General Principles And Processes Of Isolation MCQs NEET
NEET Chemistry For General Principles And Processes Of Isolation Of Elements Multiple Choice Questions
Question 1. Match List 1 and List 2
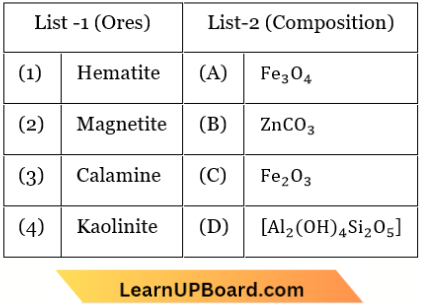
Choose the correct answer from the options given below:
- (1) -(A), (2) -(B), (3) -(C), (4) -(D)
- (1) -(C), (2) -(A), (3) -(B), (4) -(D)
- (1) -(C), (2) -(A), (3) -(D), (4) -(B)
- (1) -(A), (2) -(C), (3) -(h), (4) -(D)
Answer: 2. (1) -(C), (2) -(A), (3) -(B), (4) -(D)
Haematite \(-\mathrm{Fe}_2 \mathrm{O}_3\)
Magnetite –\(\mathrm{Fe}_3 \mathrm{O}_4\)
Calamine –\(\mathrm{ZnCO}_3\)
Kaolinite –\(\mathrm{Al}_2 \mathrm{O}_3 \cdot 2 \mathrm{SiO}_2 \cdot 2 \mathrm{H}_2 \mathrm{O}\)
Question 2. Which one is malachite from the following?
- \(\mathrm{CuCO}_3 \cdot \mathrm{Cu}(\mathrm{OH})_2\)
- \(\mathrm{CuFeS}_2\)
- \(\mathrm{Cu}(\mathrm{OH})_2\)
- \(\mathrm{Fe}_3 \mathrm{O}_4\)
Answer: 1. \(\mathrm{CuCO}_3 \cdot \mathrm{Cu}(\mathrm{OH})_2\)
Malachite: CuCO3 · Cu(OH)2
Question 3. Identify the incorrect statement.
- The scientific and technological process used for isolation of the metal from its ore is known as metallurgy.
- Minerals are naturally occurring chemical substances in the earth’s crust.
- Ores are minerals that may contain a metal.
- Gangue is an ore contaminated with undesired materials.
Answer: 4. Gangue is an ore contaminated with undesired materials.
An ore rarely contains only a desired substance. it is usually contaminated with earthly or undesired materials known as gangue.
Question 4. “Metals are usually not found as nitrates in their ores.” Out of the following two (1 and 2) reasons which is/are true for the above observation?
- Metal nitrates are highly unstable.
- Metal nitrates are highly soluble in water.
- 1 is false but 2 is true.
- 1 is true but 2 is false.
- 1 and 2 are true.
- 1 and 2 are false
Answer: 1. 1 is false but 2 is true.
All nitrates are soluble in water and are quite stable as they do not decompose easily when heated.
Read and Learn More NEET MCQs with Answers
Question 5. Which one of the following is a mineral of iron?
- Malachite
- Cassiterite
- Pyrolusite
- Magnetite
Answer: 4. Magnetite
Magnetite is Fe3O4 and contains up to 70% of iron metal.
Isolation Of Elements NEET Questions
Question 6. Cassiterite is an ore of
- Sb
- Ni
- Mn
- Sn
Answer: 4. Sn
Cassiterite is also known as tin stone (SnO2), an ore of tin (Sn).
Question 7. Sulphide ores of metals are usually concentrated by the froth floatation process. Which one of the following sulphide ores offers an exception and is concentrated by chemical leaching?
- Galena
- Copper pyrite
- Sphalerite
- Argentite
Answer: 4. Argentite
The leaching process involves the treatment of the ore with a suitable reagent as to make it soluble while impurities remain insoluble. The ore is recovered from the solution by a suitable chemical method.
Argentite or silver glance, Ag2S is an ore of silver. Silver is extracted from argentite by the MacArthur and Forest cyanide process (leaching process).
⇒ \(\mathrm{Ag}_2 \mathrm{~S}+4 \mathrm{NaCN} \longrightarrow 2 \mathrm{Na}\left[\mathrm{Ag}(\mathrm{CN})_2\right]+\mathrm{Na}_2 \mathrm{~S}\)
⇒ \(2 \mathrm{Na}\left[\mathrm{Ag}(\mathrm{CN})_2\right]+\mathrm{Zn} \longrightarrow \mathrm{Na}_2\left[\mathrm{Zn}(\mathrm{CN})_4\right]+2 \mathrm{Ag}\)
Question 8. The roasting of sulphides gives the gas X as a byproduct. This is a colourless gas with a choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as a reducing agent and its acid has never been isolated. The gas X is
- \(\mathrm{CO}_2\)
- \(\mathrm{SO}_3\)
- \(\mathrm{H}_2 \mathrm{~S}\)
- \(\mathrm{SO}_2\)
Answer:
The roasting of sulphides gives the gas X as a byproduct. This is a colourless gas with a choking smell of burnt sulphur and causes great damage to respiratory organs as a result of acid rain. Its aqueous solution is acidic, acts as a reducing agent and its acid has never been isolated.
4. \(\mathrm{SO}_2\)
Metallurgy NEET MCQs
Question 9. The reaction that does not take place in a blast furnace between 900 K to 1500 K temperature range during extraction of iron is
- \(\mathrm{C}+\mathrm{CO}_2 \rightarrow 2 \mathrm{CO}\)
- \(\mathrm{CaO}+\mathrm{SiO}_2 \rightarrow \mathrm{CaSiO}_3\)
- \(\mathrm{Fe}_2 \mathrm{O}_3+\mathrm{CO} \rightarrow 2 \mathrm{FeO}+\mathrm{CO}_2\)
- \(\mathrm{FeO}+\mathrm{CO} \rightarrow \mathrm{Fe}+\mathrm{CO}_2\)
Answer: 3. \(\mathrm{Fe}_2 \mathrm{O}_3+\mathrm{CO} \rightarrow 2 \mathrm{FeO}+\mathrm{CO}_2\)
Question 10. The maximum temperature that can be achieved in a blast furnace is
- Upto 5000 K
- Upto 1200 K
- Upto 2200 K
- Upto 1900 K.
Answer: 3. Upto 2200 K
It can withstand up to approximately 2000°C or 2200K.
Question 11. Considering the Ellingham diagram, which of the following metals can be used to reduce alumina?
- Fe
- Zn
- Mg
- Cu
Answer: 3. Mg
Any metal oxide with lower value of ΔG° is more stable than a metal oxide with higher ΔG°. This implies that the metal oxide placed higher in the Eliingham diagram can be reduced by the metal involved in the formation of the oxide placed lower in the diagram.
The relative tendency of the various metals to act as reducing agents is: Ca>Mg>AI>Zn>Fe>Cu
Thus, Mg being more reducing in nature, can reduce aluminium oxide (alumina) to aluminium.
Extraction Of Metals NEET Practice Questions
Question 12. In the extraction of copper from its sulphide ore, the metal is finally obtained by the reduction of cuprous oxide with
- Carbon monoxide
- Copper (1) sulphide
- Sulphur dioxide
- Iron (2) sulphide.
Answer: 2. Copper (1) sulphide
It is an example of auto reduction. \(2 \mathrm{Cu}_2 \mathrm{O}+\mathrm{Cu}_2 \mathrm{~S} \longrightarrow 6 \mathrm{Cu}+\mathrm{SO}_2\)
Question 13. The metal oxide which cannot be reduced to metal by carbon is
- Al2O3
- PbO
- ZnO
- Fe2O3
Answer: 1. Al2O3
Oxides of less reactive metals (like PbO, ZnO, Fe2O3) can be reduced by carbon, while oxides of very reactive metals (like Al2O3) can be reduced only by the electrolytic method.
Question 14. Which of the following elements is present as the impurity to the maximum extent in the pig iron?
- Manganese
- Carbon
- Silicon
- Phosphorus
Answer: 2. Carbon
Pig iron contains about 470 carbon and many impurities such as S, Mn, P, Si, etc’ in smaller amounts.
Question 15. The following reactions take place in the blast furnace in the preparation of impure iron. Identify the reaction pertaining to the formation of the slag.
- \(\mathrm{Fe}_2 \mathrm{O}_{3(s)}+3 \mathrm{CO}_{(g)} \rightarrow 2 \mathrm{Fe}_{(b)}+3 \mathrm{CO}_{2(g)}\)
- \(\mathrm{CaCO}_{3(s)} \rightarrow \mathrm{CaO}_{(s)}+\mathrm{CO}_{2(g)}\)
- \(\mathrm{CaO}_{(s)}+\mathrm{SiO}_{2(s)} \rightarrow \mathrm{CaSiO}_{3(s)}\)
- \(2 \mathrm{C}_{(s)}+\mathrm{O}_{2(g)} \rightarrow 2 \mathrm{CO}_{(g)}\)
Answer: 3. \(\mathrm{CaO}_{(s)}+\mathrm{SiO}_{2(s)} \rightarrow \mathrm{CaSiO}_{3(s)}\)
Slag is formed by the reaction \(\mathrm{CaO}+\mathrm{SiO}_2 \rightarrow \mathrm{CaSiO}_3\)
Question 16. Which of the following statements, about the advantage of roasting of sulphide ore before reduction is not true?
- The ΔGf° of the sulphide is greater than those for CS2 and H2S.
- The ΔGf° is negative for roasting of sulphide ore to oxide.
- Roasting of the sulphide to the oxide is thermodynamically feasible.
- Carbon and hydrogen are suitable reducing agents for metal sulphides.
Answer: 4. Carbon and hydrogen are suitable reducing agents for metal sulphides.
The standard free energies of formation (ΔGf°) of most of the sulphides are greater than those of CS2 and H2S. Hence, neither carbon nor hydrogen can reduce metal sulphides to metal. The standard free energies of the formation of oxides are much lower than those of SO2 Therefore, oxidation of metal sulphides to metal oxides is thermodynamically favourable. Hence, sulphide ore is roasted to the oxide before reduction.
General Principles OOf Extraction NEET MCQs
Question 17. Nitriding is the process of surface hardening of steel by treating it in an atmosphere of
- NH3
- O3
- N2
- H2S
Answer: 1. NH3
When steel is heated in the Presence of NH3, iron nitride on the surface of the steel is formed which imparts a hard coating. This process is called nitriding.
Question 18. Aluminium is extracted from alumina (Al2O3) by electrolysis of a molten mixture of
- \(\mathrm{Al}_2 \mathrm{O}_3+\mathrm{HF}+\mathrm{NaAlF}_4\)
- \(\mathrm{Al}_2 \mathrm{O}_3+\mathrm{CaF}_2+\mathrm{NaAlF}_4\)
- \(\mathrm{Al}_2 \mathrm{O}_3+\mathrm{Na}_3 \mathrm{AlF}_6+\mathrm{CaF}_2\)
- \(\mathrm{Al}_2 \mathrm{O}_3+\mathrm{KF}+\mathrm{Na}_3 \mathrm{AlF}_6\)
Answer: 3. \(\mathrm{Al}_2 \mathrm{O}_3+\mathrm{Na}_3 \mathrm{AlF}_6+\mathrm{CaF}_2\)
The electrolytic mixture contains alumina(Al2O3), cryolite(Na3AlF6) and fluorspar(CaF2) in the ratio of 20:40:20. Due to the presence of these, the conductivity of alumina increases and fusion temperature decreases from 2000°C to 900°c
Question 19. The purification of aluminium, by electrolytic refining, is known as
- Hoopes process
- Baeyers process
- Hall’s process
- Serpecks process.
Answer: 1. Hoopes process
Aluminium metal obtained from the Hoopet electrolytic refining process is about 99.9% pure. The cell used for this process consists of three layers. The upper layer is pure Al, which acts as the cathode, and the middle layer is a mixture of fluorides of Al and Ba, which acts as an electrolyte. The lowest layer is impure Ali which acts as anode. On electrolysis, pure Al is transferred from the bottom to the top layer, through the middle layer.
Question 20. Calcium is obtained by
- Reduction of calcium chloride with carbon
- Electrolysis of molten anhydrous calcium chloride
- Roasting of limestone
- Electrolysis of solution of calcium chloride in H2O
Answer: 2. Electrolysis of molten anhydrous calcium chloride
Calcium is obtained by the electrolysis of a fused mixture of anhydrous CaCl2 and CaF2 in a graphite-lined tank which serves as anode. The cathode is a hollow movable iron rod which is kept cool. During electrolysis, calcium is deposited at the cathode while Cl2 is liberated at the anode.
Isolation Of Elements Multiple Choice Questions
Question 21. Extraction of gold and silver involves leaching with CN– ion. Silver is later recovered by
- Distillation
- Zone refining
- Displacement with Zn
- Liquation.
Answer: 3. Displacement with Zn
Extraction of gold and silver involves leaching the metal with CN– and the metals silver and gold are later recovered by displacement method.
⇒ \(4 M_{(s)}+8 \mathrm{CN}_{(a q)}^{-}+2 \mathrm{H}_2 \mathrm{O}_{(a q)}+\mathrm{O}_{2(g)} \rightarrow 4\left[M(\mathrm{CN})_2\right]_{(a q)}^{-}+4 \mathrm{OH}_{(a q)}^{-}\)
⇒ \(2\left[\mathrm{M}(\mathrm{CN})_2\right]_{(\mathrm{miq})}^{-}+\mathrm{Zn}_{(s)} \rightarrow 2 \mathrm{M}_{(x)}+\left[\mathrm{Zn}(\mathrm{CN})_4\right]^{2-}{ }_{(\omega q)}\)
Question 22. Which one of the following methods can be used to obtain highly pure metal which is liquid at room temperature?
- Zone refining
- Electrolysis
- Chromatography
- Distillation
Answer: 4. Distillation
NEET MCQs On Metallurgy
Question 23. Identify the correct statement from the following
- Wrought iron is impure iron with 4% carbon.
- Blister copper has a blistered appearance due to the evolution of CO2.
- Vapour phase refining is carried out for nickel by the van Arkel method.
- Pig iron can be moulded into a variety of shapes.
Answer: 4. Wrought iron is impure iron with 4% carbon.
- Pig iron is impure iron with 4% carbon
- Blister copper has a blistered appearance due to the evolution of SO2.
- Vapour phase refining is carried out for the nickel bv Mondt process.
- Pig iron can be moulded into a variety of shapes
Question 24. Match items of Column 1 with the items of Column 2 and assign the correct code:
Answer: 3
Question 25. Which of the following pairs of metals is purified by the van Arkel method?
- Ga and In
- Zr and Ti
- Ag and Au
- Ni and Fe
Answer: 2. Zr and Ti
van Arkel method is used for the purification of Zr and Ti.
Extraction Processes NEET Quiz
Question 26. The method of zone refining of metals is based on the principle of
- Greater mobility of the pure metal than that of the impurity
- Higher melting point of the impurity than that of the pure metal
- The greater noble character of the solid metal than that of the impurity
- Greater solubility of the impurity in the molten state than in the solid.
Answer: 4. Greater solubility of the impurity in the molten state than in the solid.
Elements which are used as semiconductors such as Si, Ge, Ga, etc. are refined by this method, which is based on the difference in solubility of impurities in the molten and solid state of the metal.
