NEET Organic Chemistry Questions
NEET Chemistry For Haloalkanes And Haloarenes Multiple Choice Questions
Question 1. The given compound
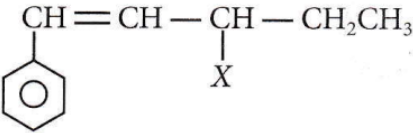
is an example of ______
- Allylic halide
- Vinylic halide
- Benzylic halide
- Aryl halide
Answer: 1. Allylic halide
The compound where the halogen group is attached to a sp³ hybridised carbon atom next to the carbon which is adjacent to the carbon-carbon double bond is known as allylic halide.
Hence, the given molecule is an alkyl halide.
Question 2. The correct sequence of bond enthalpy of the ‘C — X’ bond is
- CH3 – Cl > CH3 – F > CH3 – Br > CH3 -I
- CH3 – F < CH3– Cl < CH3– Br < CH3 – I
- CH3 – F > CH3— Cl > CH3— Br > CH – I
- CH3 – F < CH3– Cl > CH3 – Br > CH3 – I
Answer: 3. CH3 – F > CH3 – Cl > CH3 – Br > CH – I
The correct order of bond enthalpy is CH3– F > CH3 – Cl > CH3 – Br > CH3 – I
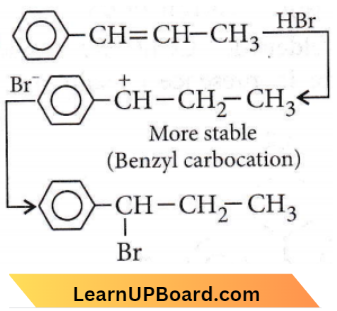
Question 3. The reaction of C6H5CH=CHCH3 with HBr produces
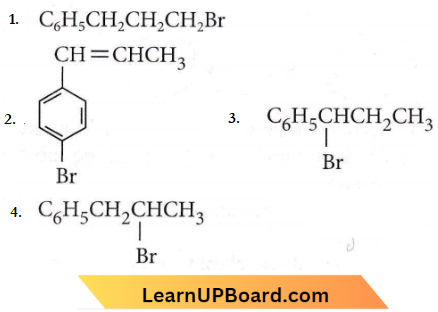
Answer: 3
Question 4. In the replacement reaction ![]() . The reaction will be most favourable if M happens to be
. The reaction will be most favourable if M happens to be
- Na
- K
- Rb
- Li
Answer: 3. Rb
Tertiary halide shows SN1 mechanism i.e., ionic mechanism. In the given reaction negative ions will attack on carbocation. Thus greater the tendency of ionisation (greater ionic character in the M – F bond) more favourable the reaction. The most ionic bond is Rb – F in the given examples thus most favourable reaction will be with Rb-F.
Read and Learn More NEET MCQs with Answers
NEET organic chemistry questions
Question 5. When chlorine is passed through propene at 400°C, which of the following is formed?
- PVC
- Allyl chloride
- Propyl chloride
- 1, 2-Dichloroethane
Answer: 2. Allyl chloride
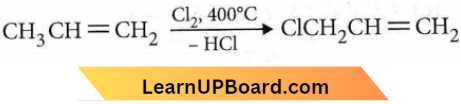
At 400°C temperature, substitution occurs instead of addition.
NEET organic chemistry questions
Question 6. Which of the following is suitable to synthesize chlorobenzene?
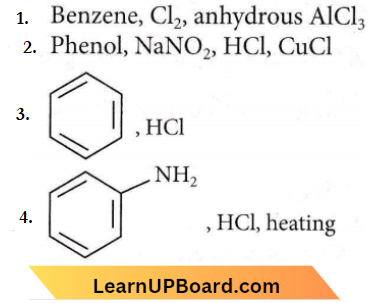
Answer: 1
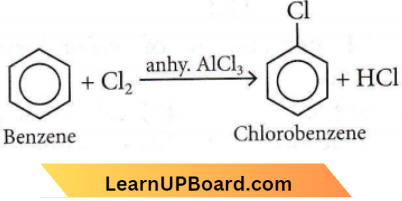
Arenes react with halogens in the presence of a Lewis acid like anhydrous FeCl3, FeBr3 or AICI3 to yield haloarenes, for example,
Question 7. The incorrect statement regarding chirality is
- SN¹ reaction yields a 1:1 mixture of both enantiomers
- The product obtained by the SN² reaction of haloalkane having chirality at the reactive site shows an inversion of configuration
- Enantiomers are superimposable mirror images of each other
- A racemic mixture shows zero optical rotation.
Answer: 3. Enantiomers are superimposable mirror images of each other
Enantiomers are non-superimposable mirror images of each other. Enantiomers possess identical physical properties namely, melting point, boiling point, refractive it dex, etc. Then, only differ with respect to the rotation of plane polarised 1ight. If one of the enantiomers is dextrorotatory, then the other will be laevorotatory.
Haloalkanes and haloarenes NEET MCQs
Question 8. The major product formed in the dehydrohalogenation reaction of 2-bromopentane is pent-2-ene. This product formation is based on the?
- Huckel’s Rule
- Saytzeff s Rule
- Hund’s Rule
- Hofmann Rule
Answer: 2. Saytzeff s Rule
It is an example of B-elimination, as the major product is 2-pentene (more substituted) not 1-pentene, hence it follows the Saytzeffb rule
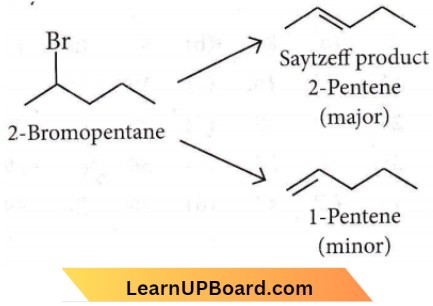
NEET organic chemistry questions
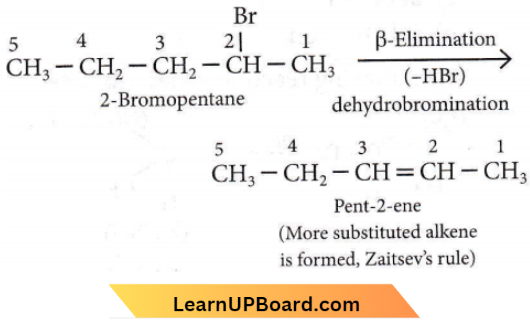
Question 9. The elimination reaction of 2-bromopentane to form pent-2-ene is
- β-Elimination reaction
- Follows Zaitsev rule
- Dehydrohalogenation reaction
- Dehydration reaction.
- (1), (2), (3)
- (1), (3), (4)
- (2), (3), (4)
- (1), (2), (4)
Answer: 1. (1), (2), (3)
Question 10. The hydrolysis reaction that takes place at the slowest rate, among the following is
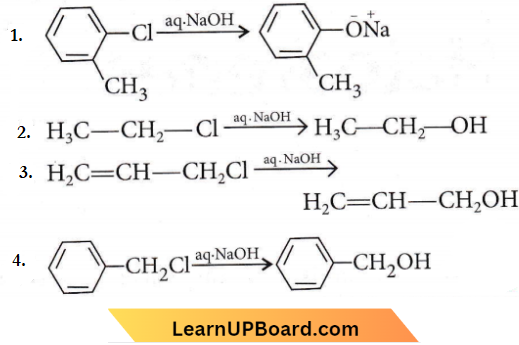
Answer: 1
Aryl halides are less reactive as compared to alkyl halides as the halogen atom in these compounds is firmly attached and cannot be replaced by nucleophiles such as OH–, NH2– etc. In chlorobenzene, the electron pair of the chlorine atom is in conjugation with n-electrons of the benzene ring. Thus C-Cl bond acquires a double bond character and is difficult to break
NEET chemistry MCQs
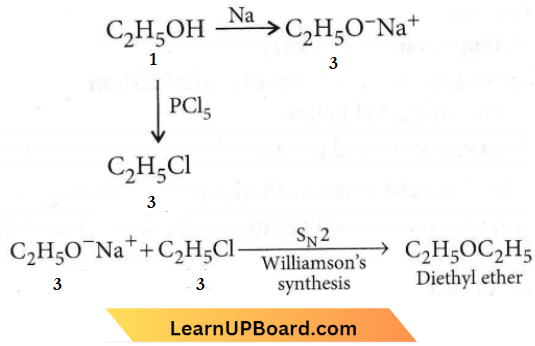
Question 11. The compound A on treatment with Na gives B, and with PCl5 gives C. B and C react together to give diethyl ether. A, B and C are in the order
- \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}, \mathrm{C}_2 \mathrm{H}_6, \mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}\)
- \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{ONa}\)
- \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}, \mathrm{C}_2 \mathrm{H}_6, \mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}\)
- \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{ONa}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}\)
Answer: 4. \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{ONa}, \mathrm{C}_2 \mathrm{H}_5 \mathrm{Cl}\)
The compound A on treatment with Na gives B, and with PCl5 gives C. B and C react together to give diethyl ether.
Haloalkanes and haloarenes NEET MCQs
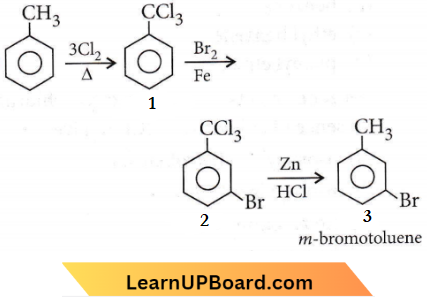
Question 12. The compound C7H8 undergoes the following reactions:
⇒ \(\mathrm{C}_7 \mathrm{H}_8 \underrightarrow{3 \mathrm{Cl}_2 / \Delta}\) A \(\underrightarrow{\mathrm{Br}_2 / \mathrm{Fe}}\) B \(\underrightarrow{\mathrm{Zn} / \mathrm{HCl}}\) C
The product C is
- m-bromotoluene
- ο-bromotoluene
- 3-bromo-2,4,6-trichlorotoluene
- p-bromotoluene.
Answer: 1. The product C is
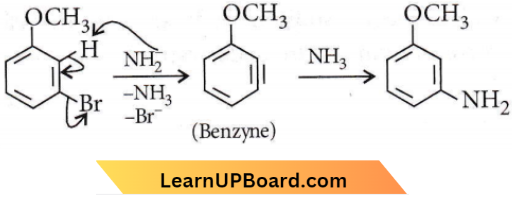
Question 13. Identify A and predict the type of reaction.
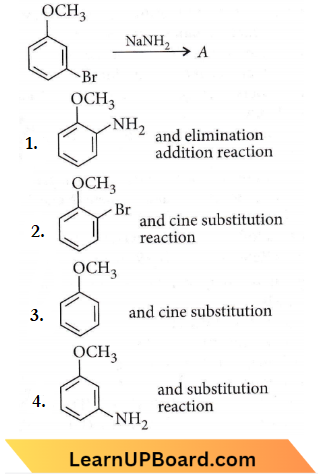
Answer: 4
m-Bromoanisole gives only the respective meta-substituted aniline. This is a substitution reaction which goes by an elimination-addition pathway.
Question 14. Consider the reaction, \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br}+\mathrm{NaCN} \longrightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CN}+\mathrm{NaBr}\)
This reaction will be the fastest in
- Ethanol
- Methanol
- N, N’ -dimethylformamide (DMF)
- Water.
Answer: 3. N, N’ -dimethylformamide (DMF)
The reaction \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br}+\mathrm{NaCN} \longrightarrow \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CN}+\mathrm{NaBr}\) follows SN² mechanism which is favoured by polar aprotic solvent i.e., N, N’-dimethylformamide (DMF),
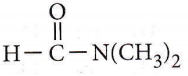
NEET chemistry MCQs
Haloalkanes and haloarenes NEET MCQs
Question 15. Which of the following biphenyls is optically active?
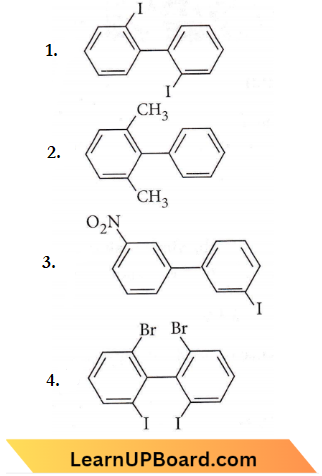
Answer: 4
o-Substituted biphenyls are optically active as both the rings are not in one plane and their mirror images are non-superimposable.
Question 16. For the following reactions:
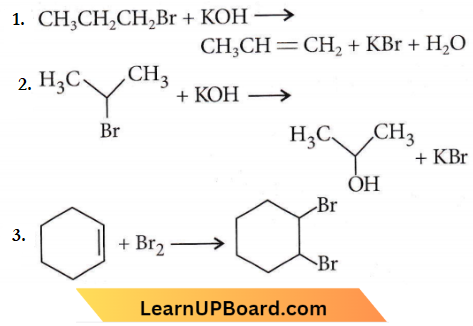
Which of the following statements is correct?
- (1) is elimination, (2) and (3) are substitution reactions.
- (1) is a substitution, (2) and (3) are addition reactions.
- (1) and (2) are elimination reactions and (3) is an addition reaction.
- (1) is elimination, (2) is substitution and (3) is addition reaction.
Haloalkanes and haloarenes practice MCQs
Answer: 4. (1) is the elimination, (2) is substitution and (3) is addition reaction.
⇒ \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Br}+\mathrm{KOH} \longrightarrow \mathrm{CH}_3 \mathrm{CH} =\mathrm{CH}_2\) + \(\mathrm{KBr}+\mathrm{H}_2 \mathrm{O}\)
The saturated compound is converted into the unsaturated compound by the removal of a group of atoms hence, it is an elimination reaction.
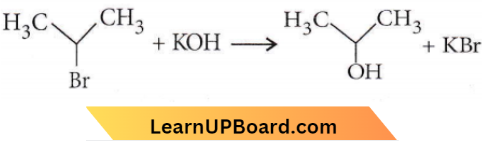
-Br group is replaced by – OH group hence, it is a substitution reaction.
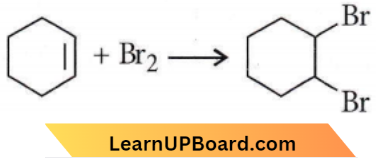
The addition of Br2 converts an unsaturated compound into a saturated compound hence, it is an addition reaction.
NEET Chemistry MCQs
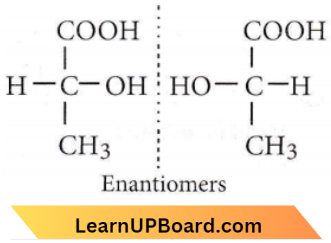
Question 17. Two possible stereo-structures of CH3CHOHCOOH, which are optically active, are called
- Atropisomers
- Enantiomers
- Mesomers
- Diastereomers.
Answer: 2. Enantiomers
Question 18. In an SN1 reaction on chiral centres, there is
- Inversion more than retention leads to partial racemisation
- 100% retention
- 100% inversion
- 100% racemisation.
Answer: 1. Inversion more than retention leading to partial racemisation
In the case of optically active alkyl halides, SN1 reaction is accompanied by racemisation. The carbocation formed in the slow step being sp² hybridised is planar and an attack of nucleophiles may take place from either side resulting in a mature of products, one having the same configuration and the other having an inverted configuration.
The isomer corresponding to inversion is present in slight excess because SN1 also depends upon the degree of shielding of the front side of the reacting carbon.
Haloalkanes and haloarenes practice MCQs
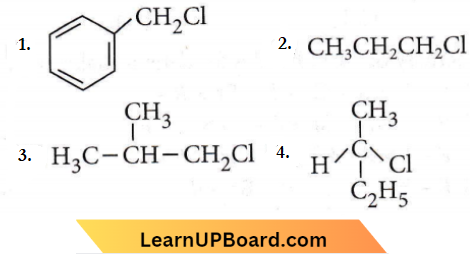
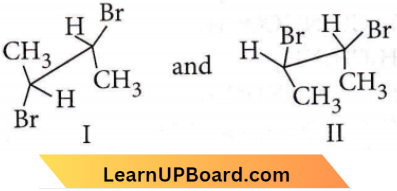
Question 19. Which of the following compounds will undergo racemisation when solution of KOH hydrolysis?
- (1) and (2)
- (2) and (4)
- (3) and (4)
- (1) and (4)
Answer: None
Due to chirality 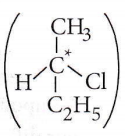 only compound (4) will undergo racemisation.
only compound (4) will undergo racemisation.
Hence, all the given options are incorrect.
NEET chemistry practice questions
Question 20. Given:
1 and 2 are
- Identical
- A pair of conformers
- A pair of geometrical isomers
- A pair of optical isomers.
Answer: 2. A pair of conformers
1 and 2 are staggered and eclipsed conformers.
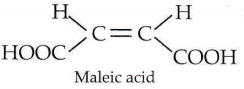
Question 21. Which of the following acids does not exhibit optical isomerism?
- Maleic acid
- α-Amino acids
- Lactic acid
- Tartaric acid
Answer: 1. Maleic acid
Maleic acid shows geometrical isomerism and not optical isomerism.
Question 22. Consider the reactions :
- \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{CH}_2 \mathrm{Br}\) \(\underrightarrow{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}\) \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{CH}_2 \mathrm{OC}_2 \mathrm{H}_5+\mathrm{HBr}\)
- \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{CH}_2 \mathrm{Br}\) \(\underrightarrow{\mathrm{C}_2 \mathrm{H}_5 \mathrm{O}^{-}}\) \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{CH}_2 \mathrm{OC}_2 \mathrm{H}_5+\mathrm{Br}^{-}\)
The mechanisms of reactions (1) and (2) are respectively
- SN1 and SN2
- SN1 and SN1
- SN2 and SN2
- SN2 and SN1
Answer: 3. SN2 and SN2
If the reaction is SN1, there will be the formation of carbocation and the rearrangement takes place. In these reactions there is no rearrangement hence both are SN2 mechanisms.
Haloalkanes and haloarenes practice MCQs
NEET chemistry practice questions
Question 23. Which of the following compounds undergoes nucleophilic substitution reaction most easily?
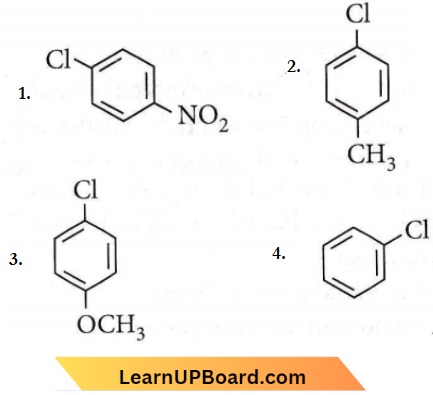
Answer: 1
Electron withdrawing groups like – NO2 facilitate nucleophilic substitution reactions in chlorobenzene.
Question 24. Which one is most reactive towards SN1 reaction?
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}\left(\mathrm{C}_6 \mathrm{H}_5\right) \mathrm{Br}\)
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}\left(\mathrm{CH}_3\right) \mathrm{Br}\)
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{C}\left(\mathrm{CH}_3\right)\left(\mathrm{C}_6 \mathrm{H}_5\right) \mathrm{Br}\)
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}\)
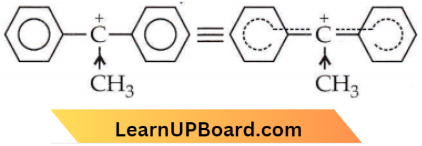
Answer: 3. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{C}\left(\mathrm{CH}_3\right)\left(\mathrm{C}_6 \mathrm{H}_5\right) \mathrm{Br}\)
SN1 reactions proceed via the formation of a carbocation intermediate.
The more stable is the carbocation more reactive the alkyl aryl halide towards SN1.
In C5H5C+(CH3)(C6H6) carbocation, the two phenyl rings by their +R effect and -CH, by its +I effect diminish the positive charge and make it stable.
Haloalkanes and haloarenes objective questions
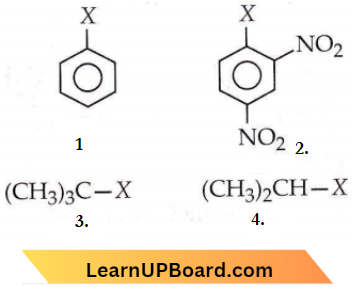
Question 25. The correct order of increasing reactivity of
NEET chemistry chapter-wise questions
C—X bond towards nucleophiles in the following compounds is
- 1 < 2 < 4 < 3
- 2 < 3 < 1 < 4
- 4 < 3 < 1 < 2
- 3 < 2 < 1 < 4
Answer: 1. 1 < 2 < 4 < 3
The order of reactivity is dependent on the stability of the intermediate carbocation formed by the cleavage of the C-X bond. The 3° carbocation (formed from 3) will be more stable than its 2° counterpart (formed from 4) which in turn will be more stable than the arenium ion (formed from 1).
Also, the aryl halide has a double bond character in the C-X bond which makes the cleavage more difficult. However, in spite of all the stated factors, 2 will be more reactive than I due to the presence of the electron-withdrawing -NO2 group. C-Xbond becomes weak and undergoes a nucleophilic substitution reaction.
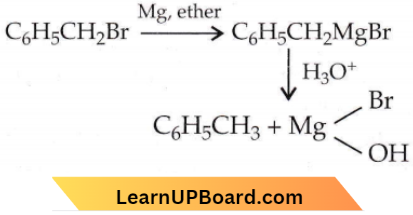
Question 26. In the following reaction, 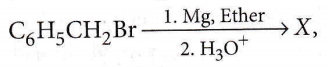 the product ‘X’ is
the product ‘X’ is
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OCH}_2 \mathrm{C}_6 \mathrm{H}_5\)
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{OH}\)
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_3\)
- \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{C}_6 \mathrm{H}_5\)
Answer: 3. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_3\)
NEET chemistry chapter-wise questions
Question 27. Which of the following reactions is an example of a nucleophilic substitution reaction?
- 2RX + 2Na → R – R + 2NaX
- RX + H2 → KH + HX
- RX + Mg → RMgX
- RX + KOH→ ROH + KX
Answer: 4. RX + KOH → ROH + KX
Haloalkanes and haloarenes objective questions
Question 28. How many stereoisomers does this molecule have? CH3CH = CHCH2CHBrCH3
- 8
- 2
- 4
- 6
Answer: 3. 4
The given compound may be written as
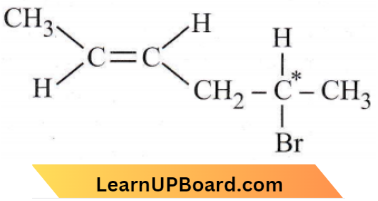
Both geometrical isomerism (cis-trans form) and optical isomerism is possible in the given compound.
No. of optical isomer = 2n = 21 = 2
(where n = no. of asymmetric carbon)
Hence total no. of stereoisomers = 2 + 2 = 4
Haloalkanes and haloarenes objective questions NEET
Question 29. In an SN2 substitution reaction of the type \(R-\mathrm{Br}+\mathrm{Cl}^{-}\) \(\underrightarrow{\mathrm{DMF}}\) \(\mathrm{R}-\mathrm{Cl}+\mathrm{Br}^{-}\) which one of the following has the highest relative rate?
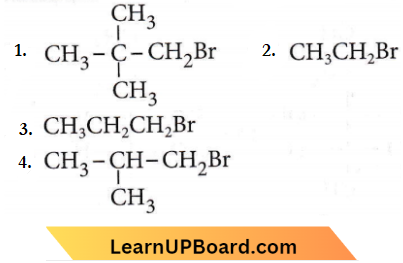
Answer: 2
In an SN2 substitution reaction of the type \(R-\mathrm{Br}+\mathrm{Cl}^{-}\) \(\underrightarrow{\mathrm{DMF}}\) \(\mathrm{R}-\mathrm{Cl}+\mathrm{Br}^{-}\)
SN2 mechanism is followed in the case of primary and secondary alkyl halides i.e. SN2 reaction is favoured by small groups on the carbon atoms attached to halogen so, CH3 – X > R – CH2 – X > R2CH – X > R3C – X. Primary is more reactive than secondary and tertiary alkyl halides
Question 30. If there is no rotation of plane polarised light by a compound in a specific solvent, though to be chiral, it may mean that
- The compound is certainly meso
- There is no compound in the solvent
- The compound may be a racemic mixture
- The compound is certainly a chiral.
Answer: 1. The compound is certainly meso
Meso compound does not rotate plane polarised light. The compound which contains tetrahedral atoms with four different groups but the whole molecule is achiral, is known as meso compound. It possesses a plane of symmetry and is optically inactive.
One of the asymmetric carbon atoms turns the plane of polarised light to the right and the other to the left and to the same extent so that the rotation due to the upper half is compensated by the lower half, i.e., internally compensated, and finally, there is no rotation of plane polarised light
Haloalkanes and haloarenes NEET MCQs
Question 31. CH3 – CHCl – CH2 – CH3 has a chiral centre. Which one of the following represents its R-configuration?
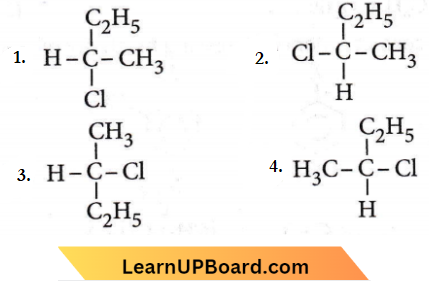
Answer: 2
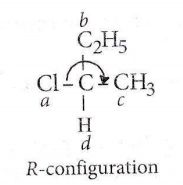
Question 32. Which of the following is not chiral?
- 2-Hydroxypropanoic acid
- 2-Butanol
- 2,3-Dibromopentane
- 3-Bromopentane
Answer: 4. 3-Bromopentane
Due to the absence of an asymmetric carbon atom.
Haloalkanes and haloarenes objective questions NEET
Question 33. Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
- Ethyl chloride
- Isopropyl chloride
- Chlorobenzene
- Benzyl chloride
Answer: 4. Benzyl chloride
SN1 reaction is favoured by heavy (bulky) grouPs on the carbon atom attached to halogens and the nature of carbonium ion in the substrate is
Benzyl > Aliyl > Tertiarv > Secondary > Primary > Methyl halides.
Question 34. The chirality of the compound
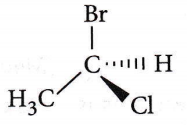
- R
- S
- E
- Z
Answer: 1. R
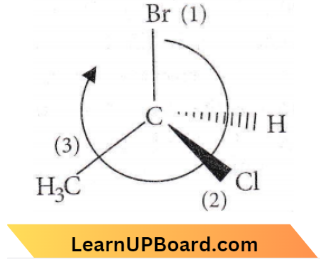
The lowest priority atom is always away from the viewer. Priority is seen on the basis of atomic no. and if atomic no are the same then on the basis of atomic mass.
If clockwise then it is R, if anticlockwise then it is S.
Name of the moiecule is, (R) 1-bromo-1-chioroethane.
Haloalkanes and haloarenes NEET MCQs
Question 35. Which of the following is least reactive in a nucleophilic substitution reaction?
- (CH3)3C – Cl
- CH2 = CHCl
- CH3CH2Cl
- CH2 = CHCH2Cl
Answer: 2. CH2 = CHCl
The non-reactivity of the chlorine atom in vinyl chloride can be explained from the molecular orbital point of view as follows. If the chlorine atom has sp² hybridisation, the C – Cl bond will be a σ-bond and the two lone pairs of electrons will occupy the other two sp² orbitals. This would leave a p orbital containing a lone pair, and this orbital could now conjugate with the π-bond of the ethylenic link.
Thus two M’O’s will be required to accommodate these four π-electrons ‘Furthermore’ since chlorine is more electronegative than carbon, the electrons will tend to be found in the vicinity of the chlorine atom. Nevertheless, the chlorine atom has now lost full control of the lone pair and so, is less negative than it would have been had there been no conjugation.
Since two carbon atoms have acquired a share in their pair each carbon atom acquires a small negative charge. Hence, owing to the delocalisation of bonds (through conjugation) the vinyl chloride molecule has an increased stability. Before the chlorine atom can be placed by some other group the lone pair must be localised again on the chlorine atom. This requires energy, and so the chlorine is more firmly bound.
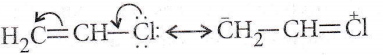
Question 36. Which of the following pairs of compounds are enantiomers?
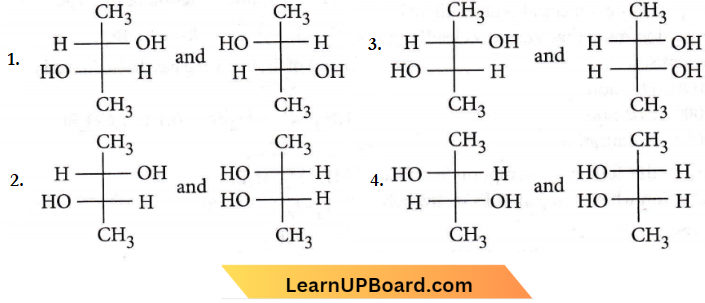
Answer: 1
These two are non-superimposable mirror images of each other, so they are enantiomers.
Haloalkanes and haloarenes MCQs with answers
Question 37. The reactivity order of halides for dehydrohalogenation is
- R-F>R-Cl>R-Br>R-I
- R-I>R-Br>R-Cl>R-F
- R-I>R-Cl>R-Br>R-F
- R-F>R-I>R-Br>R-Cl
Answer: 2. R-I>R-Br>R-Cl>R-F
I > Br > Cl > F → atomic radii
F, Ci, Br, I belong to the same group orderly. Atomic radii go on increasing as the nuclear charge increases in preceding downwards in a group. The decreasing order of bond length is C – I > C – Br > C – Cl > C – F.
The order of bond dissociation energy R- F >R- Cl>R- Br> R – I. During dehydrohalogenation C – I bond breaks more easily than the C – F bond. So reactivity order of halides is, R – I > R – Br > R – Cl > R – F.
Question 38. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{Cl}\) \(\underrightarrow{\mathrm{NaCN}}\) x \(\underrightarrow{{\mathrm{Ni}}/{\mathrm{H}_2}}\) Y \(\underrightarrow{\text { acetic anhydride }}\) Z
Z in the above reaction sequence is
- \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NHCOCH}_3\)
- \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NH}_2\)
- \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CONHCH}_3\)
- \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CONHCOCH}_3\)
Answer: 1. \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NHCOCH}_3\)
Question 39. CH3-CH2-CH-CH3 obtained by chlorination of n-butane will be
- Meso form
- Racemic mixture
- d-form
- l-form
Answer: 2. Racemic mixture
Chlorination of n-butane takes place via free radical formation. i.e \(\mathrm{Cl}_2 \longrightarrow \dot{\mathrm{Cl}}+\dot{\mathrm{Cl}}\)
⇒ \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_3\) \(\underrightarrow{\dot{\mathrm{C}}}\) \(\mathrm{CH}_3 \dot{\mathrm{C}} \mathrm{HCH}_2 \mathrm{CH}_3+\mathrm{HCl}\)
sp² hybrid planar shape intermolecular and Cl may attack from either side to give
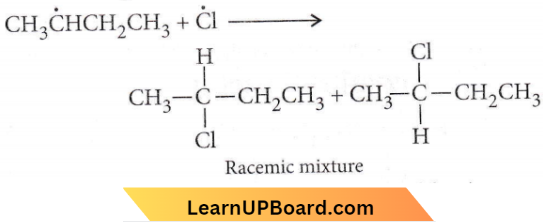
Haloalkanes and haloarenes MCQs with answers
Question 40. An organic compound A(C4H9Cl) in reaction with Na/diethyl ether gives a hydrocarbon which on monochlorination gives only one chloro derivative then, A is
- t-butyl chloride
- s-butyl chloride
- iso-butyl chloride
- n-butyl chloride.
Answer: 1. t-butyl chloride
An organic compound A(C4H9Cl) on reaction with Na/diethyl ether gives a hydrocarbon which on monochlorination gives only one chloro derivative
Wurtz reaction: It involves the reaction of alkyl halides with Na in ether to form higher alkanes.
2R – X + 2Na → R – R + 2NaX
In the given problem, \(2 \mathrm{C}_4 \mathrm{H}_9 \mathrm{Cl}+2 \mathrm{Na}\) \(\underrightarrow{\text { Ether }}\) \(\mathrm{C}_4 \mathrm{H}_9-\mathrm{C}_4 \mathrm{H}_9+2 \mathrm{NaCl}\)
Compound A is t-butyl chloride, in this compound all – CH, groups have primary hydrogen only and are able to give only, one chloro derivative.
⇒ \(\left(\mathrm{CH}_3\right)_3 \mathrm{CC}\left(\mathrm{CH}_3\right)_3\) \(\underrightarrow{\mathrm{Cl}_2}\) \( \mathrm{CH}_2 \mathrm{Cl}\left(\mathrm{CH}_3\right)_2 \mathrm{C}-\mathrm{C}\left(\mathrm{CH}_3\right)_3\)
Haloarenes practice questions NEET
Question 41. A compound of molecular formula C7H16 shows optical isomerism, compound will b
- 2,3-dimethyl pentane
- 2,2-dimethylbutane
- 2-methyl hexane
- None of these.
Answer: 1. 2,3-dimethyl pentane
Organic compounds exhibit the property of enantiomerism (optical isomerism) only when their molecules are chiral. Most chiral compounds have a chiral centre which is an atom bonded to four different atoms or groups.
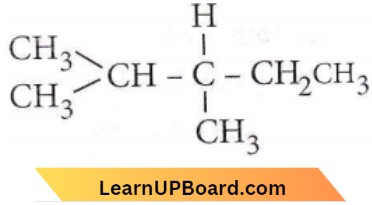
2,3-Dimethylpentane has one chiral C-atom and does not have any symmetric element.
Question 42. Which of the following compounds is not chiral?
- \(\mathrm{CH}_3 \mathrm{CHDCH}_2 \mathrm{Cl}\)
- \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHDCl}\)
- \(\mathrm{DCH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Cl}\)
- \(\mathrm{CH}_3 \mathrm{CHClCH}_2 \mathrm{D}\)
Answer: 3. \(\mathrm{DCH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{Cl}\)
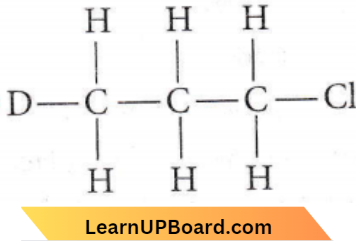
The above compound has no chiral ‘C’-atom’ All the ‘C’ atoms are attached to two identical ‘H’ atoms, so they are not symmetrical.
Haloarenes practice questions NEET
Question 43. Replacement of Cl of chlorobenzene to give phenol requires drastic conditions. But chlorine of 2,4-dinitrochlorobenzene is readily replaced because
- NO2 donates e– at meta position
- NO2 withdraws e– from ortho/para positions
- NO2 makes ring electrons rich at ortho and para
- NO2 withdraws e– from meta position.
Answer: 2. NO2 withdraws e– from ortho/para positions
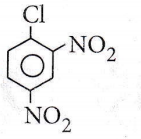
The withdrawal of electrons by -NO2 groups from orthopara positions causes easier removal of -Cl atoms due to the development of positive charge in o- and p-positions.
Haloalkanes and haloarenes MCQs with answers
Question 44. The alkyl halide is converted into an alcohol by
- Elimination
- Dehydrohalogenation
- Addition
- Substitution.
Answer: 4. Substitution.
⇒ \(\underset{\mathrm{Ethyl bromide (aqueous)}}{{\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}}}+\mathrm{KOH} \longrightarrow \underset{\mathrm{Ethyl alochol}}{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}}+\mathrm{KBr}\)
Question 45. The following reaction is described as
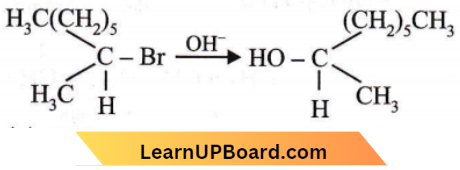
Answer: 1
SN2 reactions are bimolecular reactions where the rate of reaction depends on the concentration of both substrate and nucleophile. When OH– attacks the substrate from the opposite side of the leaving group i.e., Br– a transition state results, in which both OH and Br are partially bonded to a carbon atom.
Question 46. The reaction of t-butyl bromide with sodium methoxide produces
- Sodium t-butoxide
- t-butyl methyl ether
- Isobutane
- Isobutylene
Answer: 4. Isobutylene
Isobuythylene is obtained.
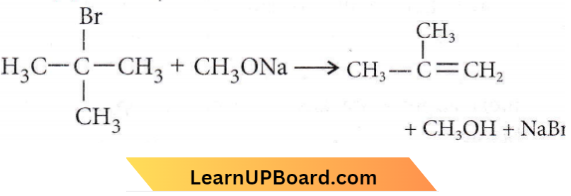
Thus, the reaction produces isobutylene.
Question 47. Grignard reagent is prepared by the reaction between
- Magnesium and alkane
- Magnesium and aromatic hydrocarbon
- Zinc and alkyl halide
- Magnesium and alkyl halide
Answer: 4. Magnesium and alkyl halide
Grignard reagent is prepared by heating an alkyl halide with dry magnesium powder in dry ether.
⇒ \(R-X+\mathrm{Mg}\) \(\underrightarrow{\text { Dry ether }}\) \(\underset{\mathrm{(Grignard Reagent )}}{{R-\mathrm{Mg}-X}}\)
NEET chemistry chapter-wise MCQs
Question 48. Chlorobenzene reacts with Mg in dry ether to give a compound (A) which further reacts with ethanol to yield
- Phenol
- Benzene
- Ethylbenzene
- Phenyl ether.
Answer: 2. Benzene
⇒ \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl}\) \(\underrightarrow{\mathrm{Mg}}\) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{MgCl}\) \(\underrightarrow{\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}}\) \(\mathrm{C}_6 \mathrm{H}_6+\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OMgCl}\)
Haloalkanes and haloarenes MCQs with answers
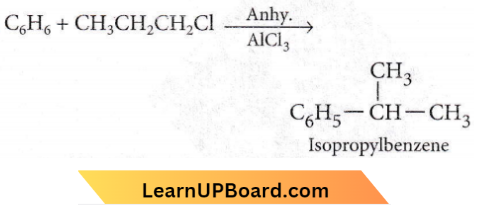
Question 49. Benzene reacts with n-propyl chloride in the presence of anhydrous AlCl3 to give
- 3-propyl-1-chlorobenzene
- n-propylbenzene
- No reaction
- Isopropylbenzene.
Answer: 4. Isopropylbenzene.
Question 50. Which chloro derivative of benzene among the following would undergo hydrolysis most readily with aqueous sodium hydroxide to furnish the corresponding hydroxy derivative?
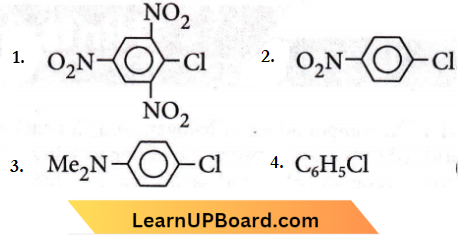
Answer: 1
Cl in 2, 4,6- trinitrochloro benzene is activated by three -NO2 groups at o-and p-positions and hence undergoes hydrolysis most readily.
Question 51. Which of the following is an optically active compound?
- 1-Butanol
- 1-Propanol
- 2-Chlorobutane
- 4-Hydroxyheptane
Answer: 3. 2-Chlorobutane
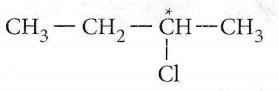
2-Chlorolrutane contains a chiral carbon atom and hence it is an optically active compound.
Haloalkanes and haloarenes practice questions
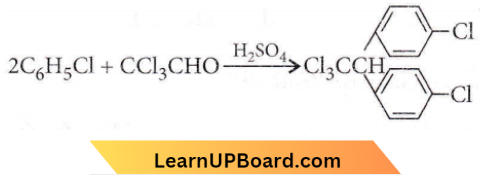
Question 52. Trichloroacetaldehyde, CCl3CHO reacts with chlorobenzene in the presence of sulphuric acid and produces
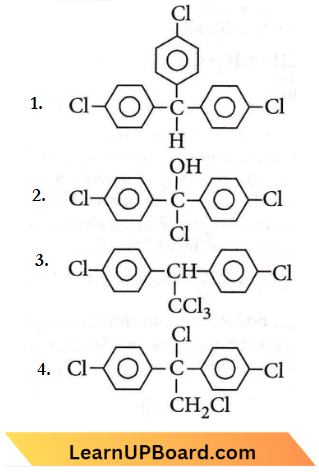
Answer: 2
It gives D.D.T
(p,p di chlorodiphenyltrichloroethane).
Haloalkanes and haloarenes practice questions
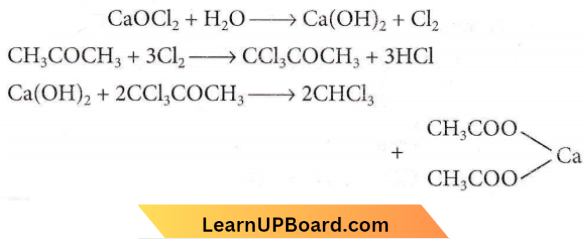
Question 53. Industrial preparation of chloroform employs acetone and
- Phosgene
- Calcium hypochlorite
- Chlorine gas
- Sodium chloride.
Answer: 3. Chlorine gas
NEET chemistry chapter-wise MCQs
Question 54. Phosgene is a common name for
- Phosphoryl chloride
- Thionyl chloride
- Carbon dioxide and phosphine
- Carbonyl chloride.
Answer: 4. Carbonyl chloride.
