{Cl}_3\)
Answer: 3. \(\left[\mathrm{Fe}\left(\mathrm{PPh}_3\right)_3 \mathrm{NH}_3 \mathrm{ClBr}\right] \mathrm{Cl}\)
[Fe(PPh3)3NH3ClBr]Cl can give two optical and two geometrical isomers. While other complexes do not form geometrical isomers.
Question 35. The total number of possible isomers for the complex compound [Cu2(NH3)4] [Pt2Cl4] are
- 5
- 6
- 3
- 4
Answer: 4. 4
The isomers of the complex compound \(\left[\mathrm{Cu}^{\mathrm{II}}\left(\mathrm{NH}_3\right)_4\right] \left[\mathrm{Pt}^{11} \mathrm{Cl}_4\right]\) are:
- \(\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}\right]\left[\mathrm{Pt}\left(\mathrm{NH}_3\right) \mathrm{Cl}_3\right]\)
- \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}\right]\left[\mathrm{Cu}\left(\mathrm{NH}_3\right) \mathrm{Cl}_3\right]\)
- \(\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_4\right]\left[\mathrm{CuCl}_4\right]\)
So, the total no. of isomers is = 4
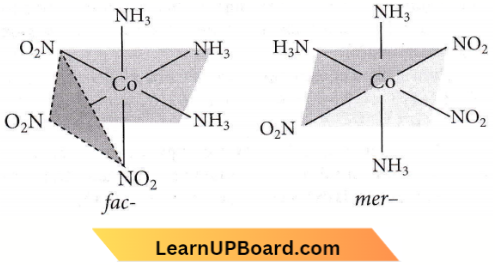
Question 36. The number of geometrical isomers of the complex [Co(NO2)3(NH3)3] is
- 4
- 0
- 2
- 3
Answer: 3. 2
Possible geometrical isomers are :
Question 37. The number of geometrical isomers for [Pt(NH3)2Cl2] is
- 3
- 4
- 1
- 2
Answer: 4. 2
Question 38. The order of energy absorbed which is responsible for the colour of complexes
- \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_2(e n)_2\right]^{2+}\)
- \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_4(\text { en })\right]^{2+}\) and
- \(\left[\mathrm{Ni}(e n)_3\right]^{2+}\) is
- (1) > (2) > (3)
- (C) > (2) > (1)
- (3) > (1) > (2)
- (2)> (1) > (3)
Answer: 3. (3) > (1) > (2)
Chelating ligand increases the stability of the complex compounds and the higher the number of chelating ligands, the higher the stability. The stronger is the strength of the ligand, the greater the energy absorbed by the complex.
Hence, the order is : \(\left[\mathrm{Ni}(e n)_3\right]^{2+}>\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_2(e n)_2\right]^{2+}>\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_4(e n)\right]^{2+}\)
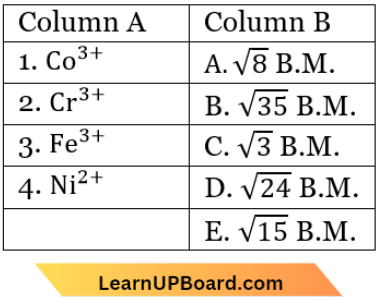
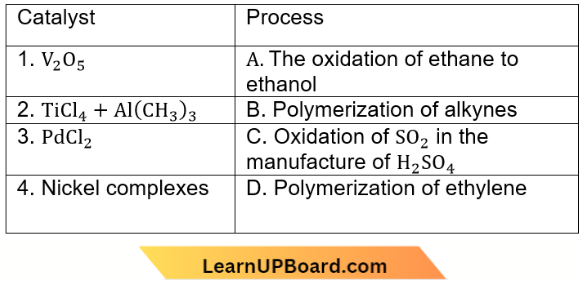
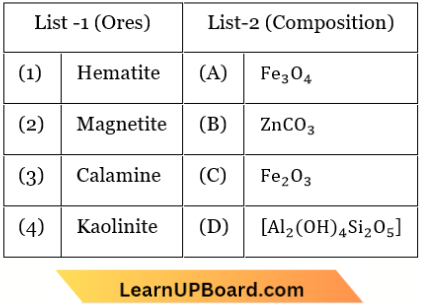
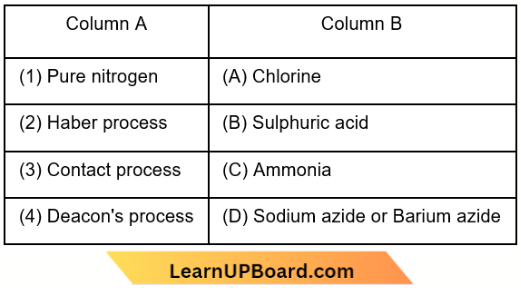
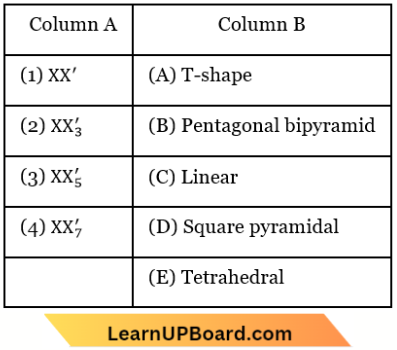
Question 39. Match List-1 with List-2
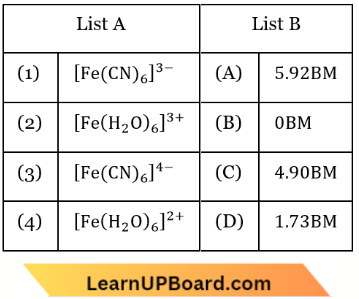
Choose the correct answer from the options given below.
- (1) – (D), (2) – (A), (3) – (B), (4) – (C)
- (1) – (D), (2) – (B), (3) – (A), (4) – (C)
- (1) – (B), (2) – (D), (3) – (C), (4) – (A)
- (1) – (A), (2) – (C), (3) – (D), (4) – (B)
Answer: 1. (1) – (D), (2) – (A), (3) – (B), (4) – (C)
⇒ \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\):
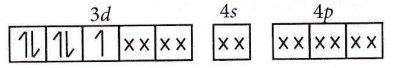
Spin only magnetic moment = \(\sqrt{n(n+2)}\)
where n = number of unpaired electrons.
Hence, n = 1.
μ = \(\sqrt{1(1+2)}=\sqrt{3}=1.73\) B.M
n = 5, \(\mu=\sqrt{5(5+2)}=\sqrt{35}=5.92\) B.M.
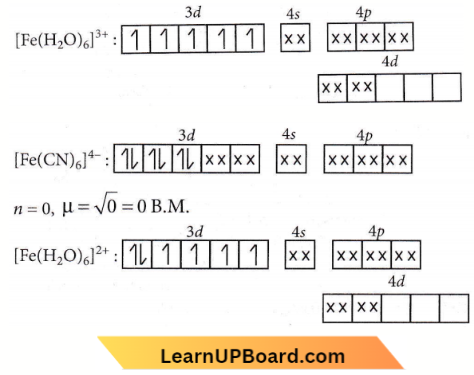
n = 4, \(\mu=\sqrt{4(4+2)}=\sqrt{24}=4.90\) B.M
Question 40. Which of the following is the correct order of increasing the field strength of ligands to form coordination compounds?
- \(\mathrm{SCN}^{-}<\mathrm{F}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}<\mathrm{CN}^{-}\)
- \(\mathrm{SCN}^{-}<\mathrm{F}^{-}<\mathrm{CN}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}\)
- \(\mathrm{F}^{-}<\mathrm{SCN}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}<\mathrm{CN}^{-}\)
- \(\mathrm{CN}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}<\mathrm{SCN}^{-}<\mathrm{F}^{-}\)
Answer: 1. \(\mathrm{SCN}^{-}<\mathrm{F}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}<\mathrm{CN}^{-}\)
According to the spectrochemical series, the order of increasing field strength is : \(\mathrm{SCN}^{-}<\mathrm{F}^{-}<\mathrm{C}_2 \mathrm{O}_4^{2-}<\mathrm{CN}^{-}\)
Question 41. What is the correct electronic configuration of the central atom in K4[Fe(CN)6] based on the crystal field
Answer: 3
In K4[Fe(CN)6] complex, Fe is in +2 oxidation state.
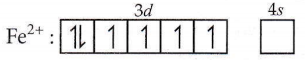
As CN– is a strong field ligand, it causes pairing of electrons therefore, electronic configuration of \(\mathrm{Fe}^{2+}\) in \(\mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]\) is \(t_{2 \mathrm{~g}}^6 e_{g^6}^0\)
Question 42. Aluminium chloride in acidified aqueous solution forms a complex ‘A’, in which the hybridisation state of ‘A’ is ‘B’. What are ‘A’ and ‘B’ respectively?
- \(\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}, s p^3 d^2\)
- \(\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{3+}, s p^3\)
- \(\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{3+}, d s p^2\)
- \(\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}, d^2 s p^3\)
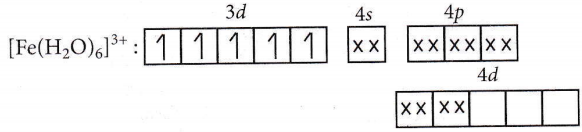
Answer: 1. \(\left[\mathrm{Al}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}, s p^3 d^2\)
Question 43. The crystal field stabilisation energy (CFSE) for will be CoCl6]4- is 18000 cm-1 The CFSE for [CoCl4]2-
- 6000 cm-1
- 16000 cm-1
- 18000 cm-1
- 8000 cm-1
Answer: 4. 8000 cm-1
⇒ \(\Delta_t=\frac{4}{9} \Delta_o=\frac{4}{9} \times 18000=8000 \mathrm{~cm}^{-1}\)
Question 44. The geometry and magnetic behaviour of the complex [Ni(CO)4] are
- Square planar geometry and diamagnetic
- Tetrahedral geometry and diamagnetic
- Square planar geometry and paramagnetic
- Tetrahedral geometry and paramagnetic.
Answer: 2. Tetrahedral geometry and diamagnetic
Ni(28) : [Ar]3d84s2
CO is a strong field ligand, so, unpairedelectrons get paired.
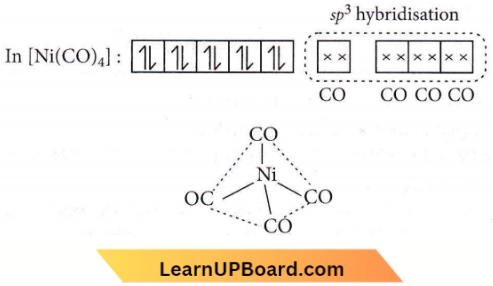
Thus, the complex is sp3 hybridised with tetrahedral geometry and is diamagnetic in nature.
Question 45. Correct increasing order for the wavelengths of absorption in the visible region for the complexes of Co3+ is
- \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+},\left[\mathrm{Co}(e n)_3\right]^{3+},\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+},\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{Co}(e n)_3\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{Co}(e n)_3\right]^{3+},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}(en)_3\right]^{3+},\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}(2017)\)
Answer: 4. \(\left[\mathrm{Co}(en)_3\right]^{3+},\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}(2017)\)
Increasing order of crystal field splitting energy is \(\mathrm{H}_2 \mathrm{O}<\mathrm{NH}_3<\text { en }\)
Thus, increasing order of crystal field splitting energy for the given complexes is : \({\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}<\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}<\left[\mathrm{Co}(e n)_3\right]^{3+}}\)
As, E = \(\frac{h c}{\lambda}\)
Thus, increasing order of wavelength of absorption is : \(\left[\mathrm{Co}(\mathrm{en})_3\right]^{3+}<\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}<\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
Question 46. Pick out the correct statement with respect to [Mn(CN)6]3-.
- It is sp³d2 hybridised and tetrahedral.
- It is d2sp³ hybridised and octahedral.
- It is dsp2 hybridised and square planar.
- It is sp³d2 hybridised and octahedral.
Answer: 2. It is d2sp³ hybridised and octahedral.
[Mn(CN)6]3-: Letoxidation state of Mn be x.
x+6x(-1)=-3 + x=+3
Electronic configuration of Mn : [Ar]4s2 3d5
Electronic configuration of Mn3+ : [Ar]3d4
CN– is a strong field ligand thus, it causes the pairing of electrons in the 3d-orbital.
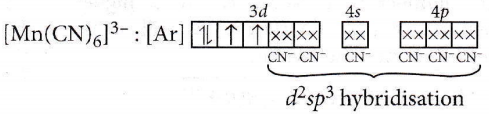
Thus, [Mn(CN)6]3- has d²sp³ hybridization and has octahedral geometry.
Question 47. Jahn-Teller effect is not observed in high spin complexes of
- d7
- d8
- d4
- d9
Answer: 2. d8
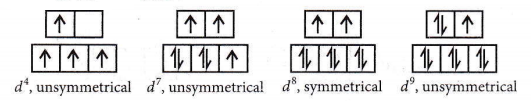
Jahn-Teller distortion is usually significant for asymmetrically occupied eg orbitals since they are directed towards the ligands and the energy gain is considerably greater. In the case of unevenly occupied t2g, orbitals, the fan-teller distortion is very weak since the t2g, set does not point directly at the ligands and therefore, the energy gain is much less.
High spin complexes:
Question 48. The hybridization involved in complex [Ni(CN)4]2- is (At. No. Ni = 28)
- sp³
- d²sp²
- d²sp³
- dsp²
Answer: 4. dsp²
[Ni(CN)4]2-: Oxidation number of Ni = +2
Electronic configuration of Ni2+: [Ar]3d84s0
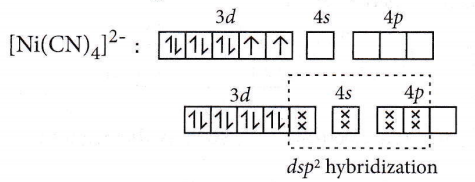
The pairing of electrons in the d-orbital takes place due to the presence of a strong field ligand (CN–).
Question 49. Among the following complexes the one which shows zero crystal field stabilization energy (CFSE) is
- \(\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
- \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
Answer: 2. \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
H2O is a weak field ligand, hence Δ0 < pairing energy.
CFSE=(-0.4x+0.6y)Δ0
where r and y are no. of electrons occupying t2g and eg orbitals respectively.
For [Fe(H2O)6]3+ complex ion,
Fe3+ (3d5) = t³2g e²g=-0.4×3+0.6 x2= 0.0or 0 Δ0
Question 50. A magnetic moment at 1.73 BM will be shown by one of the following
- \(\mathrm{TiCl}_4\)
- \(\left[\mathrm{CoCl}_6\right]^{4-}\)
- \(\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}\)
- \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
Answer: 3. \(\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right]^{2+}\)
Oxidation state of Cu in I Cu(NH3)4)2+ is + 2
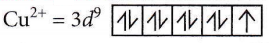
It has one unpaired electron (n = 1)
μ = \(\sqrt{n(n+2)}=\sqrt{1(1+2)}=\sqrt{3}=1.73 \mathrm{BM}\)
Question 51. Crystal field splitting energy for high spin d4 octahedral complex is
- – 1.2 Δ0
- – 0.6 Δ0
- -0.8 Δ0
- – 1.6 Δ0
Answer: 2. – 0.6 Δ0
CFSE = (- 0.4 x + 0.6 y) Δ0
where, x = No. of electrons occupying t2g orbitals
y = no. of electrons occupying eg orbitals
= (- 0.4 x 3 + 0.6 x 1)Δ0 [High spin d4 =t2g3eg1)
= (- 1.2 + 0.6)Δ0 = -0.6 Δ0
Question 52. Which among the following is a paramagnetic complex?
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Pt}(e n) \mathrm{Cl}_2\right]\)
- \(\left[\mathrm{CoBr}_4\right]^{2-}\)
- \(\mathrm{Mo}(\mathrm{CO})_6\)
(Atomic number Mo = 42, Pt = 78)
Answer: 3. \(\left[\mathrm{CoBr}_4\right]^{2-}\)
Co2+ in [CoBr4]2- has 3d74s0 configuration and Br– is a weak field ligand. Thus, it has 3 unpaired electrons and hence, paramagnetic.
Question 53. Which is diamagnetic?
- \(\left[\mathrm{CoF}_6\right]^{3-}\)
- \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
- \(\left[\mathrm{NiCl}_4\right]^{2-}\)
- \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\)
Answer: 2. \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
In [Ni(CN)4]2- all orbitals are doubly occupied, hence, it is diamagnetic
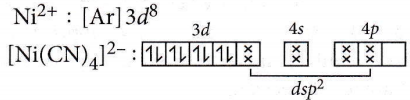
CN– is a strong field ligand and causes the pairing of 3d-electrons of Ni2+
Question 54. Which one of the following is an outer orbital complex and exhibits paramagnetic behaviour?
- \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
- \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
Answer: 1. \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
⇒ \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}: s p^3 d^2\) (outer), octahedral, paramagnetic
⇒ \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}: s p^3 d^2\) (outer), octahedral, diamagnetic
⇒ \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}: d^2 s p^3\) (inner), octahedral, paramagnetic
⇒ \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}: d^2 s p^3\) (inner), octahedral, diamagnetic
Question 55. The low spin complex of d6-cation in an octahedral field will have the following energy
- \(\frac{-12}{5} \Delta_o+P\)
- \(\frac{-12}{5} \Delta_o+3 P\)
- \(\frac{-2}{5} \Delta_o+2 P\)
- \(\frac{-2}{5} \Delta_o+P\)
(Δ0 = crystal field splitting energy in an octahedral field, P = Electron pairing energy)
Answer: 2. \(\frac{-12}{5} \Delta_o+3 P\)
CFSE = (-0.4x + 0.6y)Δ0 + zP
where x = number of electrons occupying t2g orbital
y = number of electrons occupying eg orbital
z = number of pairs of electrons
For low spin d6 complex electronic configuration
= \(t_{2 g}{ }^6 e_g^0\) or \(t_{2 g}{ }^{2,2,2} e_g^0\)
∴ x=6, y=0, z=3
CFSE = \((-0.4 \times 6+0 \times 0.6) \Delta_0+3 P\)
= \(\frac{-12}{5} \Delta_o+3 P\)
Question 56. A red precipitate is obtained when an ethanol solution of dimethylglyoxime is added to ammoniacal Ni(2). Which of the following statements is not true?
- The red complex has a square planar geometry.
- The complex has symmetrical H-bonding.
- The red complex has a tetrahedral geometry.
- Dimethylglyoxime functions as a bidentate ligand.
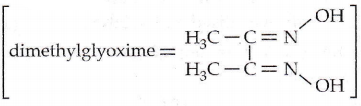
Answer: 3. Red complex has a tetrahedral geometry.
[Ni(dmg)2] is square planar in structure not tetrahedral.
Question 57. Of the following complex ions, which is diamagnetic in nature?
- \(\left[\mathrm{NiCl}_4\right]^{2-}\)
- \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
- \(\left[\mathrm{CuCl}_4\right]^{2-}\)
- \(\left[\mathrm{CoF}_6\right]^{3-}\)
Answer: 2. \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
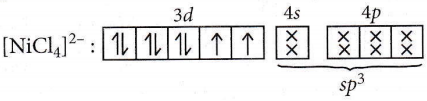
Number of unpaired electrons = 2
Hence, [NiCl4]2- is paramagnetic
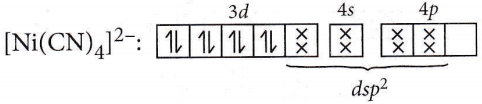
Number of unpaired electrons = 0, so it is diamagnetic in nature.
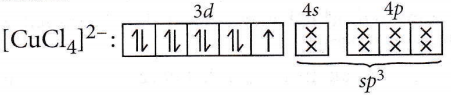
No. of unpaired electron = 1, so it is paramagnetic.
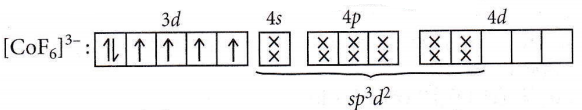
No. of unpaired electrons = 4, so it is paramagnetic.
Question 58. The d-electron configurations of Cr2+, Mn2+, Fe2+ and Co2+ are d4, d5, d6 and d7 respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
- \(\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
(Atomic number Cr = 24, Mn = 25, Fe = 26, Co = 27)
Answer: 3. \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
The d-electron configurations of Cr2+, Mn2+, Fe2+ and Co2+ are d4, d5, d6 and d7 respectively.
∴ \(\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}: \mathrm{Mn}^{2+}=3 d^6\)
Number of unpaired electrons =5
∴ \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+} ; \mathrm{Fe}^{2+}=3 d^6\)
Number of unpaired electrons =4
∴ \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}: \mathrm{Co}^{2+}=3 d^7\)
Number of unpaired electrons =3
∴ \(\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}: \mathrm{Cr}^{2+}=3 d^4\)
Number of unpaired electrons =4
Minimum paramagnetic behaviour is shown by \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
Question 59. Which of the following complex compounds will exhibit the highest paramagnetic behaviour?
- \(\left[\mathrm{Ti}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
(Atomic number Ti = 22, Cr = 24, Co = 27, Zn = 30)
Answer: 2. \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
Ti\(:[\mathrm{Ar}] 3 d^2 4 s^2, \mathrm{Ti}^{3+}:[\mathrm{Ar}] 3 d^1 4 s^0\) (1 unpaired electron)
Cr:\([\mathrm{Ar}] 3 d^4 4 s^2, \mathrm{Cr}^{34}:[\mathrm{Ar}] 3 d^3 4 s^0\) (3 unpaired electrons)
Co: \([\mathrm{Ar}] 3 d^7 4 s^2, \mathrm{Co}^{3+} ;[\mathrm{Ar}] 3 d^6 4 s^0\)
(No unpaired electrons because of pairing)
Zn:\([\mathrm{Ar}] 3 d^{10} 4 s^2, \mathrm{Zn}^{2+}:[\mathrm{Ar}] 3 d^{10}\) (No unpaired electrons)
∴ \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\) exhibits the highest paramagnetic behaviour as it contains 3 unpaired electrons.
Question 60. Which of the following complex ions is not expected to absorb visible light?
- \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
Answer: 1. \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\)
A transition metal complex absorbs visible light only if it has unpaired electrons.
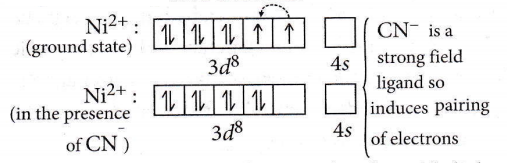
No unpaired electron so does not absorb visible light.
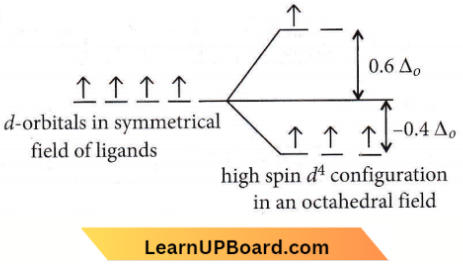
Question 61. Crystal field stabilization energy for high spin d4 octahedral complex is
- – 1.8 Δ0
- – 1.6 Δ0 + P
- – 1.2 Δ0
- – 0.6 Δ0
Answer: 4. – 0.6 Δ0
Question 62. Out of \(\mathrm{TiF}_6^{2-}, \mathrm{CoF}_6^{3-}, \mathrm{Cu}_2 \mathrm{Cl}_2\) and \(\mathrm{NiCl}_4^{2-}\) (Z of Ti = 22, Co = 27, Cu = 29, Ni = 28) the colourless species are
- \(\mathrm{Cu}_2 \mathrm{Cl}_2\) and \(\mathrm{NiCl}_4{ }^{2-}\)
- \(\mathrm{TiF}_6{ }^{2-}\) and \(\mathrm{Cu}_2 \mathrm{Cl}_2\)
- \(\mathrm{CoF}_6{ }^{3-}\) and \(\mathrm{NiCl}_4{ }^{2-}\)
- \(\mathrm{TiF}_6{ }^{2-}\) and \(\mathrm{CoF}_6{ }^{3-}\)
Answer: 2. \(\mathrm{TiF}_6{ }^{2-}\) and \(\mathrm{Cu}_2 \mathrm{Cl}_2\)
A species is coloured when it contains unpaired d-electrons which are capable of understanding d-d transition on adsorption of light of a particular wavelength.
In \(\mathrm{TiF}_6{ }^{2-}, \mathrm{Ti}^{4+}: 3 d^0,\) colourless
In \(\mathrm{CoF}_6{ }^{3-}, \mathrm{Co}^{3+}: 3 d^6\), coloured
In \(\mathrm{Cu}_2 \mathrm{Cl}_2, \mathrm{Cu}^{+}: 3 d^{10},\) colourless
In \(\mathrm{NiCl}_4^{2-}, \mathrm{Ni}^{2+}: 3 d^8,\) coloured
Thus, \(\mathrm{TiF}_6^{2-}\left(3 d^0\right)\) and \(\mathrm{Cu}_2 \mathrm{Cl}_2\left(3 d^{10}\right)\) with empty and fully filled d-orbitals appear colourless as they are not capable of undergoing d-d transition
Question 63. Which of the following complex ions is expected to absorb visible light?
- \(\left[\mathrm{Ti}(e n)_2\left(\mathrm{NH}_3\right)_2\right]^{4+}\)
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
- \(\left[\mathrm{Sc}\left(\mathrm{H}_2 \mathrm{O}\right)_3\left(\mathrm{NH}_3\right)_3\right]^{3+}\)
[Atomic number Zn = 30, Sc = 21, Ti = 22, Cr = 24]
Answer: 2. \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
⇒ \(\mathrm{Ti}^{4+} \rightarrow 3 d^0, \mathrm{Cr}^{3+} \rightarrow 3 d^3\)
⇒ \(\mathrm{Zn}^{2+} \rightarrow 3 d^{10}, \mathrm{Sc}^{3+} \rightarrow 3 d^0\)
Question 64. Which of the following complexes exhibits the highest paramagnetic behaviour?
- \(\left[\mathrm{Co}(o x)_2(\mathrm{OH})_2\right]^{-}\)
- \(\left[\mathrm{Ti}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{V}(\mathrm{gl} \text { ) })_2(\mathrm{OH})_2\left(\mathrm{NH}_3\right)_2\right]^{+}\)
- \(\left[\mathrm{Fe}(e n)(b p y)\left(\mathrm{NH}_3\right)_2\right]^{2+}\)
where gly = glycine, en = ethylenediamine and bpv = bipyridyl moities. (At. nos. Ti = 22, V = 23, USB Fe = 26, Co = 27)
Answer: 1. \(\left[\mathrm{Co}(o x)_2(\mathrm{OH})_2\right]^{-}\)
Question 65. In which of the following coordination entities the magnitude of Δ0 (CFSE in the octahedral field) will be maximum?
- \(\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}\)
- \(\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}\)
- \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
(Atomic number Co = 27)
Answer: 1. \(\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}\)
When the ligands are arranged ln order of the magnitude of crystal field splitting, the arrangement, thus, obtained is called a spectrochemical series.
Arranged in increasing field strength as \(\mathrm{I}^{-}<\mathrm{Br}^{-}<\mathrm{Cl}^{-}<\mathrm{NO}_3^{-}<\mathrm{F}^{-}<\mathrm{OH}^{-}<\mathrm{C}_2 \mathrm{O}_4{ }^{2-}<\mathrm{H}_2 \mathrm{O}<\mathrm{NH}_3<\text { en }\) \(<\mathrm{NO}_2^{-}<\mathrm{CN}^{-}<\mathrm{CO}\)
It has been observed that ligands before H2O are weak field ligands while ligands after H2O are strong field ligands.
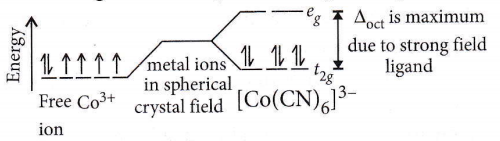
CFSE in an octahedral field depends upon the nature of ligands.
The stronger the ligands larger the value of Δoct.
Question 66. The d electron configurations of \(\mathrm{Cr}^{2+}, \mathrm{Mn}^{2+}, \mathrm{Fe}^{2+}\) and \(\mathrm{Ni}^{2+}\) are \(3 d^4, 3 d^5, 3 d^6\) and \(3 d^8\) respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour?
- \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
- \(\left[\mathrm{Mn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
(Atomic number Cr = 24, Mn = 25, Fe = 26, Ni = 28)
Answer: 2. \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
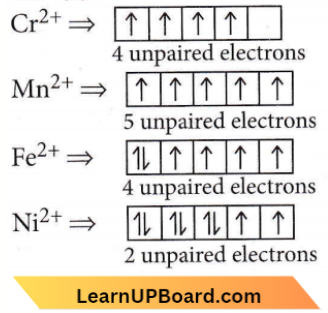
The greater the number of unpaired electrons, the higher is the paramagnetism. Hence, [Ni(H2O)6]2+ will exhibit the minimum paramagnetic behaviour.
Question 67. [Cr(H2O)6]Cl3 (Atomic number of Cr = 24) has a magnetic moment of 3.83 B.M. The correct distribution of 3d electrons in the chromium of the complex is
- \(3 d_{x y}^1, 3 d_{y z}^1, 3 d_{z^2}^1\)
- \(3 d_{\left(x^2-y^2\right)}^1, 3 d_{z^2}^1, 3 d_{x z}^1\)
- \(3 d_{x y}^1, 3 d_{\left(x^2-y^2\right)}^1, 3 d_{y z}^1\)
- \(3 d_{x y}^1, 3 d_{y z}^1, 3 d_{x z}^1\)
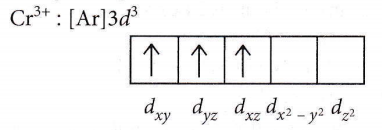
Answer: 4. \(3 d_{x y}^1, 3 d_{y z}^1, 3 d_{x z}^1\)
Magneti moment = \(\sqrt{n(n+2)}\)
3.83 = \(\sqrt{n(n+2)} \Rightarrow(3.83)^2=n(n+2)\)
14.6689 = \(n^2+2 n\)
On solving the equation, n=3
Question 68. Which one of the following is an inner orbital complex as well as diamagnetic in behaviour?
- \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{34}\)
- \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}\)
(Atomic number : Zn = 30, Cr = 24, Co = 27, Ni = 28)
Answer: 3. \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{34}\)
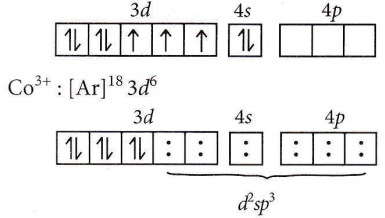
⇒ \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+} ; \mathrm{Co}(27):[\mathrm{Ar}]^{18} 3 d^7 4 s^2\)
electron pair from six ligands \(\left(\mathrm{NH}_3\right)\)
⇒ \(d^2 s p^3 \rightarrow\) inner octahedral complex and diamagnetic.
⇒ \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_6\right]^{2+} \rightarrow s p^3 d^2\) (outer)and diamagnetic.
⇒ \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+} \rightarrow d^2 s p^3\) (inner) and paramagnetic.
⇒ \(\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+} \rightarrow s p^3 d^2\) (outer)and paramagnetic.
Question 69. Among \(\left[\mathrm{Ni}(\mathrm{CO})_4\right],\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-},\left[\mathrm{NiCl}_4\right]^{2-}\) species, the hybridisation states at the Ni atom are, respectively
- \(s p^3, d s p^2, d s p^2\)
- \(s p^3, d s p^2, s p^3\)
- \(s p^3, s p^3, d s p^2\)
- \(d s p^2, s p^3, s p^3\)
[Atomic number of Ni = 28]
Answer: 2. \(s p^3, d s p^2, s p^3\)
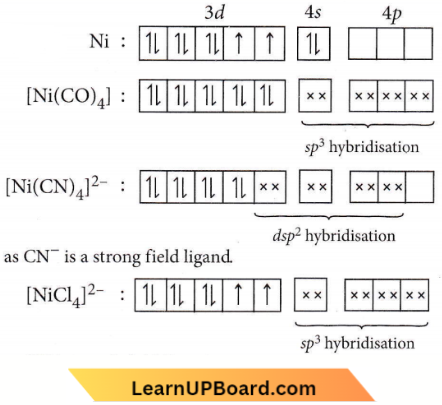
Question 70. CN– is a strong field ligand. This is due to the fact that
- It carries a negative charge
- It is a pseudohalide
- It can accept electrons from metal species
- It forms high-spin complexes with metal species.
Answer: 2. It is a pseudohalide
Cyanide ion is a strong field ligand because it is a pseudohalide ion. Pseudohalide ions are stronger coordinating ligands and they have the ability to form σ-bond (from the pseudohalide to the metal) and π bond (from the metal to pseudohalide).
Question 71. Considering H2O as a weak field ligand, the number of unpaired electrons in [Mn(H2O)6]2+ will be (atomic number of Mn = 25)
- Three
- Five
- Two
- Four.
Answer: 2. Five
Mn (25):3d54s2
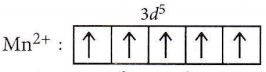
In the presence of a weak field ligand, there will be no pairing of electrons. So, it will form a high spin complex, i.e., the number of unpaired electrons = 5.
Question 72. In an octahedral structure, the pair of d orbitals involved in d²sp³ hybridisation is
- \(d_{x^2-y^2}, d_{z^2}\)
- \(d_{x z}, d_{x^2-y^2}\)
- \(d_z, d_{x z}\)
- \(d_{x y}, d_{y z}\)
Answer: 1. \(d_{x^2-y^2}, d_{z^2}\)
In the formation of d²sp³ hybrid orbitals, two (n – 1) d orbitals of eg set i.e. (n -1)d²z and (n – 1)dx²-y² orbitals), one ns and three np(npx npy and npz) orbitals combine together and form six d²sp³ hybrid orbitals.
Question 73. The number of unpaired electrons in the complex ion [CoF6]3- is
- 2
- 3
- 4
- Zero.
(Atomic number Co = 27)
Answer: 3. 4
⇒ \(\left[\mathrm{CoF}_6\right]^{3-}\)
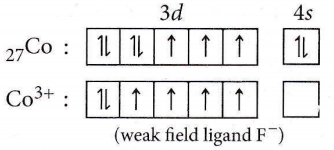
Thus, the number of unpaired electrons = 4
Question 74. The atomic numbers of Cr and Fe are respectively 24 and 26, which of the following is paramagnetic with the spin of an electron?
- \(\left[\mathrm{Cr}(\mathrm{CO})_6\right]\)
- \(\left[\mathrm{Fe}(\mathrm{CO})_5\right]\)
- \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}\)
- \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
Answer: 4. \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\)
Odd electrons, ions and molecules are paramagnetic.
In Cr(CO)6 molecule 12 electrons are contributed by the CO group and it contains no odd electron.
Cr: \(3 d^6 4 s^1\)
∴ \(\mathrm{Fe}(\mathrm{CO})_5\) molecule also does not contain odd electron.
Fe: \(3 d^6 4 s^2\)
In \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}\) ion, \(\mathrm{Fe}(+2): 3 d^6 4 s^0\)
∴ No odd electrons,
In \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+}\) ion, \(\mathrm{Cr}(+3): 3 d^3 4 s^0\)
This ion contains odd electrons so it is paramagnetic.
Question 75. Which statement is incorrect?
- \(\mathrm{Ni}(\mathrm{CO})_4\) – tetrahedral, paramagnetic
- \(\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}\) – square planar, diamagnetic
- \(\mathrm{Ni}(\mathrm{CO})_4\) – tetrahedral, diamagnetic
- \(\left[\mathrm{NiCl}_4\right]^{2-}\) – tetrahedral, paramagnetic
Answer: 1. \(\mathrm{Ni}(\mathrm{CO})_4\) – tetrahedral, paramagnetic
In Ni(CO)4 complex, Ni(0) will have 3d10 configuration
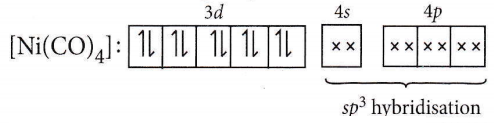
Hence, [Ni(CO)4] will have tetrahedral geometry and diamagnetic as there are no unpaired electrons.
Question 76. Iron carbonyl, Fe(CO)5 is
- Tetranuclear
- Mononuclear
- Trinuclear
- Dinuclear.
Answer: 2. Mononuclear
Based on the number of metal atoms present in a complex, they are classified as :
Example, \(\mathrm{Fe}(\mathrm{CO})_5:\) mononuclear; \(\mathrm{Co}_2(\mathrm{CO})_8\): dinuclear ; \(\mathrm{Fe}_3(\mathrm{CO})_{12}\) : trinuclear
Question 77. An example of a sigma-bonded organometallic compound is
- Grognards reagent
- Ferrocene
- Cobaltocene
- Ruthenocene.
Answer: 1. Grognard reagent
In sigma-bonded organometallic complexes, the metal atom and carbon atom of the ligand are joined together with a sigma bond, i.e., the ligand contributes one electron and is therefore, called one electron donor, for example, Grignard’s reagent R-Mg-X
Question 78. Which of the following has the longest C—O bond length? (Free C—O bond length in CO is 1.128 Å)
- \(\left[\mathrm{Fe}(\mathrm{CO})_4\right]^{2-}\)
- \(\left[\mathrm{Mn}(\mathrm{CO})_6\right]^{+}\)
- \(\mathrm{Ni}(\mathrm{CO})_4\)
- \(\left[\mathrm{Co}(\mathrm{CO})_4\right]^{-}\)
Answer: 1. \(\left[\mathrm{Fe}(\mathrm{CO})_4\right]^{2-}\)
The greater the negative charge on the carbonyl complex, the more easy it would be for the metal to permit its electrons to participate in the back bonding, the higher the M-C bond order and simultaneously there would be a larger reduction in the C-O bond order. Thus, [Fe(CO)4]2- has the lowest C-O bond order means the longest bond length.
Question 79. Which of the following carbonyls will have the strongest C – O bond?
- \(\mathrm{Mn}(\mathrm{CO})_6^{+}\)
- \(\mathrm{Cr}(\mathrm{CO})_6\)
- \(\mathrm{V}(\mathrm{CO})_6^{-}\)
- \(\mathrm{Fe}(\mathrm{CO})_5\)
Answer: 1. \(\mathrm{Mn}(\mathrm{CO})_6^{+}\)
The presence of a positive charge on the metal carbonyl would resist the flow of the metal electron charge to π orbitals of CO. This would increase the CO bond order and hence, CO in a metal carbonyl cation would absorb at a higher frequency compared to its absorption in a neutral metal carbonyl.
Question 80. Which of the following does not have a metal-carbon bond?
- \(\mathrm{Al}\left(\mathrm{OC}_2 \mathrm{H}_5\right)_3\)
- \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{MgBr}\)
- \(\mathrm{K}\left[\mathrm{Pt}\left(\mathrm{C}_2 \mathrm{H}_4\right) \mathrm{Cl}_3\right]\)
- \(\mathrm{Ni}(\mathrm{CO})_4\)
Answer: 1. \(\mathrm{Al}\left(\mathrm{OC}_2 \mathrm{H}_5\right)_3\)
AI(OC2H5)3 contains bonding through O and thus it does not have a retail-carbon bond.
Question 81. Among the following which is not the π-bonded organometallic compound?
- \(\mathrm{K}\left[\mathrm{PtCl}_3\left(\eta^2-\mathrm{C}_2 \mathrm{H}_4\right)\right]\)
- \(\mathrm{Fe}\left(\eta^5-\mathrm{C}_5 \mathrm{H}_5\right)_2\)
- \(\mathrm{Cr}\left(\eta^6-\mathrm{C}_6 \mathrm{H}_6\right)_2\)
- \(\left(\mathrm{CH}_3\right)_4 \mathrm{Sn}\)
Answer: 4. \(\left(\mathrm{CH}_3\right)_4 \mathrm{Sn}\)
π-bonded organometallic compounds include organometallic compounds of alkenes, alkynes and some other carbon-containing compounds having electrons in their p-orbitals.
Question 82. Which of the following organometallic compounds is σ and π-bonded?
- \(\left[\mathrm{Fe}\left(\eta^5-\mathrm{C}_5 \mathrm{H}_5\right)_2\right]\)
- \(\mathrm{K}\left[\mathrm{PtCl}_3\left(\eta^2-\mathrm{C}_2 \mathrm{H}_4\right)\right]\)
- \(\left[\mathrm{Co}(\mathrm{CO})_5 \mathrm{NH}_3\right]^{2+}\)
- \(\mathrm{Fe}\left(\mathrm{CH}_3\right)_3\)
Answer: 3. \(\left[\mathrm{Co}(\mathrm{CO})_5 \mathrm{NH}_3\right]^{2+}\)
[Co(CO)5NH3]2+: In this complex, Co-atom is attached with NH3 through o bonding and with CO through dative π-bond.
Question 83. Shape of Fe(CO)5 is
- Octahedral
- Square planar
- Trigonal bipyramidal
- Square pyramidal.
Answer: 3. Trigonal bipyramidal
Question 84. In metal carbonyl having general formula M(CO)x where M = metal, x = 4 and the metal is bonded to
- Carbon and oxygen
- C ≡O
- Oxygen
- Carbon.
Answer: 4. Carbon.
In M(CO)4, metal is bonded to the ligands via carbon atoms with both σ and πbond character. Both metal-to-ligand and ligand-to-metal bonding are possible.
Question 85. Which of the following complexes is used to be as an anticancer agent?
- mer- \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_3 \mathrm{Cl}_3\right]\)
- cis- \(\left[\mathrm{PtCl}_2\left(\mathrm{NH}_3\right)_2\right]\)
- cis- \(\mathrm{K}_2\left[\mathrm{PtCl}_2 \mathrm{Br}_2\right]\)
- \(\mathrm{Na}_2 \mathrm{CoCl}_4\)
Answer: 2. cis- \(\left[\mathrm{PtCl}_2\left(\mathrm{NH}_3\right)_2\right]\)
Question 86. Copper sulphate dissolves in excess of KCN to give
- \(\mathrm{Cu}(\mathrm{CN})_2\)
- \(\mathrm{CuCN}\)
- \(\left[\mathrm{Cu}(\mathrm{CN})_4\right]^{3-}\)
- \(\left[\mathrm{Cu}(\mathrm{CN})_4\right]^{2-}\)
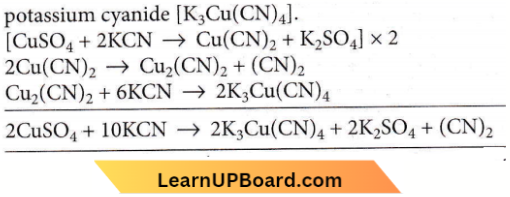
Answer: 3. \(\left[\mathrm{Cu}(\mathrm{CN})_4\right]^{3-}\)
First cupric cyanide is formed which decomposes to give cuprous cyanide and cyanogen gas. Cuprous cyanide dissolves in excess of potassium cyanide to form a complex, potassium cyanide \(\left[\mathrm{K}_3 \mathrm{Cu}(\mathrm{CN})_4\right]\)
Question 87. Which of the following is considered to be an anticancer species?
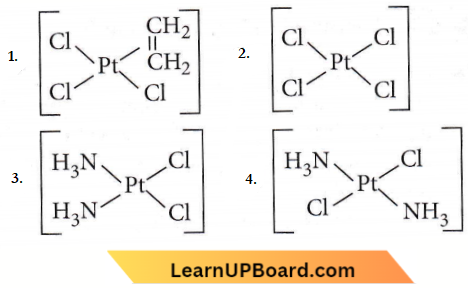
Answer: 3
cis-platin is cls-[PtCl2NH3)2] is used as an anticancer agent.
Question 88. In the silver plating of copper, K[Ag(CN)2] is used instead of AgNO3. The reason is
- A thin layer of Ag is formed on Cu
- More voltage is required
- Ag+ ions are completely removed from the solution
- Less availability of Ag+ ions, as Cu cannot displace Ag from [Ag(CN)–2] ion.
Answer: 4. Less availability of Ag+ ions, as Cu cannot displace Ag from [Ag(CN)2]
Copper being more electropositive readily precipitates silver from their salt (Ag+) solution
⇑ \(\mathrm{Cu}+2 \mathrm{AgNO}_3 \rightarrow \mathrm{Cu}\left(\mathrm{NO}_3\right)_2+\mathrm{Ag}\)
In \(\mathrm{K}\left[\mathrm{Ag}(\mathrm{CN})_2\right]\) solution, a complex anion \(\left[\mathrm{Ag}(\mathrm{CN})_2\right]^{-}\) is
Question 89. CuSO4 when reacts with KCN forms CuCN, which is insoluble in water. It is soluble in excess of KCN, due to the formation of the following complex
- \(\mathrm{K}_2\left[\mathrm{Cu}(\mathrm{CN})_4\right]\)
- \(\mathrm{K}_3\left[\mathrm{Cu}(\mathrm{CN})_4\right]\)
- \(\mathrm{CuCN}_2\)
- \(\mathrm{Cu}\left[\mathrm{KCu}(\mathrm{CN})_4\right]\)
Answer: 2. \(\mathrm{K}_3\left[\mathrm{Cu}(\mathrm{CN})_4\right]\)
Copper sulphate reacts with potassium cyanide giving a white precipitate of cuprous cyanide and cyanogen gas. The cuprolls cyanide dissolves in excess of KCN forming potassium cuprocyanide K3[Cu(CN)2].
⇒ \(2 \mathrm{CuSO}_4+4 \mathrm{KCN} \rightarrow 2 \mathrm{CuCN}+(\mathrm{CN})_2+2 \mathrm{~K}_2 \mathrm{SO}_4\)
⇒ \(\mathrm{CuCN}+3 \mathrm{KCN} \rightarrow \mathrm{K}_3\left[\mathrm{Cu}(\mathrm{CN})_4\right]\)
Question 90. Hypo is used in photography to
- Reduce AgBr grains to metallic silver
- Convert metallic silver to silver salt
- Remove undecomposed silver bromide as a soluble complex
- Remove reduced silver
Answer: 3. Remove undecomposed silver bromide as a soluble complex
Undecomposed AgBr forms a soluble complex with hypo and the reaction is given as:
⇒ \(\mathrm{AgBr}+2 \mathrm{Na}_2 \mathrm{~S}_2 \mathrm{O}_3 \rightarrow \underset{\mathrm{ Soluble complex}}{\mathrm{Na}_3\left[\mathrm{Ag}\left(\mathrm{S}_2 \mathrm{O}_3\right)_2\right]}+\mathrm{NaBr}\)
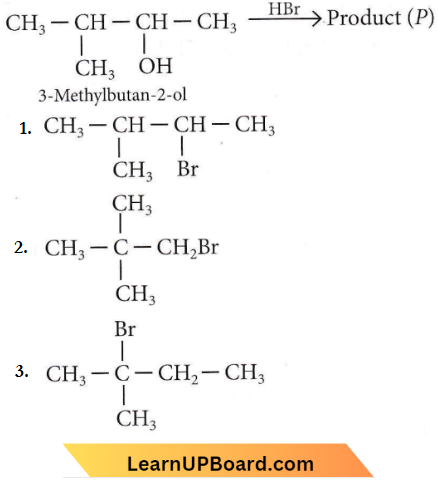
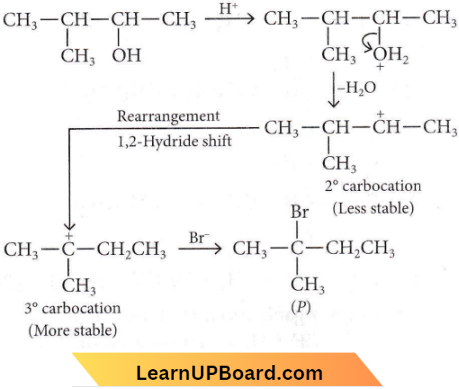
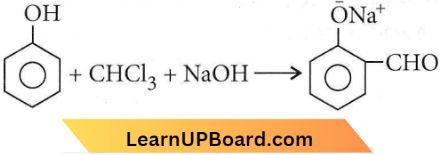
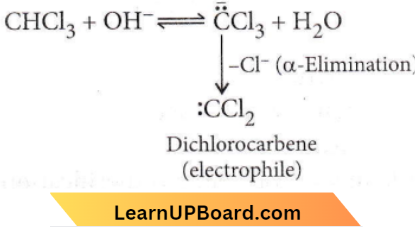
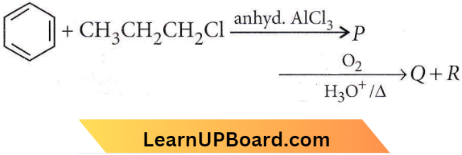
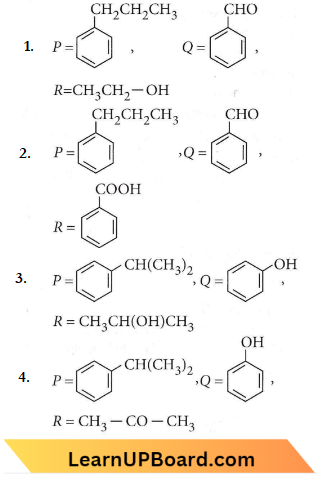
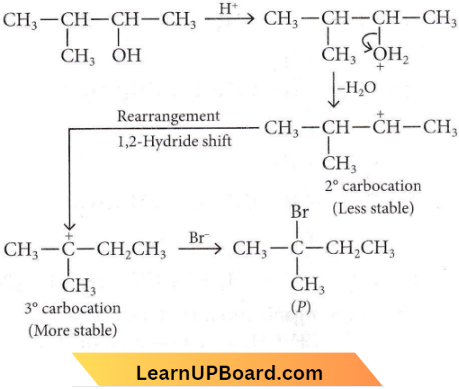
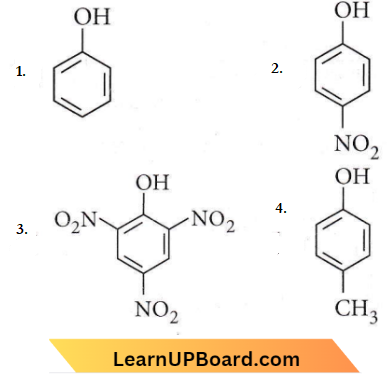
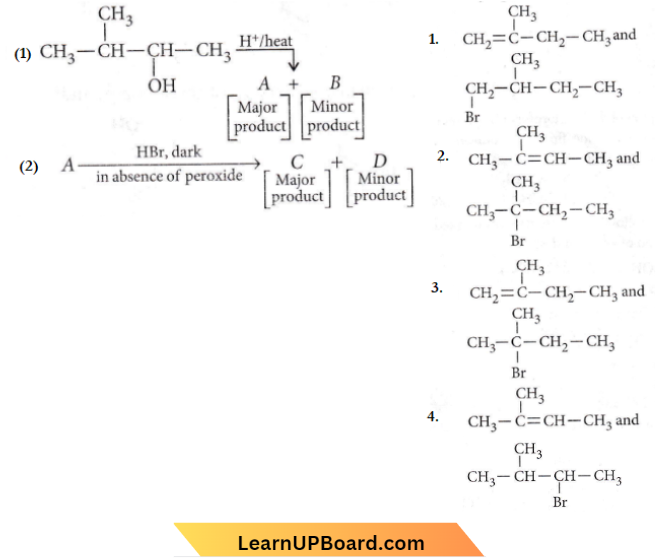
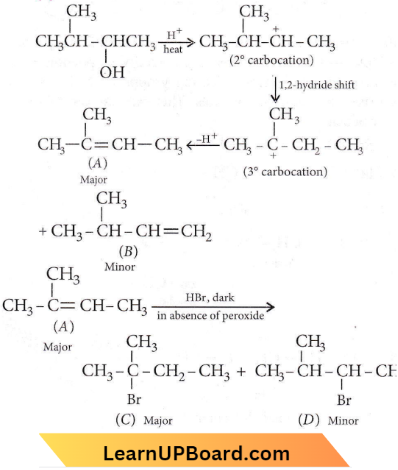
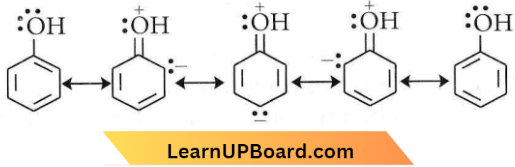
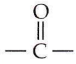 in the -COOH group polarises the O-H bond and increases the acidic strength. Acetic acid is therefore more acidic than phenol or cyclohexanol.
in the -COOH group polarises the O-H bond and increases the acidic strength. Acetic acid is therefore more acidic than phenol or cyclohexanol.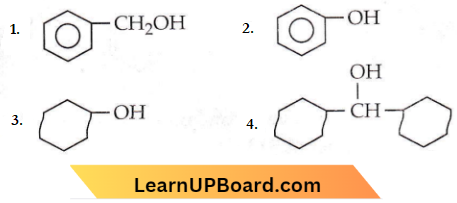
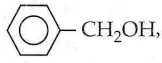
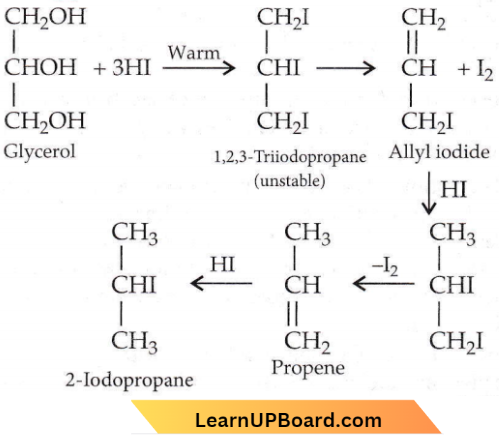
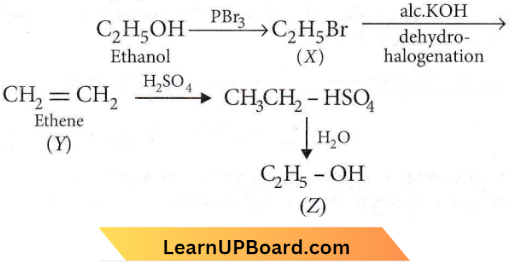
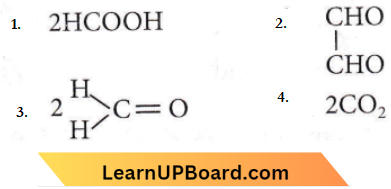
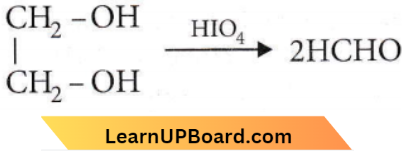
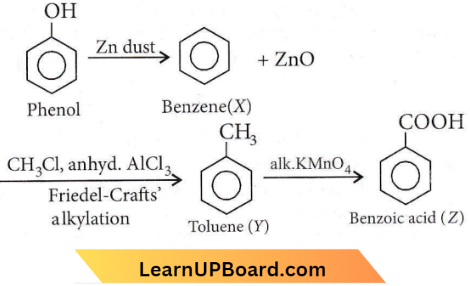
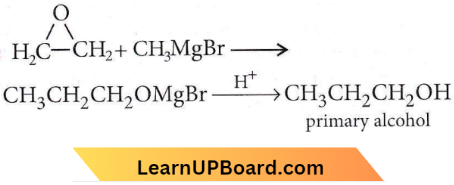
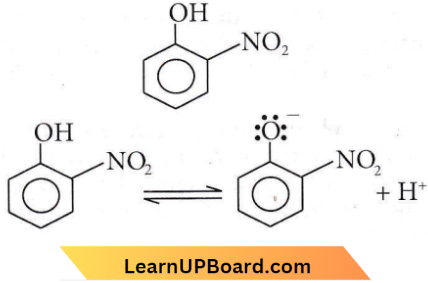
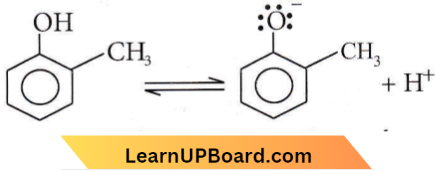
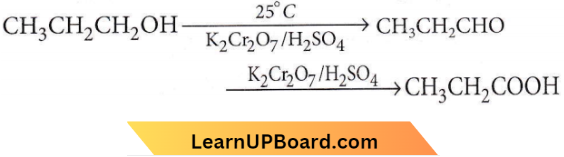
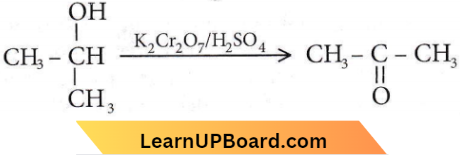
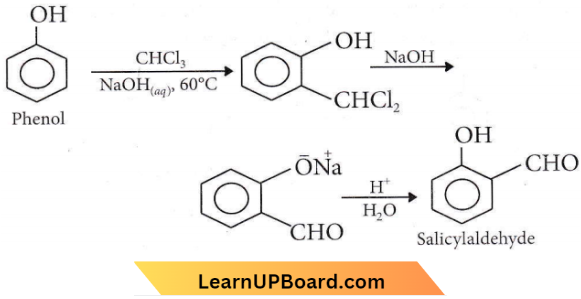
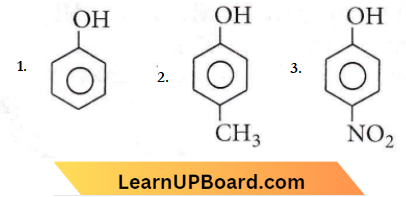
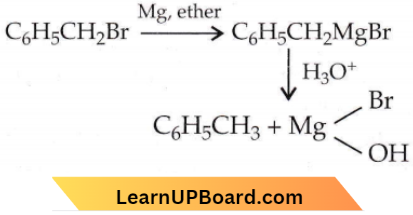
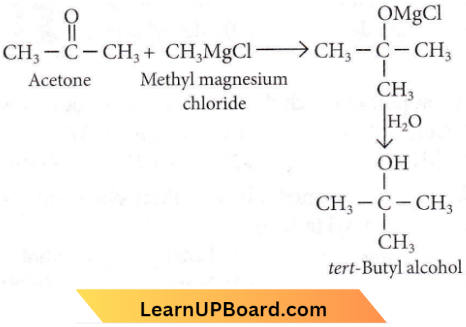
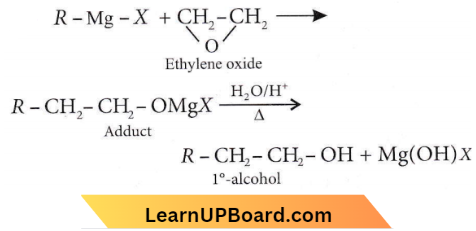
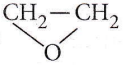 with RMgX leads to the formation of
with RMgX leads to the formation of
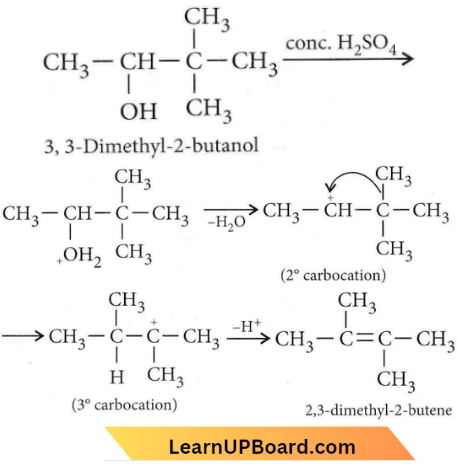
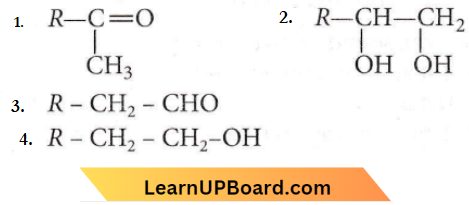
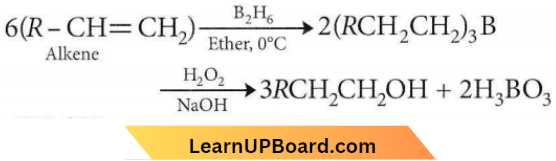
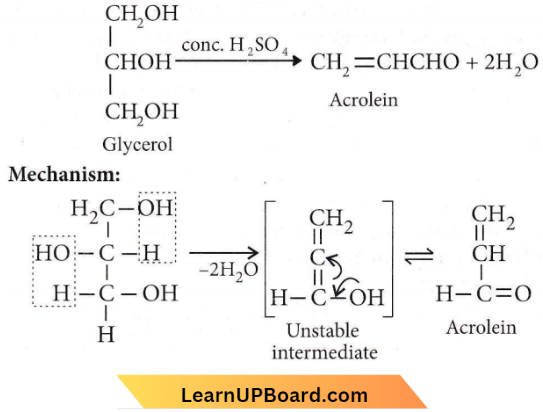
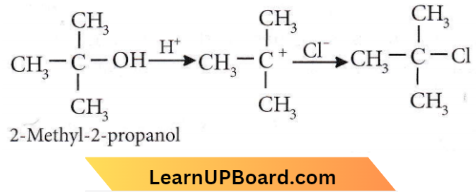
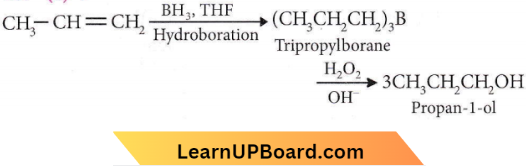
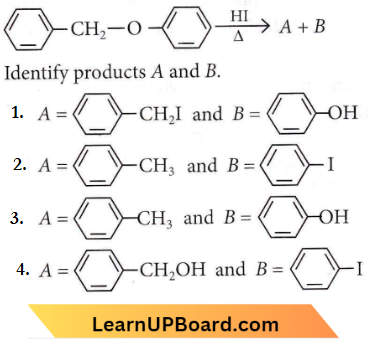
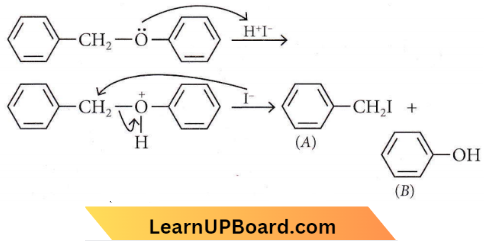
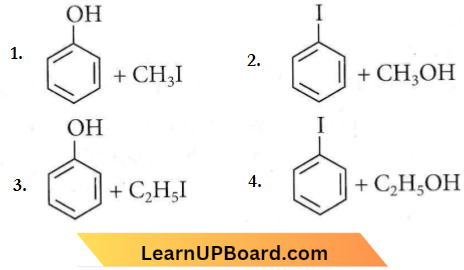
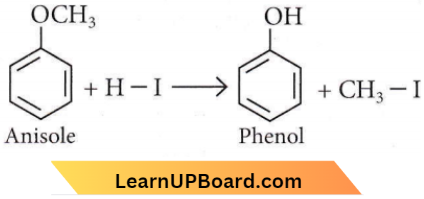
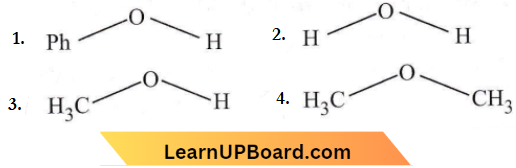
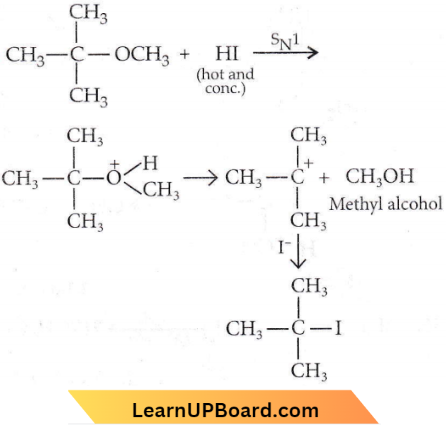
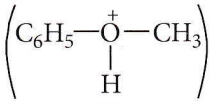 is formed by protonation of ether. The O-CH3 bond is weaker than O-C6H5 bond as O-C6H5 has a partial double bond character. Therefore, the attack by I– ion breaks O-CH3 bond to form CH3l.
is formed by protonation of ether. The O-CH3 bond is weaker than O-C6H5 bond as O-C6H5 has a partial double bond character. Therefore, the attack by I– ion breaks O-CH3 bond to form CH3l.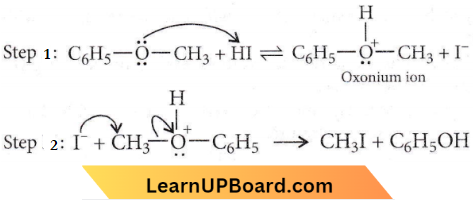
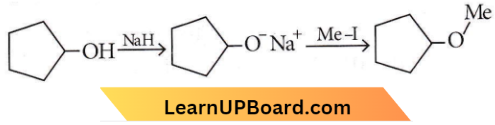
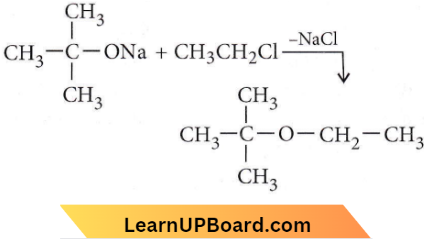
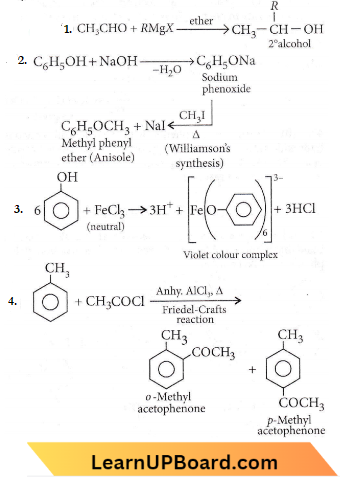
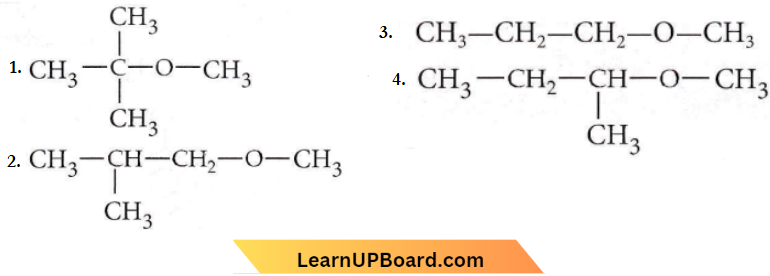
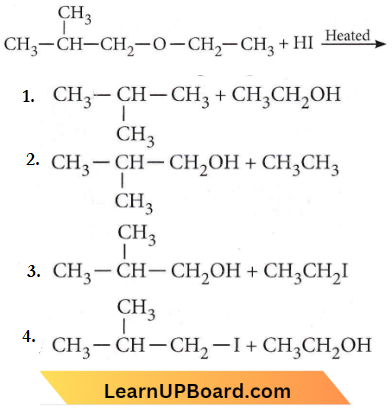
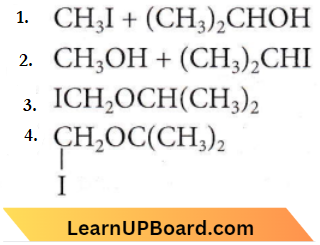

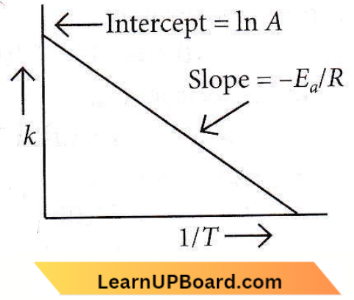
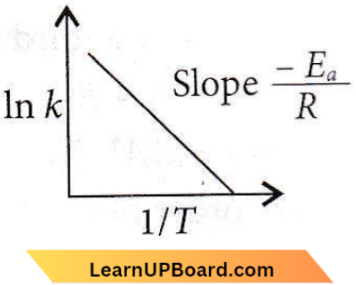
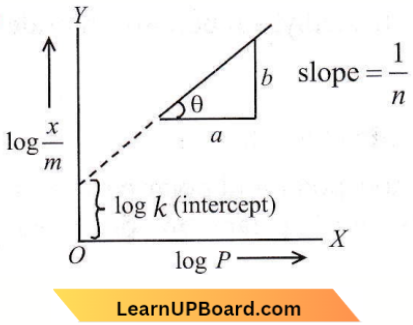
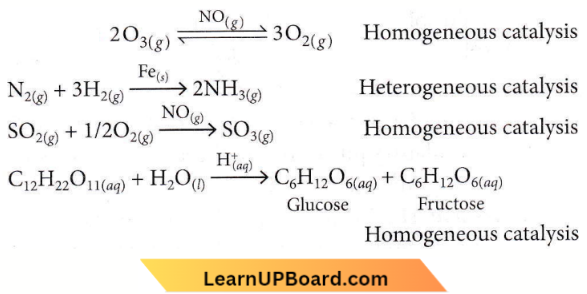
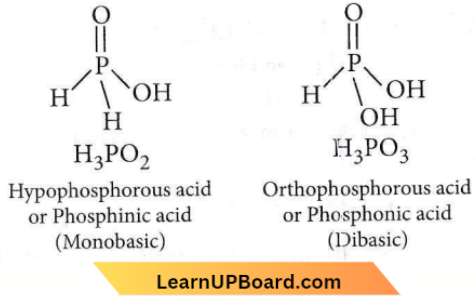
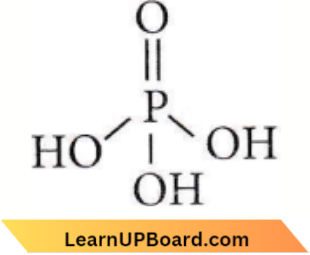
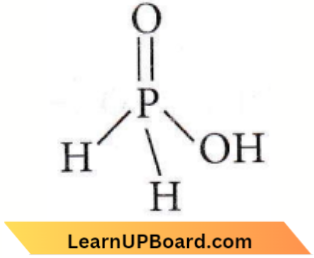
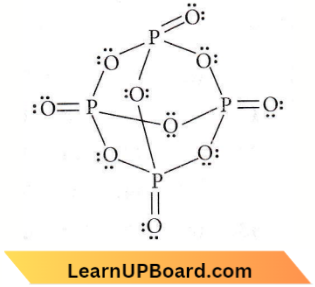
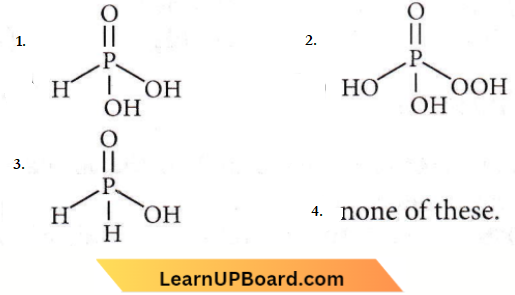
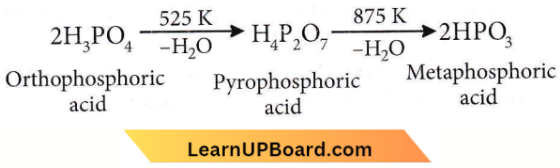
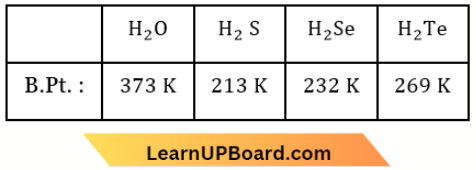
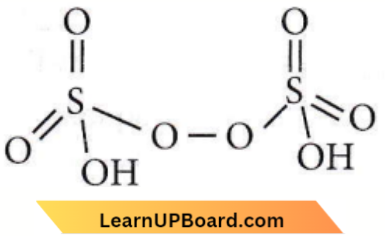
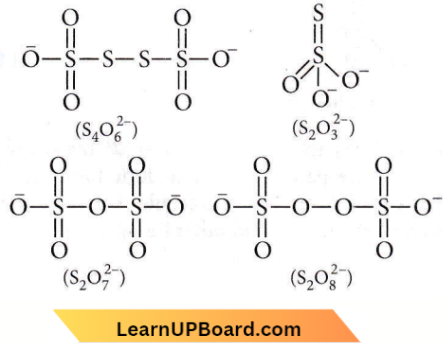
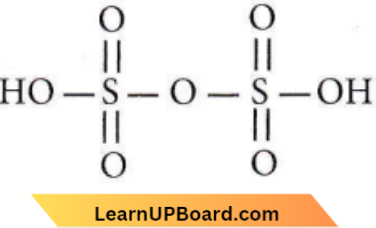

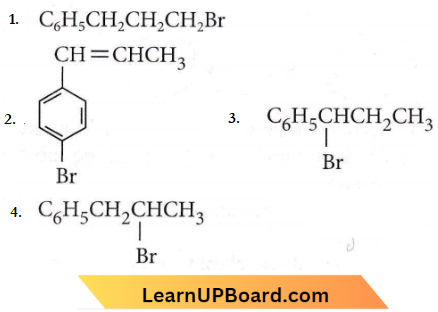
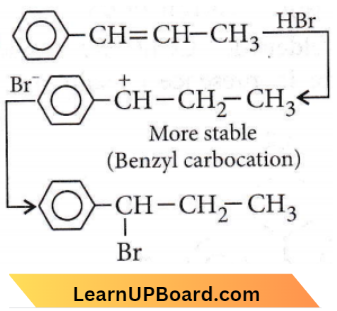
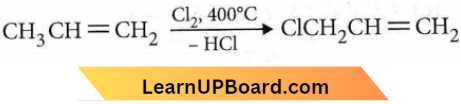
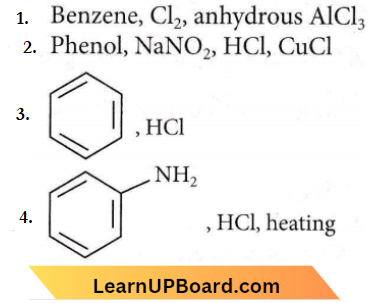
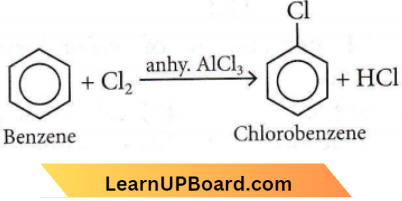
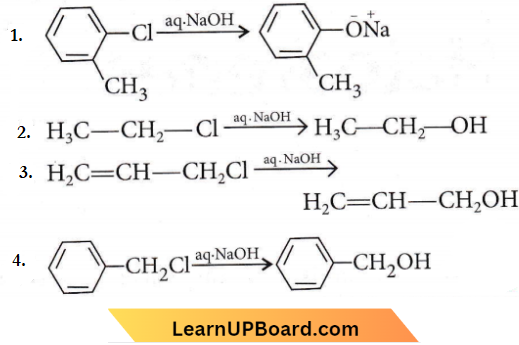
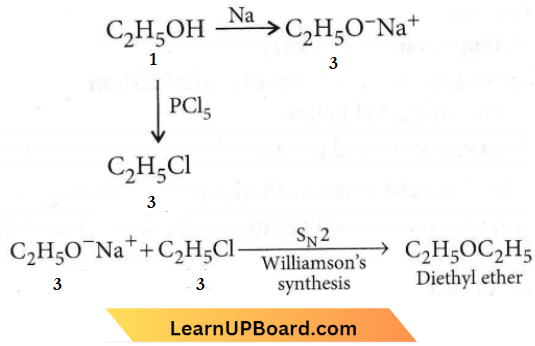
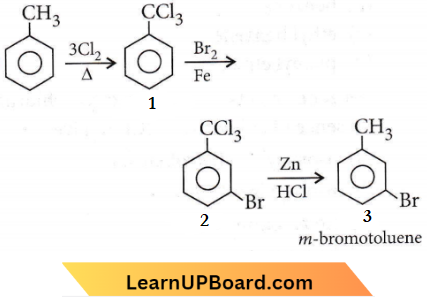
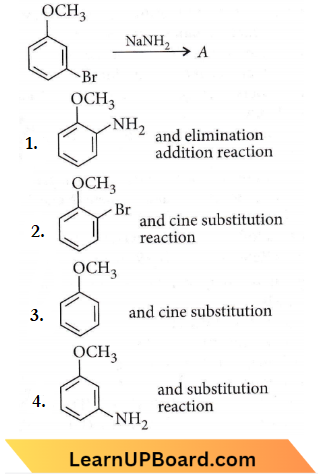
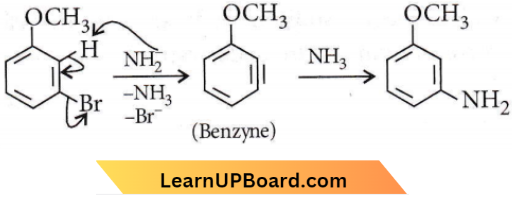
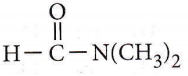
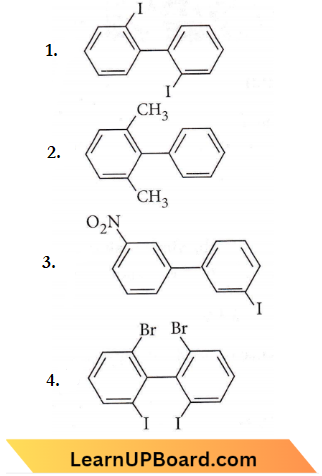
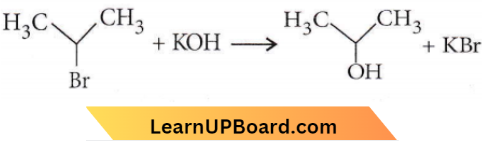
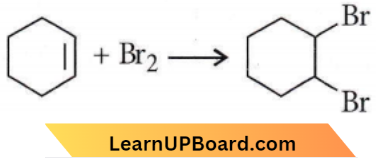
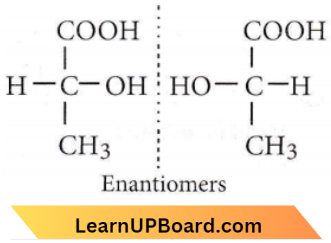
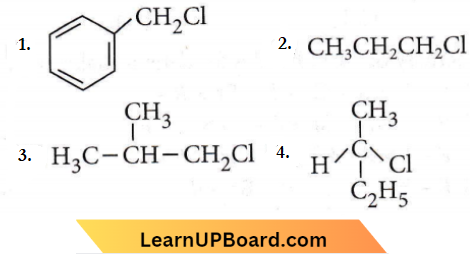
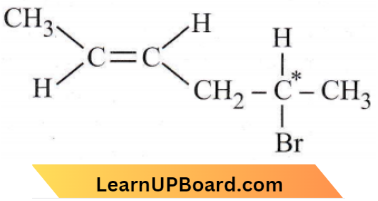
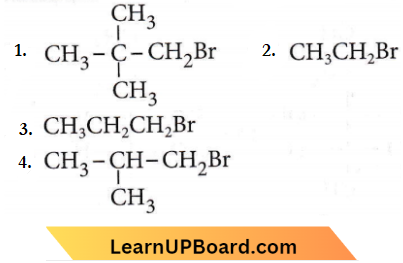
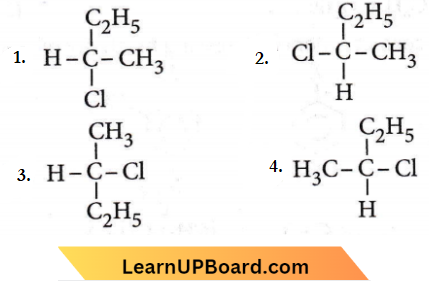
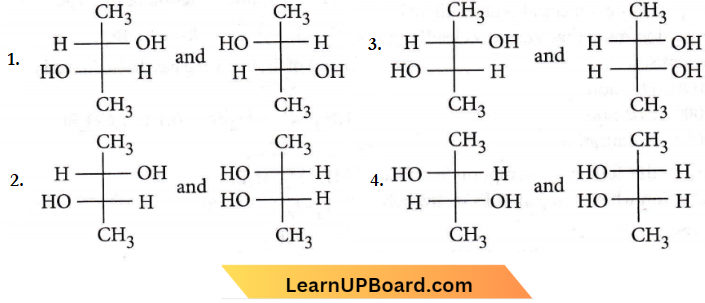
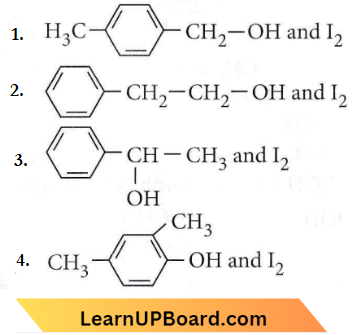
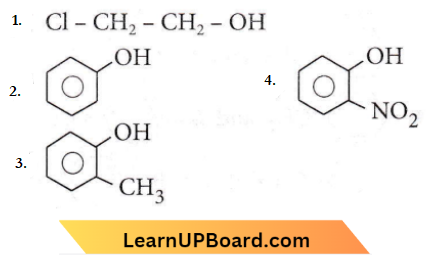
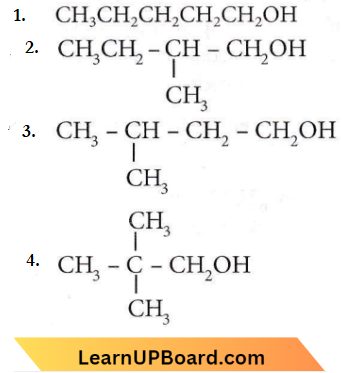
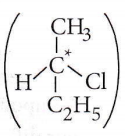 only compound (4) will undergo racemisation.
only compound (4) will undergo racemisation.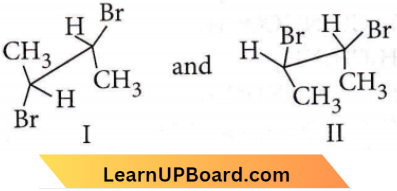
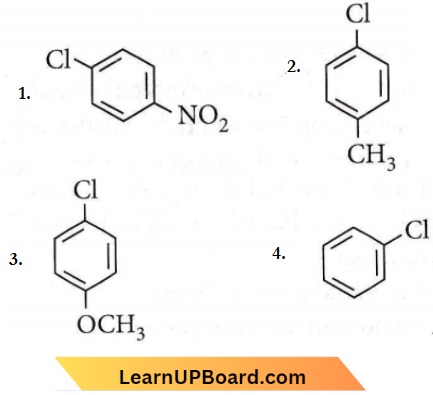
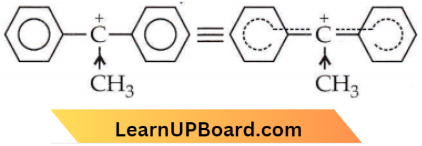
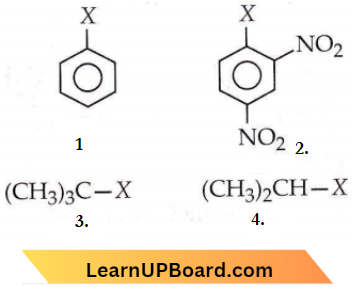
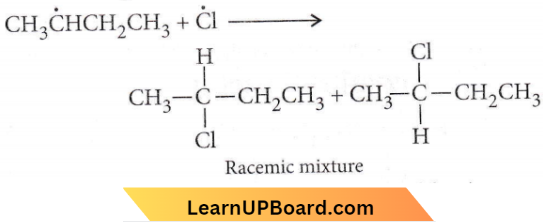
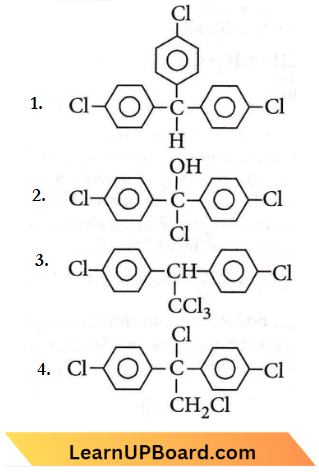
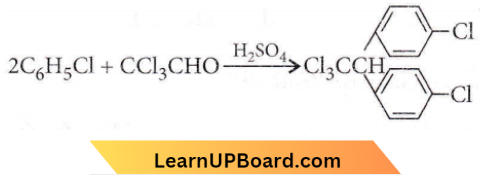
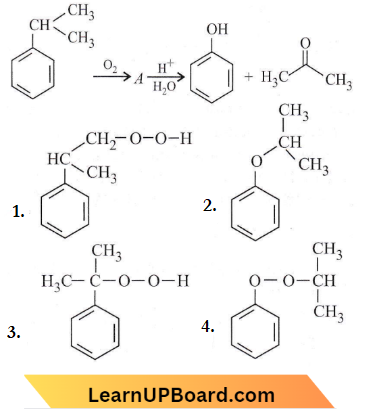
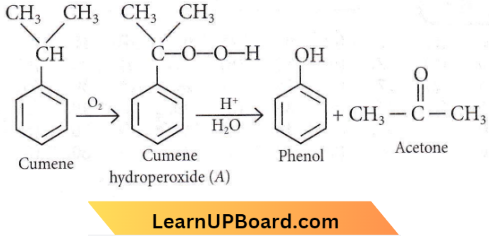
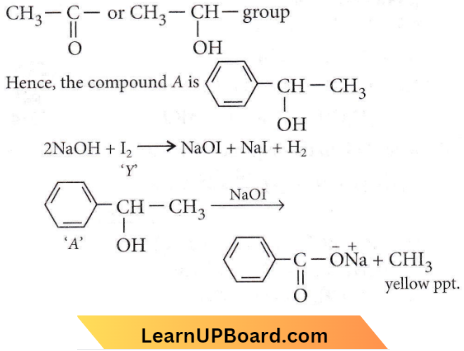
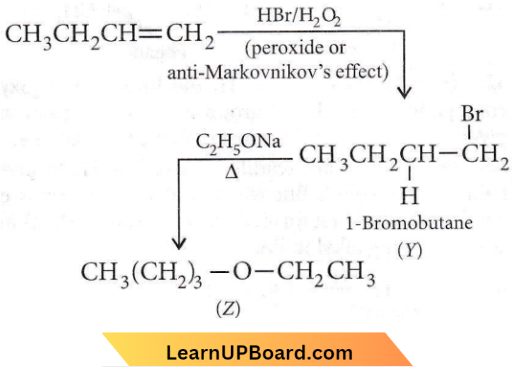
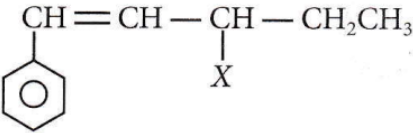
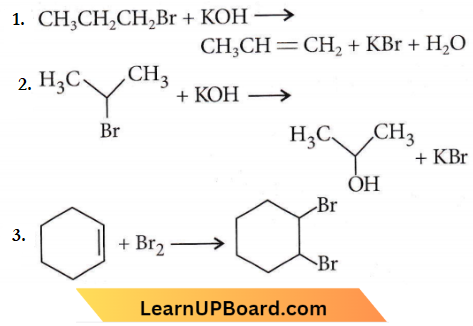
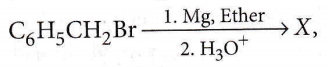 the product ‘X’ is
the product ‘X’ is