Current Electricity MCQs For NEET
NEET Physics For Dual Nature Of Matter And Radiation Multiple Choice Questions
Question 1. In a discharge tube ionization of enclosed gas is produced due to collisions between:
- neutral gas atoms/molecules
- positive ions and neutral atoms/molecules
- negative electrons and neutral atoms/molecules
- photons and neutral atoms/molecules
Answer: 3. negative electrons and neutral atoms/molecules
Negative electrons and neutral atoms collide and cause the ionization of gas enclosed in a discharge tube.
Question 2. J.J. Thomson’s cathode ray tube experiment demonstrated that:
- cathode rays are streams of negatively charged ions
- all the mass of an atom is essentially in the nucleus
- the EM of electrons is much greater than the e/m,
- the EM ratio of the cathode-ray particle changes when a different gas is placed in the discharge tube
Answer: 3. the EM of electrons is much greater than the e/m,
⇒ \(\left(\frac{e}{m}\right)_{\text {electron }} \gg\left(\frac{e}{m}\right)_{\text {proton }}\)
∴ \({\left[\left(\frac{e}{m}\right)_{\text {enti }}=\frac{1}{1837}\left(\frac{e}{m}\right)_{\text {electron }}\right]}\)
Question 3. Which of the following is not the property of cathode rays?
- It produces a heating effect
- It does not deflect in an electric field
- It casts shadow
- It produces fluorescence
Answer: 2. It does not deflect in an electric field
Cathode rays do not deflect in an electric field.
Read and Learn More NEET Physics MCQs
Question 4. A source of light is placed at a distance of 50 cm from a photo cells and the stopping potential is found to be F0. If the distance between the light source and photocells is 25 cm, the new stopping potential will be:
- \(V_0 / 2\)
- \(V_0\)
- \(4 V_0\)
- \(2 V_0\)
Answer: 2. \(V_0\)
Given
A source of light is placed at a distance of 50 cm from a photo cells and the stopping potential is found to be F0. If the distance between the light source and photocells is 25 cm
Stopping potential is independent of distance hence new stopping potential will remain unchanged in required.
Stopping potential = \(V_0\)
Current Electricity MCQs For NEET
Question 5. In the photoelectric emission process from a metal of work function 1.8 eV, the kinetic energy of most energetic electrons is 0.5 eV. The corresponding stopping potential is:
- 1.3 V
- 0.5 V
- 2.3 V
- 1.8 V
Answer: 2. 0.5 V
Given
In the photoelectric emission process from a metal of work function 1.8 eV, the kinetic energy of most energetic electrons is 0.5 eV.
In a photoelectric effect, the maximum kinetic energy of the electron,
Stopping potential = Maximum kinetic energy
e \(\mathrm{~V} =\mathrm{KE}_{\max } \)
∴ \(\mathrm{V} =\frac{\mathrm{KE}_{\max }}{e}=\frac{0.5 e v}{e}=0.5 \mathrm{v}\)
Question 6. Photoelectric emission occurs only when the incident light has more than a certain minimum:
- wavelength
- intensity
- frequency
- power
Answer: 3. frequency
From Einstein’s photoelectric equation
E =\(h\left(v-v_0\right) \)
⇒ \(\frac{1}{2} m v^2 =h\left(v-v_0\right)\)
v \(\geq v_0\)
Question 7. The potential difference that must be applied to stop the fastest photoelectrons emitted by a nickel surface, having work function 5.01 eV, when ultraviolet light of 200 nm falls on it, must be:
- 2.4 V
- – 1.2 V
- – 2.4 V
- 1.2 V
Answer: 4. 1.2 V
We know that the energy of incident light
E=\(e V=6.2 \mathrm{eV}\)
Using Einstein’s photoelectric equation
E =W+e\( V_0 \)
e \(V_0 =E-W=(6.2-5.01)=1.2 \mathrm{eV} \)
∴ \(V_0 =1.2 \mathrm{~V}\)
Question 8. When monochromatic radiation of intensity l falls on a metal surface, the number of photoelectrons and their maximum kinetic energy are N and T respectively. If the intensity of radiation is 21, the number of emitted electrons and their maximum kinetic energy are respectively:
- A and 2T
- 2N and T
- 2N and 2T
- A and T
Answer: 2. 2N and T
The kinetic energy of photoelectrons depends on the frequency of incident radiation and a number of photoelectrons depends upon the intensity.
Question 9. The figure shows a plot of photocurrent versus anode potential for a photo-sensitive surface for three different radiations. Which one of the following is a correct statement?
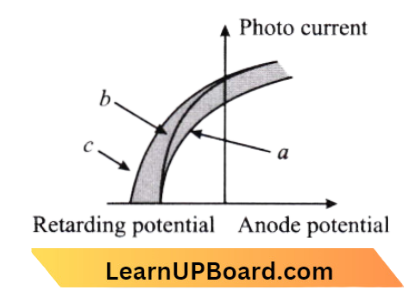
- Curves a and b represent incident radiations of different frequencies and different intensities
- Curves a and b represent incident radiations of the same frequency but of different intensities
- Curves b and c represent incident radiations of different frequencies and different intensities
- Curves b and c represent incident radiations of the same frequency having the same intensity
Answer: 2. Curves a and b represent incident radiations of the same frequency but of different intensities
Curves a and b represent incident radiations of the same frequency but of different intensities.
Current Electricity Questions NEET
Question 10. The number of photoelectrons emitted for light of a frequency v (higher than the threshold frequency v0) is proportional to:
- v = v0
- threshold frequency (v0)
- intensity of light
- frequency of light (v)
Answer: 3. intensity of light
The number of photoelectrons emitted is directly proportional to the intensity of light.
Question 11. A 5-watt source emits monochromatic light of wavelength 5000 Å. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. When the source is moved to a distance of 1.0 m, the number of photoelectrons liberated will be reduced by a factor of:
- 8
- 16
- 2
- 4
Answer: 4. 4
Given
A 5-watt source emits monochromatic light of wavelength 5000 Å. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. When the source is moved to a distance of 1.0 m
Intensity is given by \(\frac{p}{4 \pi d^2}\)
Where p = Power of source
d = distance
I \(\propto \frac{1}{d^2} \)
or \(\frac{I_1}{I_2} =\frac{d_2{ }^2}{d_1{ }^2}\)
or \(\frac{I_1}{I_2} =\left(\frac{1}{0.5}\right)^2 \)
⇒ \(\frac{I_1}{I_2}=4\)
or \(I_2 =\frac{I_1}{4}\)
We know (assume) that light spreads out uniformly in all directions i.e. spherical source
Question 12. A photocell employs the photoelectric effect to convert:
- change in the frequency of light into a change in the electric current.
- change in the frequency of light into a change in electric voltage.
- change in the intensity of illumination into a change in photoelectric current.
- change in the intensity of illumination into a change in the work function of the photocathode.
Answer: 3. change in the intensity of illumination into a change in photoelectric current.
Photocell employs the photoelectric effect to convert the change in the intensity of illumination into a charge in photoelectric current.
Question 13. A photoelectric cell is illuminated by a point source of light 1 m away. When the source is shifted to 2 m then:
- each emitted electron carries one-quarter of the initial energy.
- number of electrons emitted in half the initial number.
- each emitted electron carries half the initial energy.
- number of electrons emitted is a quarter of the initial number.
Answer: 4. number of electrons emitted is a quarter of the initial number.
For a point source, I \(\propto \frac{1}{r^2}\)
NEET Current Electricity MCQs
Question 14. When the intensity of incident light increases:
- photocurrent increase
- photocurrent decreases
- the kinetic energy of emitted photoelectrons increases
- the kinetic energy of emitted photoelectrons decreases
Answer: 1. photocurrent increase
Einstein’s photoelectric effect theory states that a single incident photon ejects a single electron. As a result, as the intensity increases, the number of incident photons increases, and the number of emitted electrons increases, the photocurrent increases.
We have, maximum energy of electrons =\(\frac{1}{2} m v^2 max\)
⇒ \(\frac{1}{2} m v^2{ }_{\max }=e V_0 \)
where \(V_0\) is stopping potential.
Thus, since, the stopping potential is unaffected by increasing ray intensity, the maximum kinetic energy of the electrons is independent of the intensity of the incident rays.
Question 15. The cathode of a photoelectric cell is changed such that the work function changes from W1 to W2 (W2 > W1). If the current before and after changes are I1 and I2, all other conditions remaining unchanged, then (assuming hv> W2)
- \(I_1=I_2\)
- \(I_1<I_2\)
- \(I_1>I_2\)
- \(I_1<I_2<2 I_1\)
Answer: 1. \(I_1=I_2\)
By the work function of a metal, it means that the minimum energy required for the electron in the highest level of the conduction band to get out of the metal. The work function does not affect photoelectric current as long as hv > W0. The photoelectric current is proportional to the intensity of incident light. Since there is no change in the intensity of light, hence I1 = I2
Question 16. The threshold frequency for the photoelectric effect on sodium corresponds to a wavelength of 5000Å. Its work function is:
- 4 x 10-19 J
- 1 J
- 2 x 10-19 J
- 3 x 10-19 J
Answer: 1. 4 x 10-19 J
When a photon of light with frequency v strikes a photosensitive metal surface, its energy (hv) is consumed in two ways. A portion of the photon’s energy is utilized to liberate the electron from the metal surface, which is equal to the metal’s work function \(W_0\). metal.
⇒ \(W_0 = hv_0\)
(where, \(v_0\) is threshold frequency)
⇒ \(W_0 =\frac{h c}{\lambda_0}\)
Here,\(\lambda_0 =5000 Å \)
= 5000 \(\times 10^{-10} \mathrm{~m} \)
⇒ \(W_0 =\frac{6.63 \times 10^{-34} \times 3 \times 10^8}{5000 \times 10^{-10}} \)
= 4 \(\times 10^{-19} \mathrm{~J}\)
Question 17. When two monochromatic light of frequency v and \(\frac{v}{2}\) are incident on a photoelectric metal, their stopping potential becomes \(\frac{V_{\mathrm{s}}}{2}\) and \(V_{\mathrm{s}}\)respectively. The threshold frequency for this metal is:
- 2v
- 3v
- \(\frac{2}{3} v\)
- \(\frac{3}{2} v\)
Answer: 4. \(\frac{3}{2} v\)
According to the photoelectric effect,
E =\(h v_o+e v_o \)
⇒ \(h v =h v_o+\frac{e v_s}{2}\) → Equation 1
⇒ \(\frac{h v}{2} =h v_o+e v_s \) → Equation 2
⇒ \(\frac{h v}{2} =\frac{-e v_s}{2}\)
⇒ –\(h v =e v_s \) → Equation 3
Putting (3) in eq (1), we get
∴ h v=\(h v_o-\frac{h v}{2}\) , So \(v_o=\frac{3}{2} v\)
Question 18. The work function of a photosensitive material is 4.0 eV. The longest wavelength of light that can cause photon emission from the substance is (approximately):
- 3100 nm
- 3600 nm
- 31 nm
- 310 nm
Answer: 4. 310 nm
The work function of the material is
⇒ \(\mathrm{W}=h v=\frac{h c}{\lambda}\)
Here, h= Planck’s constant
h=6.63 \(\times 10^{-34} \mathrm{~J} / \mathrm{s}\)
c = speed of light =\(3 \times 10^8 \mathrm{~m} / \mathrm{s}\)
⇒ \(\lambda\)= wavelength of light
And \(\mathrm{W}=v=4 \times 1.6 \times 10^{-19} \mathrm{~K}\)
From eq. (1) and (2) we can write
4 \(\times 1.6 \times 10^{-19} =\frac{6.63 \times 10^{-34} \times 3 \times 10^8}{\lambda}\)
⇒ \(\lambda =\frac{6.63 \times 10^{-34} \times 3 \times 10^8}{4 \times 1.6 \times 10^{-19}}\)
∴ \(\lambda =3.108 \times 10^{-7} \mathrm{~m}=310 \mathrm{~nm}\)
NEET Current Electricity MCQs
Question 19. When the light 2v0 (where v0 is threshold frequency), in an incident on a metal plate, the maximum velocity of electrons emitted is v1. When the frequency of the incident radiation is increased to 5v0. the maximum velocity of electrons emitted from the same plate is v2. The ratio of V1 to v2 is:
- 4: 1
- 1: 4
- 1: 2
- 2: 1
Answer: 3. 1: 2
From Einstein’s photoelectric equation, we can write
E=\(W_0+\frac{1}{2} m v^2\) → Equation 1
Where, \(W_0\)= work function
⇒ \(\frac{1}{2} m v^2\)= Kinetic energy
and E=h v
When the incident frequency of light is v=2\( v_0 \)then eq.
(1) become
h\(\left(2 v_0\right) =h v_0+\frac{1}{2} m v_1^2\)
⇒ \(h v_0 =\frac{1}{2} m v_1^2\) → Equation 2
When the incident frequency of light is v=2 \(v_0\) then eq.
(1) becomes
⇒ \(h\left(5 v_0\right) =h v_0+\frac{1}{2} m v_2^2 \)
⇒ \(4 h v_0 =\frac{1}{2} m v_2^2\) → Equation 3
From eq. (2) and eq. (3) we have
⇒ \(\frac{h v_0}{4 h v_0}=\frac{\frac{1}{2} m v_1^2}{\frac{1}{2} m v_2{ }^2}\)
⇒ \(\frac{v_1{ }^2}{v_2{ }^2}=\frac{1}{4} \)
∴ \(\frac{v_1}{v_2}=1: 2 \)
Question 20. The photoelectric threshold wavelength of silver is 3250 x 10-10 m. The velocity of the electron ejected from a silver surface by ultraviolet light of wavelength 2536 x 10-10 m is (Given, h = 4.14 x 10-15 eVS and c = 3 x 108 ms-1):
- = 6 x 105 ms-1
- = 0.6 x 106 ms-1
- = 61 x 103 ms-1
- = 0.3 x 106 ms-1
Answer: 1. = 6 x 105 ms-1
Given : Threshold wavelength \(\lambda_0=3250 \times 10^{-10} \mathrm{~m}\)
Wavelength of UV light \(\lambda=2536 \times 10^{-10} \mathrm{~m}\)
We know that Einstein’s photoelectric equation is.
E =k+W
h v =\(\frac{1}{2} m v^2+h v_0 \)
W = work function =h \(v_0\)
⇒ \(\frac{1}{2} m v^2 =h v-h v_0=h\left(v-v_0\right)\)
=\(h c\left(\frac{1}{\lambda}-\frac{1}{\lambda_0}\right)\)
velocity , v=\(\sqrt{\frac{2 h c}{m_0}\left(\frac{1}{\lambda}-\frac{1}{\lambda_0}\right)}\)
= \(\sqrt{\frac{2 \times 6.02 \times 10^{-34} \times 3 \times 10^8}{9.1 \times 10^{-31}}\left(\frac{3250-2536}{3250 \times 2536}\right)} \)
= 0.6 \(\times 10^6 \mathrm{~ms}^{-1}\)
= 6 \(\times 10^5 \mathrm{~ms}^{-1}\)
Current Electricity Mcqs With Answers
Question 21. Photons with energy 5 eV are incident on a cathode C in a photoelectric cell. The maximum energy of emitted photoelectrons is 2 eV. When photons of energy 6 eV are incident on C, no photoelectrons will reach the anode A, if the stopping potential of A relative C is:
- – 3 V
- + 4 V
- – 1 V
- – 3 V
Answer: 4. – 3 V
From the photoelectric equation, we have
⇒ \(h v=W+\frac{1}{2} m v_0^2\)
where W= work function
⇒ \(\mathrm{KE}=h v-W\)
putting the given values
⇒ \(2 e \mathrm{~V} =5 e \mathrm{~V}-W \)
W =3 e v
=\(3 \mathrm{~V}\)
⇒ \(V_{\text {cathod }}-V_{\text {anode }} =3 \mathrm{~V}\)
∴ \(V_{\text {anode }}-V_{\text {cathod }} =-3 \mathrm{~V}\)
Question 22. A photoelectric surface is illuminated successively by monochromatic light of wavelength \(\lambda\) and \(\frac{\lambda}{2}\). If the maximum kinetic energy of the emitted photoelectrons in the second case is 3 times that in the first case, the work function of the surface of the material is: (h = Planck’s constant, c = speed of light)
- \(\frac{h c}{2 \lambda}\)
- \(\frac{h c}{\lambda}\)
- \(\frac{2 h c}{\lambda}\)
- \(\frac{h c}{3 \lambda}\)
Answer: 1. \(\frac{h c}{2 \lambda}\)
From Einstein’s photoelectric equation
h v=W+\(K_{\max }\)
where, W= work function
⇒ \(K_{\max }\)= kinetic energy
⇒ \(K_{\max }\)=h v-W
\(K_{\max }=\frac{h c}{\lambda}-W\) → Equation 1
In the question, the maximum kinetic of the emitted electron is 3 times that in the first case.
⇒ \(K_{\max }=\frac{h c}{\frac{\lambda}{2}}-W\) → Equation 2
From eq. (1) and (2),
∴ \(\mathrm{W}=\frac{h c}{2 \lambda}\)
Question 23. When the energy of the incident radiation is increased by 20%, the kinetic energy of the photoelectrons emitted from a metal surface increases from 0.5 eV to 0.8 eV. The work function of the metal is :
- 0.65 eV
- 1.0 eV
- 1.3 eV
- 1.5 eV
Answer: 2. 1.0 eV
From Einstein’s electrons photoelectric equation
h v =\(W-(\mathrm{KE})_{\max }\)
K.E.\( _{\max } =h v-W\) → Equation 1
In the first condition, 0.5=E-W → Equation 2
In the second condition, 0.8=1.2 E-W → Equation 3
From (2) and (3),
-0.3 =-0.2 E
⇒ \(\mathrm{E} =\frac{0.3}{0.2}=1.5 \mathrm{eV}\)
Putting in eq. (2) we get,
0.5 =1.5-W
W =1.5-0.5=1 eV
Question 24. For photoelectric emission from certain metal the cut-off frequency is v. If radiation of frequency 2v impinges on the metal plate, the maximum possible velocity of the emitted electron will be (m is the electron mass):
- \(\sqrt{\frac{h v}{(2 m)}}\)
- \(\sqrt{\frac{h v}{m}}\)
- \(\sqrt{\frac{2 h v}{m}}\)
- 2 \(\sqrt{\frac{h v}{m}}\)
Answer: 3. \(\sqrt{\frac{2 h v}{m}}\)
We know that, \(\frac{1}{2} m v_{\max }{ }^2\)=h v
⇒ \(v_{\max }^2=\frac{2 h v}{m}\)
∴ \(v_{\max }=\sqrt{\frac{2 h v}{m}}\)
Current Electricity Mcqs With Answers
Question 25. Two radiations of photon energies 1 eV and 2.5 eV, successively illuminate a photosensitive metallic surface of work function 0.5 eV. The ratio of the maximum speeds of the emitted electron is:
- 1:4
- 1: 2
- 1: 1
- 1: 5
Answer: 2. 1: 2
⇒ \(v_{\max } =\sqrt{\frac{2}{m}\left(K E_{\max }\right)}\)
= \(\sqrt{\frac{2}{m}\left(E_{p h}-W\right)}\)
∴ \(\frac{v_1}{v_2} =\sqrt{\frac{1-0.5}{25-0.5}}=\sqrt{\frac{0.2}{2}}\)=1: 2
Question 26. The threshold frequency for a photo-sensitive metal is 3.3 x 1014Hz. If the light of frequency 8.2 x 1014 Hz is incident on this metal, the cut-off voltage for the photoelectric emission is near:
- 2 V
- 13 V
- 5 V
- 1V
Answer: 1. 2 V
Given,
Threshold frequency \(v_0=3.3 \times 10^{14} \mathrm{~Hz}\) and light of frequency v=8.2 \(\times 10^{14} \mathrm{~Hz}\)
Then we know from Einstein’s photoelectric equation
⇒ \(e V_0=E-V\)
⇒ \(V_0=\frac{E-V}{e}\)
⇒ \(V_0=\frac{h\left(V-V_0\right)}{e}\)
⇒ \(V_0=\frac{6.62 \times 10^{-34}\left(8.2 \times 10^{14}-3.3 \times 10^{14}\right)}{1.6 \times 10^{-19}}\)
⇒ \(V_0=\frac{6.62 \times 10^{-34}}{1.6} \times 4.9 \times 10^{33} \)
⇒ \(V_0=\frac{6.02 \times 4.9 \times 10^{-1}}{1.6}\)
∴ \(V_0\)=2 volt
Current Electricity Mcqs With Answers
Question 27. The work function of the surface of a photosensitive material is 6.2 eV. The wavelength of the incident radiations for which the stopping potential is 5 V lies in the:
- ultraviolet region
- visible region
- infrared region
- X-ray region
Answer: 1. ultraviolet region
Using Einstein’s photoelectric equation
Where, \(K E_{\max }\)=E-f
f= work function
⇒ \(K E_{\max }\)= Maximum kinetic energy
h v=\(e V_0+f\)
h v=5 \(\mathrm{eV}+6.2 \mathrm{eV}=11.2 \mathrm{eV}\)
⇒ \(\lambda=\left(\frac{12400}{11.2 \mathrm{eV}}\right)=1.104 \times 10^{-7}\)
Hence the radiation lies in the ultraviolet region.
Question 28. When photons of energy hv fall on an aluminum plate (of work function E0), photoelectrons of maximum kinetic energy K are ejected. If the frequency of radiation is doubled, the maximum kinetic energy of the ejected photoelectrons will be:
- K+hv
- K + E0
- 2K
- K
Answer: 1. K+hv
According to a given situation,
h v=\(E_0+K \text { and } 2 h v=E_0+K^{\prime} \)
\(K^{\prime}\)=K+h v
Question 29. The work functions for metal A, B, and C is respectively 1.92 eV, 2.0 eV, and 5 eV. According to Einstein’s equation, the metals which will emit photoelectrons for a radiation of wavelength 4100 Å is/are:
- none
- An only
- A and B only
- all the three metals
Answer: 3. A and B only
The energy of an electron with an associated wavelength of 4100 Å is:
⇒ \(\frac{h c}{\lambda} =4.845 \times 10^{-19} \mathrm{~J} \)
= 3.024 eV
This incident electron would emit photons from metals whose work potential is less than its energy.
Question 30. The photosensitive metallic surface has a work function hv0 fall on this surface, the electrons come out with a maximum velocity of 4 x 106 m/s. When the photon energy is increased to 5hv0. Then, the maximum velocity of photoelectrons will be:
- 2 x 107 m/s
- 2 x 106 m/s
- 8 x 106 m/s
- 8 x 105 m/s
Answer: 3. 8 x 106 m/s
Given
The photosensitive metallic surface has a work function hv0 fall on this surface, the electrons come out with a maximum velocity of 4 x 106 m/s. When the photon energy is increased to 5hv0.
According to the question
⇒ \(2 h v_0=h v_0+\frac{1}{2} m\left(4 \times 10^6\right)^2\) → Equation 1
⇒ \(5 h v_0=h v_0+\frac{1}{2} m v^2\) → Equation 2
Divide eq. (2) by eq. (1), we get
⇒ \(v_1^2 =4 v_1^2 \) or \(v_2=2 v_1 \)
⇒ \(v_2 =2 \times 4 \times 10^6 \)
= 8 \(\times 10^6 \mathrm{~m} / \mathrm{s}\)
Question 31. According to Einstein’s photoelectric equation, the graph between the kinetic energy of the photoelectron ejected and the frequency of incident radiation is:
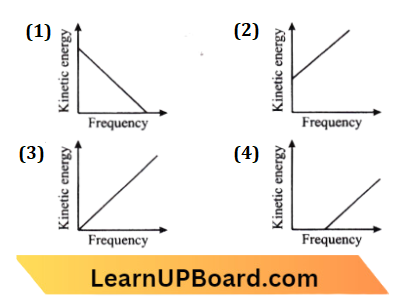
Answer: 4.
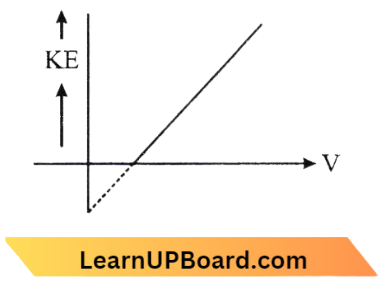
∴ \(h v=\phi+k \mathrm{E}_{\max }\)
Neet Physics Important Questions
Question 32. When ultraviolet rays incident on a metal plate then the photoelectric effect does not occur, it occurs by the incidence of:
- infrared rays
- X-rays
- radio wave
- light wave
Answer: 2. X-rays
Question 33. A light source is at a distance d from a photoelectric cell, then the number of photoelectrons emitted from the cell is n. If the distance of the light source and the cell is reduced to half, then the number of photoelectrons emitted will become:
- \(\frac{n}{2}\)
- 2n
- 4n
- n
Answer: 3. 4n
Given
A light source is at a distance d from a photoelectric cell, then the number of photoelectrons emitted from the cell is n. If the distance of the light source and the cell is reduced to half
We have, the intensity of the light source,
I=\(\frac{1}{d^2}\)
where d is the distance or light source from the cell. So, for two different cases of intensities, we have,
⇒ \(\frac{I_1}{I_2}=\left(\frac{d_2}{d_1}\right)^2=\left(\frac{1}{2}\right)^2=\frac{1}{4}\)
⇒ \(I_2=4 I_1\)
A number of photoelectrons emitted is directly proportional to intensity, so the number of photoelectrons emitted will become 4 times, i.e. 4 n
Question 34. Einstein’s work on photoelectric effect gives support to:
- \(E=m c^2\)
- E = hv
- h v=\(\frac{1}{2} m v^2\)
- E=\(\frac{h}{\lambda}\)
Answer: 2. E = hv
In 1905, Einstein observed that the photoelectric effect could be explained if the energy in light was concentrated in small packets, or photons, instead of spread out over wavefronts. The energy hv is contained in each photon of light with frequency v. Hence, Einstein’s work on the photoelectric effect supports
E = hv
Question 35. The energy of a photon of light is 3 eV. Then the wavelength of the photon must be:
- 4125 nm
- 412.5 nm
- 41250 nm
- 4nm
Answer: 2. 412.5 nm
If photon energy is expressed in (eV) and wavelength is expressed (in Å), then photon energy is given by
E=\(\frac{h c}{\lambda}=\frac{12375}{\lambda(Å)} \mathrm{eV}\)
⇒ \(\lambda =\frac{12375}{E(\mathrm{eV})}Å [ h c=12375 \mathrm{eV}-Å]\)
=\(\frac{12375}{3(\mathrm{eV})}Å=4125 Å=412.5 \mathrm{~nm}\)
Question 36. If the threshold wavelength for a certain metal is 2000 Å, then the work function of the metal is:
- 6.2 J
- 6.2 eV
- 6.2 MeV
- 6.2 KeV
Answer: 2. 6.2 eV
The work function is the minimum energy required to remove an electron from a metal surface without releasing any kinetic energy.
⇒ \(W_0=h v_0\)
Given wavelength, \(\lambda=2000 Å=2000 \times 10^{-10} \)
⇒ \(\mathrm{W}_0=\frac{6.6 \times 10^{-34} \times 3 \times 10^8}{2000 \times 10^{-10}}\)
=9.9 \(\times 10^{-19} \mathrm{~J}=\frac{9.9 \times 10^{-19}}{1.6 \times 10^{-19}} \)
⇒ \({[1 \mathrm{eV}=1.6 \times 10^{-19} \mathrm{~J}]}\)
=6.2\( \mathrm{eV}\)
Neet Physics Important Questions
Question 37. The number of photons per second on average emitted by the source of monochromatic light of wavelength 600 nm, when it delivers the power of 3.3 x 10-3 watts will be (h = 6.6 x 10-34 Js):
- 1018
- 1017
- 1016
- 1015
Answer: 3. 1016
Given, \(\frac{n}{t}\)= ?
⇒ \(\lambda=600 \mathrm{~nm} \)
⇒ \(\lambda=600 \times 10^{-9}\)
⇒ \(\lambda=6 \times 10^{-7} \mathrm{~m} \)
P=3.3 \(\times 10^{-3} watt \)
We know power, P=\(\frac{E}{t}=\frac{n h v}{t}\)
⇒ \(\frac{n}{t} =\frac{\mathrm{P}}{h v}=\frac{\mathrm{P} \lambda}{h c}\)
⇒ \(\frac{n}{t} =\frac{3.3 \times 10^{-3} \times 6 \times 10^{-7}}{6.6 \times 10^{-34} \times 3 \times 10^8}\)
∴ \(\frac{n}{t} =10^{-3-7+34-8}=10^{16}\)
Question 38. Light with an energy flux of 25 x 104 Wm-2 falls on a perfectly reflecting surface at normal incidence. If the surface area is 15 cm², the average force exerted on the surface is :
- 1.25 \(\times 10^{-6} \mathrm{~N}\)
- 2.50 x HT6 N
- 1.20 x 10-6 N
- 12.5 x 10-6 N
Answer: 1. 25 \(\times 10^{-6} \mathrm{~N}\)
We can write,
⇒ \(F_{a v} =\frac{\Delta P}{\Delta t} \)
= \(\frac{2 I}{c . A t} A\)
= \(\frac{2 \times 25 \times 10^4}{3 \times 10^8} \times 15 \times 10^{-4}\)
= \(\frac{10 \times 25 \times 10^4 \times 10^{-4}}{10^8}\)
= 1.25 \(\times 10^{-6} \mathrm{~N}\)
Question 39. A 200 W sodium street lamp emits yellow light of wavelength 0.6 μm. Assuming it to be 25% efficient in converting electrical energy to light, the number of photons of yellow light it emits per second is:
- 1.5 x 1020
- 6 x 1018
- 62 x 1020
- 3 x 1019
Answer: 1. 1.5 x 1020
Given
A 200 W sodium street lamp emits yellow light of wavelength 0.6 μm. Assuming it to be 25% efficient in converting electrical energy to light
Using the formula P=\(\frac{N}{t} \times \frac{h c}{\lambda}\)
= 200 \(\times 0.25 \)
⇒\(\frac{N}{t}=50 \times \frac{\lambda}{h c}\)
=\(\frac{50 \times 0.6 \times 10^{-6}}{6.6 \times 10^{-34} \times 3 \times 10^8}=1.5 \times 10^{20}\)
NEET Physics MCQs Current Electricity
Question 40. A source S1 is producing, 1015 photons Å of wavelength 5000 Å. Another source S1 is producing 1.02 x 1015 photons per second of wavelength 5100 Å. Then, (power of S2) (power of S1) is equal to:
- 1.00
- 1.02
- 1.04
- 0.98
Answer: 1. 1.00
Number of photons emitted per second
n=\(\frac{P}{\left(\frac{h c}{\lambda}\right)} \)
P=\(\frac{n h c}{\lambda}\)
⇒ \(\frac{P_1}{P_2}=\frac{n_2 \lambda_1}{n_1 \lambda_2}=\frac{1.02 \times 10^{15} \times 5000}{10^{15} \times 5100}\)
=1
Question 41. The momentum of a photon of energy 1 MeV in kg m/s will be:
- 5 x 10-22
- 0.33 x 106
- 7 x 10-24
- 10-22
Answer: 1. 5 x 10-22
The energy of the photon, E=1 MeV
Momentum of photon, P=\(\frac{E}{C}\)
P =\(\frac{E}{C}=\frac{1 \times 10^6 \times 1.6 \times 10^{-19}}{3 \times 10^8} \)
=5\( \times 10^{-22} \mathrm{~kg} \mathrm{~m} / \mathrm{s}\)
Question 42. The value of Planck’s constant is:
- 6.63 x 10-34 Js-1
- 6.63 x 10-34 m²s-1
- 6.63 x 10-34 kg m²s-1
- 6.63 x 10-34 J s-1
Answer: 3. 6.63 x 10-34 kg m²s-1
The value of Planck’s constant
h=6.63 \(\times 10^{-34} \mathrm{kgm}^2 \mathrm{~s}^{-1}\)
Question 43. The momentum of a photon of wavelength \(\lambda\) is:
- \(\frac{h}{\lambda}\)
- zero
- \(\frac{h \lambda}{c^2}\)
- \(\frac{h \lambda}{c}\)
Answer: 1. \(\frac{h}{\lambda}\)
We have,
The energy of light \(\mathrm{E}=\mathrm{hc} / \lambda\) → Equation 1
The energy of light \(\mathrm{E}=\mathrm{mc}^2\) → Equation 2
From (1) and (2), we get
⇒ \(\frac{h c}{\lambda}=m c^2 \)
⇒ \(\frac{h}{\lambda}\)=m c = Momentum
Thus, the momentum of the photon =\(\frac{h}{\lambda}\)
NEET Physics MCQs Current Electricity
Question 44. The wavelength of a 1 KeV photon is 1.24 x 10-9m. What is the frequency of 1 MeV photon?
- 1.24 x 1015 Hz
- 2.4 x 1020 Hz
- 1.24 x 1018 Hz
- 2.4 x 1023 Hz
Answer: 2. 2.4 x 1020 Hz
We have, E=\(h c / \lambda\)
or E \(\lambda\)=h c= constant
Therefore, \(E_1 \lambda_1=E_2 \lambda_2\)
Putting the given values, we get
1 \(\times 10^3 \mathrm{eV} \times 1.24 \times 10^{-19} =1 \times 10^6 \mathrm{eV} \times \lambda_2 \)
⇒ \(\lambda_2 =1.24 \times 10^{-12} \mathrm{~m}\)
Now, We have,
so, v=\(\mathrm{c} / \lambda \)
v=2.4 \(\times 10^{20} \mathrm{~Hz}\)
Question 45. The graph shows the variation of the de-Broglie wavelength of a particle and its associated momentum!/?) is:
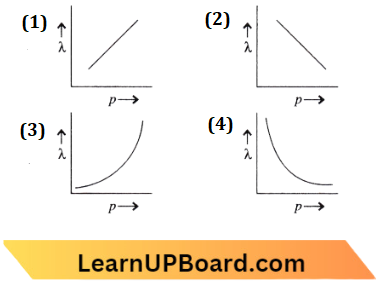
Answer: 4.
According to de-Broglie wavelength
⇒ \(\lambda=\frac{h}{p}\) or \(\lambda \propto \frac{1}{p}\)
The graph will be a rectangular hyperbola. Hence the correct option is (D).
Question 46. An electromagnetic wave of wavelength ‘\(\lambda\)’ is incident on a photosensitive surface of negligible work function. If ‘m’ mass is of photoelectron emitted from the surface has de-Broglie wave length \(\lambda_d\), then:
- \(\lambda=\left(\frac{2 m}{h c}\right) \lambda_d^2\)
- \(\lambda=\left(\frac{2 m c}{h}\right) \lambda^2\)
- \(\lambda=\left(\frac{2 m c}{h}\right) \lambda^2{ }_d\)
- \(\lambda=\left(\frac{2 h}{m c}\right) \lambda^2{ }_d\)
Answer: 3. \(\lambda=\left(\frac{2 m c}{h}\right) \lambda^2{ }_d\)
Given, Wavelength =\(\lambda\)
⇒ \(\mathrm{W}_f\) =0
mass = \(m_e\)
de-Broglie wavelength =\(\lambda_d\)
We know from Einstein’s relation
⇒ \(h v =W+\mathrm{KE}\)
Since W = 0
h v= \(\mathrm{KE}\) → Equation 1
From the de-Broglie hypothesis, we know,
\(\lambda_d=\frac{h}{p}\)and p=\(\sqrt{2 \mathrm{mkE}}\)
⇒ \(\lambda_d=\frac{h}{\sqrt{2 m k \mathrm{E}}}\) → Equation 2
From eq. (2) \(\lambda_d{ }^2=\frac{h^2}{2 m \mathrm{KE}}\)From eq. (2)\(\lambda_d{ }^2=\frac{h^2}{2 m \mathrm{KE}}\)
⇒ \(\mathrm{KE}=\frac{h^2}{2 m \lambda d^2}\) → Equation 3
From eq. (1) \(\frac{h c}{\lambda}=\mathrm{KE}\)
⇒ \(\lambda=\frac{h c}{\mathrm{KE}}\) → Equation 4
From eq. (3) and (4),
⇒ \(\lambda=\frac{h c}{h^2} 2 m \lambda_d^2 \)
⇒ \(\lambda=\frac{c}{h} 2 m \lambda_d^2 \)
∴ \(\lambda=\left(\frac{2 m c}{h}\right) \lambda_d^2\)
Current Electricity NEET Questions
Question 47. The de-Broglie wavelength of an electron moving with kinetic energy of 144 eV is nearly :
- \(102 \times 10^{-3} \mathrm{~nm}\)
- 102 \(\times 10^{-4} \mathrm{~nm}\)
- 102\( \times 10^{-5} \mathrm{~nm}\)
- 102 \(\times 10^{-2} \mathrm{~nm}\)
Answer: 4. 102 \(\times 10^{-2} \mathrm{~nm}\)
The kinetic energy of electron a=ÿ
KE = 144 eV
Means, eV= 144 eV
V= 144 V
de-Broglie wavelength,
⇒ \(\lambda=\frac{1.227}{\sqrt{V}} \)
From eq. (1),
⇒ \(\lambda =\frac{1.227}{144} \)
= \(\frac{1.227}{12} Å=0.102 \)
1.02 \(\mathrm{~nm} =102 \times 10^{-2} \mathrm{~nm}\)
Question 48. A proton and \(\alpha\)particle are accelerated from rest to the same energy. The de-Broglie wavelength \(\lambda_p\) and \(\lambda_\alpha\) are in the ratio:
- 2: 1
- 1: 1
- \(\sqrt{2}\): 1
- 4: 1
Answer: 1. 2: 1
The de-Broglie wavelength of any moving particle associated with momentum
\(\lambda=\frac{h}{p}\) → Equation 1
where h= Planck’s constant
Now K.E. =\(\frac{1}{2} m v^2\)
K.E. =\(\frac{1}{2}(m v)^2=\frac{p^2}{2 m}\)
p =\(\sqrt{2 m K E}\) → Equation 2
From (1) and (2),
⇒ \(\lambda=\frac{h}{\sqrt{2 m K E}}\)
For Proton, \(\lambda_{\mathrm{P}}=\frac{h}{\sqrt{2 m_P K E_P}}\) → Equation 3
For \(\alpha – particle, \lambda_\alpha=\frac{h}{\sqrt{2 m_\alpha K E_\alpha}}\) → Equation 4
From (3) and (4),
⇒ \(\frac{\lambda_{\mathrm{P}}}{\lambda_\alpha}=\frac{\sqrt{2 m_\alpha \mathrm{KE}_\alpha}}{2 \mathrm{M}_{\mathrm{P}}}\)
⇒ \(\mathrm{KE}_{\mathrm{p}} \)
⇒ {Since } \(\mathrm{KE}_\alpha=\mathrm{KE}_{\mathrm{P}} \) and \(m_\alpha=4 \mathrm{~m}_p \)
⇒ \(\frac{\lambda_{\mathrm{P}}}{\lambda_\alpha}=\sqrt{\frac{4 m_{\mathrm{p}}}{m_{\mathrm{P}}}}=\frac{2}{1}\)
∴ \(\lambda_{\mathrm{P}}: \lambda_\alpha\)=2: 1
Question 49. An electron of mass m with a velocity \(v=v_0 \hat{i}\left(v_0>0\right)\) enters an electric field \(E=E_0 \hat{i} \left(E_0=\text { constant }>0\right)\) at t = 0. If \(\lambda_0\) is its de-Broglie wavelength initially, then its de-Broglie wavelength at time 1 is:
- \(\lambda_0 t\)
- \(\lambda_0\left(1+\frac{e \mathrm{E}_0}{m v_0} t\right)\)
- \(\frac{\lambda_0}{\left(1+\frac{e \mathrm{E}_0}{m v_0} t\right)}\)
- \(\lambda_0\)[/latex]
Answer: 3. \(\frac{\lambda_0}{\left(1+\frac{e \mathrm{E}_0}{m v_0} t\right)}\)
From question
V=\(V_0 \hat{i}\) and \(E=-E_0 \hat{i}\)
Now initial de-Broglie wavelength is \(\lambda_0=\frac{h}{m v_0}\) → Equation 1
Acceleration of electron
a=\(\frac{e E_0}{m}\)
Velocity after t is V=\(\left(V_0+\frac{e E_0}{m} t\right)\) [This is obtained from \(V=u+a t ]\) → Equation 2
So, \(\lambda=\frac{h}{m v}=\frac{h}{m\left(v_0+\frac{e E_0}{m} t\right)}\)
⇒\(\lambda=\frac{h}{m v_0\left(1+\frac{e E_0}{m v_0} t\right)}=\frac{\lambda_0}{\left(1+\frac{e \mathrm{E}_0}{m v_0} t\right)}\)
Thus,\(\lambda=\frac{\lambda_0}{\left(1+\frac{e \mathrm{E}_0}{m v_0} \cdot t\right)}\)
Question 50. The de-Broglie wavelength of a neutron in thermal equilibrium with heavy water at a temperature T (Kelvin) and mass m, is:
- \(\frac{h}{\sqrt{m k T}}\)
- \(\frac{h}{\sqrt{3 m k T}}\)
- \(\frac{2 h}{\sqrt{3 m k T}}\)
- \(\frac{2 h}{\sqrt{m k T}}\)
Answer: 2. \(\frac{h}{\sqrt{3 m k T}}\)
de-Broglie wavelength
⇒ \(\lambda=\frac{h}{P}=\frac{h}{m v}\)
⇒ \(\lambda=\frac{h}{\sqrt{2 m(K E)}} \)
⇒ \(\lambda=\frac{h}{\sqrt{2 m\left(\frac{3}{2} K T\right)}}\)
∴ \(\lambda=\frac{h}{\sqrt{3 m K T}}\)
Current Electricity NEET Questions
Question 51. An electron of mass m and a photon have the same energy E. The ratio of de-Broglie wavelength associated with them is:
- \(\left(\frac{E}{2 m}\right)^{\frac{1}{2}}\)
- \(C(2 m E)^{1 / 2}\)
- \(\frac{1}{C}\left(\frac{2 m}{E}\right)^{\frac{1}{2}}\)
- \(\frac{1}{C}\left(\frac{E}{2 m}\right)^{\frac{1}{2}}\)
Answer: 4. \(\frac{1}{C}\left(\frac{E}{2 m}\right)^{\frac{1}{2}}\)
For electron \(\lambda_e=\frac{h}{\sqrt{2 m E}}\) for photon, E=PC
⇒ \(\lambda_{p h} =\frac{h c}{E}\)
⇒ \(\frac{\lambda_e}{\lambda_{p h}} =\frac{h}{\sqrt{2 m E}} \times \frac{E}{h c}\)
=\(\left(\frac{E}{2 m}\right)^{\frac{1}{2}} \times \frac{1}{c}\)
Question 52. Electrons of mass m with de-Broglie wavelength X fall on the target in an X-ray tube. The cut-off wavelength \(\left(\lambda_0\right)\) of the emitted X-ray is:
- \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
- \(\lambda_0=\frac{2 h}{m c}\)
- \(\lambda_0=\frac{2 m^2 c^2 \lambda^3}{h^2}\)
- \(\lambda_0=\lambda\)
Answer: 1. \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
From de-Broglie equation
⇒ \(\lambda =\frac{h}{P}\)
P =\(\frac{h}{\lambda}\)
KE of electrons, E=\(\frac{P^2}{2 m}=\frac{h^2}{2 m \lambda^2}\)
Also in X-ray, \(\lambda_0=\frac{h c}{t}\)
∴ \(\lambda_0=\frac{2 m c \lambda^2}{h}\)
Question 53. Which of the following figures represents the variation of the particle momentum and the associated de-Broglie wavelength?
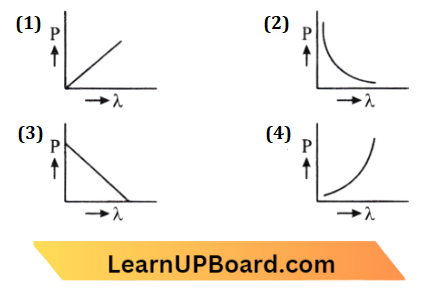
Answer: 3.
According to de-Broglie wavelength
⇒ \(\lambda =\frac{h}{p}\)
p \(\lambda \)=h
This equation looks like \(y_x\)=c which is shown in the graph (c)
Question 54. Light of wavelength 500 nm is incident on a metal with work function 2.28 eV. The de-Broglie wavelength of the emitted electron is:
- \(<2.8 \times 10^{-10} \mathrm{~m}\)
- \(<2.8 \times 10^{-9} \mathrm{~m}\)
- \(\geq 2.8 \times 10^{-9} \mathrm{~m}\)
- \(\leq 2.8 \times 10^{-12} \mathrm{~m}\)
Answer: 3. \(<2.8 \times 10^{-9} \mathrm{~m}\)
Energy of photon, (E) =\(\frac{12400}{5000}\)
=2.48 \(\mathrm{eV}\)
Work function W=2.28 \(\mathrm{eV}\)
Using Einstein’s photoelectric equation
E = \(W+K E_{\max } \)
2.48 =2.28+ \(K.E._{\max } \)
for electron \(K. E ._{\max } =0.20 \mathrm{eV} \)
⇒ \(\lambda =\frac{h}{\sqrt{2 m E}} \)
So \(\lambda =28 Å\)
∴ \(\lambda \geq 2.8 \times 10^{-9} \mathrm{~m}\)
NEET Important Questions Current Electricity
Question 55. If the kinetic energy of the particle is increased to 16 times its previous value, the percentage change in the de- Broglie wavelength of the particle is:
- 25
- 75
- 60
- 50
Answer: 2. 75
Using de-Broglie hypothesis,
⇒ \(\lambda=\frac{h}{P}\)
Hence, \(\lambda_1=\frac{h}{P}=\frac{h}{\sqrt{2 m k}}\) → Equation 1
and \(\lambda_2 =\frac{h}{2 m \times 10 k}\)
=\(\frac{h}{4 \sqrt{2 m k}}=\frac{\lambda_1}{4}\) → Equation 2
Using eq. (1)
⇒ \(\lambda_2=\frac{1}{4} \times x_1=0.25 \% of \lambda_1\)
Thus 75\% changes in the wavelength.
Question 56. The de-Broglie wavelength of neutrons in thermal equilibrium at temperature T is:
- \(\frac{3.08}{\sqrt{T}} Å\)
- \(\frac{0.308}{\sqrt{T}} Å\)
- \(\frac{0.0308}{\sqrt{T}} Å\)
- \(\frac{30.8}{\sqrt{T}} Å\)
Answer: 4. \(\frac{30.8}{\sqrt{T}} Å\)
Using de-Broglie wavelength
⇒ \(\lambda =\frac{h}{\sqrt{2 m K_B T}}\)
⇒ \(\lambda =\frac{6.63 \times 10^{-34}}{\sqrt{2 \times 1.67 \times 10^{-27} \times 1.38 \times 10^{-23} \times \mathrm{T}}}\)
= \(\frac{3.08 \times 10^{-34} \times 10^{25}}{\sqrt{T}} m=\frac{30.8}{\sqrt{T}} Å\)
Question 57. An a-particle moves in a circular path of radius 0.83 em in the presence of a magnetic field of 0.25 Wb/m². The de-Broglie wavelength associated with the particle will be:
- 1 Å
- 0.1 Å
- 10 Å
- 0.01 Å
Answer: 4. 0.01 Å
We know that, R=\(\frac{m v}{q \mathrm{~B}} and \lambda=\frac{h}{m v}\)
⇒ \(\lambda =\frac{h}{m \nu}=\frac{h}{R q B}\)
⇒ \(\lambda =\frac{6.63 \times 10^{-34}}{\sqrt{2 \times 1.67 \times 10^{-27} \times 1.38 \times 10^{-23} \times T}}\)
=0.01 Å
Question 58. If the momentum of an electron is changed by p, then the de-Broglie wavelength associated with it changes by 0.5%. The initial momentum of the electron will be:
- 200 p
- 400 p
- \(\frac{p}{200}\)
- 100 p
Answer: 1. 200 p
From de-Broglie hypothesis
P =\(\frac{h}{\lambda} \)
⇒ \(\left|\frac{\Delta P}{P}\right| =\frac{\Delta \lambda}{\lambda}\)
⇒ \(\frac{\Delta P}{P_i} =\frac{\Delta \lambda}{\lambda} \)
⇒ \(P_i =\frac{P}{\frac{0.5}{100}} \)
∴ \(P_i =\frac{1000}{5}\) P=200 P
Question 59. Electrons used in an electron microscope are accelerated by a voltage of 25 kV. If the voltage is increased to 100 kV then the de-Broglie wavelength associated with the electrons would:
- decrease by 2 times
- decrease by 4 times
- increase by 4 times
- increase by 2 times
Answer: 1. decrease by 2 times
We know that,\(\lambda=\frac{12.27}{\sqrt{V}}\)
⇒ \(\frac{\lambda_1}{\lambda_2}=\sqrt{\frac{V_2}{V_1}} \)
⇒ \(\lambda_2=\lambda_1 \sqrt{\frac{V_1}{V_2}} \)
⇒ \(\lambda_2=\lambda_1 \sqrt{\frac{25}{100}} \)
∴ \(\lambda_2=\lambda_1 \sqrt{\frac{1}{4}}=\frac{\lambda_1}{2}\)
Question 60. A particle of mass 1 mg has the same wavelength as an electron moving with a velocity 3 x \(10^6 \mathrm{~ms}^{-1}\). The velocity of the particle is:
- \(2.7 \times 10^{-18} \mathrm{~ms}^{-1}\)
- \(9 \times 10^{-2} \mathrm{~ms}^{-1}\)
- \(3 \times 10^{-31} \mathrm{~ms}^{-1}\)
- \(2.7 \times 10^{-21} \mathrm{~ms}^{-1}\)
Mass of electron =9.1 \(\times 10^{-31}\) kg
Answer: 1. \(2.7 \times 10^{-18} \mathrm{~ms}^{-1}\)
De-Broglie wavelength, associated with an electron moving with velocity v, \(\lambda=\frac{h}{m v}\)
So,\(\lambda_e=\frac{h}{9.1 \times 10^{-31} \times 3 \times 10^6}\)
The wavelength of a particle of mass 1 \(\mathrm{~kg}\) moving with velocity v.
⇒ \(\lambda_p=\frac{h}{10^{-3} \times v}\)
As given, \(\lambda_e=\lambda_p\)
⇒ \(\frac{h}{10^{-6} \times v} =\frac{h}{9.1 \times 10^{-31} \times 3 \times 10^6}\)
= 2.7 \(\times 10^{-18} \mathrm{~m} / \mathrm{s}\)
Question 61. If particles are moving with the same velocity, then the de Broglie wavelength is maximum for:
- proton
- α-particle
- neutron
- β-particle
Answer: 4. β-particle
De-Broglie wavelength is maximum for \(\beta\)-particle
Question 62. The wavelength associated with an electron accelerated through a potential difference of 100 V, is of the order of:
- 1000 Å
- 100 Å
- 10.5 Å
- 1.2 Å
Answer: 4. 1.2 Å
⇒ \(\lambda =\frac{h}{\sqrt{2 m e V}} \)
=\(\frac{6.63 \times 10^{-34}}{\sqrt{2 \times 9.1 \times 10^{-31} \times 1.6 \times 10^{-19} \times 100}}\)
=1.227 \(\times 10^{-10}=1.227 Å\)
NEET Important Questions Current Electricity
Question 63. The de-Broglie wave corresponding to a particle of mass m and velocity v has a wavelength associated with it
- \(\frac{h}{m v}\)
- HMV
- \(\frac{m h}{v}\)
- \(\frac{m}{h v}\)
Answer: 1. \(\frac{h}{m v}\)
The de-Broglie wavelength associated with the particle of mass m moving with velocity v is
–\(\lambda =\frac{h}{p}\)
⇒ \(\lambda =\frac{h}{m v}\)
Where, m and v are the mass and the velocity of the particle and h is Planck’s constant.
Question 64. The wave nature of electron was experimentally verified by:
- de-Broglie
- Hertz
- Einstein
- Davisson and Germer
Answer: 4. Davisson and Germer
The wave nature of electrons was experimentally verified by Davisson and Germer.
Question 65. In the Davisson and Germer experiment, the velocity of electrons emitted from the electron gun can be increased by:
- increasing the filament current
- decreasing the filament current
- decreasing the potential difference between the anode and filament
- increasing the potential difference between the anode and filament
Answer: 4. increasing the potential difference between the anode and filament
In the Davisson and Germer experiment, the velocity of electrons emitted from the electron gun can be increased by increasing the potential difference between the anode and filament.
