Chemical Kinetics NEET MCQs
Chemical Kinetics
Question 1. For the chemical reaction, \(\mathrm{N}_{2(g)}+3 \mathrm{H}_{2(g)} \rightleftharpoons 2 \mathrm{NH}_{3(g)}\) the correct option is
- \(3 \frac{d\left[\mathrm{H}_2\right]}{d t}=2 \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
- \(-\frac{1}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}=-\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
- \(-\frac{d\left[\mathrm{~N}_2\right]}{d t}=2 \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
- \(-\frac{d\left[\mathrm{~N}_2\right]}{d t}=\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
Answer: 4. \(-\frac{d\left[\mathrm{~N}_2\right]}{d t}=\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
For the given chemical reaction,
Rate of reaction = \(-\frac{d\left[\mathrm{~N}_2\right]}{d t}=-\frac{1}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}=\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}\)
Question 2. The rate of the reaction \(2 \mathrm{~N}_2 \mathrm{O}_5 \rightarrow 4 \mathrm{NO}_2+\mathrm{O}_2\) can be written in three ways.
- \(\frac{-d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t}=k\left[\mathrm{~N}_2 \mathrm{O}_5\right]\)
- \(\frac{d\left[\mathrm{NO}_2\right]}{d t}=k^{\prime}\left[\mathrm{N}_2 \mathrm{O}_5\right]\); \(\frac{d\left[\mathrm{O}_2\right]}{d t}=k^{\prime \prime}\left[\mathrm{N}_2 \mathrm{O}_5\right]\)
The relationship between k and k’ and between k and k” are
- k’ = 2k, k” = k
- k’ = 2k, k” = k/2
- k’ = 2k, k” = 2k
- k’ = k, k” = k
Answer: 2. k’ = 2k, k” = k/2
For the reaction, \(2 \mathrm{~N}_2 \mathrm{O}_5 \rightarrow 4 \mathrm{NO}_2+\mathrm{O}_2\)
⇒ \(-\frac{1}{2} (k) \frac{d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t} (k’) =+\frac{1}{4} \frac{d\left[\mathrm{NO}_2\right]}{d t}=+\frac{d\left[\mathrm{O}_2\right]}{d t}(k”)\)
⇒ \(\frac{1}{2} k=\frac{1}{4} k^{\prime}=k^{\prime \prime}, k^{\prime}=2 k ; k^{\prime \prime}=\frac{1}{2} k\)
Question 3. For the reaction \(\mathrm{N}_2 \mathrm{O}_{5(g)} \rightarrow 2 \mathrm{NO}_{2(g)}+1 / 2 \mathrm{O}_{2(g)}\) the value of rate of disappearance of N2O5 is given as 6.25 x 10-3 mol L-1s-1 The rate of formation of N02 and 02 is given respectively as
- \(6.25 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\) and \(6.25 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
- \(1.25 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\) and \(3.125 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
- \(6.25 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\) and \(3.125 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
- \(1.25 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\) and \(6.25 \times 10^{-3} \mathrm{moll}^{-1} \mathrm{~s}^{-4}\)
Answer: 2. \(1.25 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\) and \(3.125 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
For the reaction \(\mathrm{N}_2 \mathrm{O}_{5(g)} \rightarrow 2 \mathrm{NO}_{2(g)}+1 / 2 \mathrm{O}_{2(g)}\) the value of rate of disappearance of N2O5 is given as 6.25 x 10-3 mol L-1s-1
⇒ \(\mathrm{N}_2 \mathrm{O}_{5(g)} \rightarrow 2 \mathrm{NO}_{2(g)}+1 / 2 \mathrm{O}_{2(g)}\)
For the given reaction the rate is written as \(\frac{-d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t}=\frac{1}{2} \frac{d\left[\mathrm{NO}_2\right]}{d t}=\frac{2 d\left[\mathrm{O}_2\right]}{d t}\)
Given that \(\frac{-d\left[\mathrm{~N}_2 \mathrm{O}_5\right]}{d t}=6.25 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
∴ \(\frac{d\left[\mathrm{NO}_2\right]}{d t}=2 \times 6.25 \times 10^{-3}=1.25 \times 10^{-2} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
and \(\frac{d\left[\mathrm{O}_2\right]}{d t}=\frac{6.25 \times 10^{-3}}{2}=3.125 \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
Chemical Kinetics NEET MCQs
Question 4. For the reaction, \(\mathrm{N}_2+3 \mathrm{H}_2 \rightarrow 2 \mathrm{NH}_3\) if \(\frac{d\left[\mathrm{NH}_3\right]}{d t}=2 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\) the value of \(\frac{-d\left[\mathrm{H}_2\right]}{d t}\) would be
- \(4 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
- \(6 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
- \(1 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
- \(3 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
Answer: 4. \(3 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
For reaction, \(\mathrm{N}_2+3 \mathrm{H}_2 \rightarrow 2 \mathrm{NH}_3\)
Rate = \(\frac{1}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{1}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}=-\frac{d\left[\mathrm{~N}_2\right]}{d t}\)
Given, \(\frac{d\left[\mathrm{NH}_3\right]}{d t}=2 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
∴ \(-\frac{d\left[\mathrm{H}_2\right]}{d t}=\frac{3}{2} \frac{d\left[\mathrm{NH}_3\right]}{d t}=\frac{3}{2} \times 2 \times 10^{-4}\)
⇒ \(-\frac{d\left[\mathrm{H}_2\right]}{d t}=3 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}\)
Question 5. In the reaction: \(\mathrm{BrO}_{3(\text { aq) }}^{-}+5 \mathrm{Br}_{(\text {aq) }}+6 \mathrm{H}_{(\text {aq) }}^{+} \rightarrow 3 \mathrm{Br}_{2(t)}+3 \mathrm{H}_2 \mathrm{O}_{(l)}\). The rate of disappearance of bromide ions as
- \(\frac{d\left[\mathrm{Br}_2\right]}{d t}=-\frac{5}{3} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}\)
- \(\frac{d\left[\mathrm{Br}_2\right]}{d t}=\frac{5}{3} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}\)
- \(\frac{d\left[\mathrm{Br}_2\right]}{d t}=\frac{3}{5} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}\)
- \(\frac{d\left[\mathrm{Br}_2\right]}{d t}=-\frac{3}{5} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}\)
Answer: 4. \(\frac{d\left[\mathrm{Br}_2\right]}{d t}=-\frac{3}{5} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}\)
In the reaction: \(\mathrm{BrO}_{3(\text { aq) }}^{-}+5 \mathrm{Br}_{(\text {aq) }}+6 \mathrm{H}_{(\text {aq) }}^{+} \rightarrow 3 \mathrm{Br}_{2(t)}+3 \mathrm{H}_2 \mathrm{O}_{(l)}\).
For the given reaction, \(\mathrm{BrO}_3^{-}{ }_{(a q)}^{-}+5 \mathrm{Br}_{(a q)}^{-}+6 \mathrm{H}^{+}{ }_{(a q)} \rightarrow 3 \mathrm{Br}_{2(l)}+3 \mathrm{H}_2 \mathrm{O}_{(l)}\)
Rate of reaction in terms of \(\mathrm{Br}_2\) and \(\mathrm{Br}^{-}\) is,
Rate = \(\frac{1}{3} \frac{d\left[\mathrm{Br}_2\right]}{d t}=-\frac{1}{5} \frac{d\left[\mathrm{Br}^{-}\right]}{d t} \)
∴ \(\frac{d\left[\mathrm{Br}_2\right]}{d t}=-\frac{3}{5} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}\)
Question 6. Consider the reaction: \(\mathrm{N}_{2(g)}+3 \mathrm{H}_{2(g)} \rightarrow 2 \mathrm{NH}_{3(g)}\) The equality relationship between, \(\frac{d\left[\mathrm{NH}_3\right]}{d t}\) and \(-\frac{d\left[\mathrm{H}_2\right]}{d t}\) is
- \(\frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{d\left[\mathrm{H}_2\right]}{d t}\)
- \(\frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{1}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}\)
- \(+\frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{2}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}\)
- \(+\frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{3}{2} \frac{d\left[\mathrm{H}_2\right]}{d t}\)
Answer: 3. \(+\frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{2}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}\)
⇒ \(\mathrm{N}_{2(g)}+3 \mathrm{H}_{2(g)} \rightarrow 2 \mathrm{NH}_{3(g)}\)
Rate = \(\frac{-d\left[\mathrm{~N}_2\right]}{d t}=-\frac{d\left[\mathrm{H}_2\right]}{3 d t}=+\frac{d\left[\mathrm{NH}_3\right]}{2 d t}\)
Hence, \(+\frac{d\left[\mathrm{NH}_3\right]}{d t}=-\frac{2}{3} \frac{d\left[\mathrm{H}_2\right]}{d t}\)
Question 7. For the reaction, 2A + B → 3C + D, which of the following does not express the reaction rate?
- \(-\frac{d[A]}{2 d t}\)
- \(-\frac{d[C]}{3 d t}\)
- \(-\frac{d[B]}{d t}\)
- \(\frac{d[D]}{d t}\)
Answer: 2. \(-\frac{d[C]}{3 d t}\)
2 A+B → 3 C+D
rate = \(\frac{-d[A]}{2 d t}=-\frac{d[B]}{d t}=\frac{d[C]}{3 d t}=\frac{d[D]}{d t}\)
A negative sign shows a decrease in concentration.
Question 8. 3A → 2B, rate of reaction \(\frac{+d[B]}{d t}\) is equal to
- \(-\frac{3}{2} \frac{d[A]}{d t}\)
- \(-\frac{2}{3} \frac{d[A]}{d t}\)
- \(-\frac{1}{3} \frac{d[A]}{d t}\)
- \(+2 \frac{d[A]}{d t}\)
Answer: 2. \(-\frac{2}{3} \frac{d[A]}{d t}\)
3 A → 2 B
Rate of the reaction = \(\frac{1}{2} \frac{d[B]}{d t}=-\frac{1}{3} \frac{d[A]}{d t}\)
⇒ \(\frac{d[B]}{d t}=-\frac{2}{3} \frac{d[A]}{d t}\)
NEET questions on Chemical Kinetics
Question 9. For the reaction, \(\mathrm{H}^{+}+\mathrm{BrO}_3^{-}+3 \mathrm{Br}^{-} \longrightarrow 5 \mathrm{Br}_2+\mathrm{H}_2 \mathrm{O}\) which of the following relations correctly represents the consumption and formation of products?
- \(\frac{d\left[\mathrm{Br}^{-}\right]}{d t}=-\frac{3}{5} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
- \(\frac{d\left[\mathrm{Br}^{-}\right]}{d t}=\frac{3}{5} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
- \(\frac{d\left[\mathrm{Br}^{-}\right]}{d t}=-\frac{5}{3} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
- \(\frac{d\left[\mathrm{Br}^{-}\right]}{d t}=\frac{5}{3} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
Answer: 1. \(\frac{d\left[\mathrm{Br}^{-}\right]}{d t}=-\frac{3}{5} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
For the reaction, \(\mathrm{H}^{+}+\mathrm{BrO}_3^{-}+3 \mathrm{Br}^{-} \longrightarrow 5 \mathrm{Br}_2+\mathrm{H}_2 \mathrm{O}\)
Rate of reaction = \(-\frac{1}{3} \frac{d\left[\mathrm{Br}^{-}\right]}{d t}=+\frac{1}{5} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
∴ \(\frac{d\left[\mathrm{Br}^{-}\right]}{d t}=-\frac{3}{5} \frac{d\left[\mathrm{Br}_2\right]}{d t}\)
Question 10. For the reaction, \(\mathrm{H}_{2(g)}+\mathrm{I}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{HI}_{(\mathrm{g})}\) the rate of reaction is expressed as
- \(\frac{\Delta\left[\mathrm{H}_2\right]}{\Delta t}=\frac{1}{2} \frac{\Delta\left[\mathrm{I}_2\right]}{\Delta t}=-\frac{\Delta[\mathrm{HI}]}{\Delta t}\)
- \(-\frac{\Delta\left[\mathrm{I}_2\right]}{\Delta t}=-\frac{\Delta\left[\mathrm{H}_2\right]}{\Delta t}=\frac{1}{2} \frac{\Delta[\mathrm{HI}]}{\Delta t}\)
- \(\frac{\Delta\left[\mathrm{I}_2\right]}{\Delta t}=\frac{\Delta\left[\mathrm{H}_2\right]}{\Delta t}=\frac{\Delta[\mathrm{HI}]}{2 \Delta t}\)
- None of these.
Answer: 2. \(-\frac{\Delta\left[\mathrm{I}_2\right]}{\Delta t}=-\frac{\Delta\left[\mathrm{H}_2\right]}{\Delta t}=\frac{1}{2} \frac{\Delta[\mathrm{HI}]}{\Delta t}\)
For the reaction, \(\mathrm{H}_{2(g)}+\mathrm{I}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{HI}_{(\mathrm{g})}\)
For \(\mathrm{H}_{2(g)}+\mathrm{I}_{2(g)} \rightarrow 2 \mathrm{HI}_{(g)}\), the rate of reaction is
⇒ \(-\frac{\Delta\left[\mathrm{H}_2\right]}{\Delta t}=-\frac{\Delta\left[\mathrm{I}_2\right]}{\Delta t}=\frac{1}{2} \frac{\Delta[\mathrm{HI}]}{\Delta t}\)
The negative sign shows the disappearance of the reactant and the positive sign shows the appearance product
Question 11. For a certain reaction, the rate = k[A]²[B], when the initial concentration of A is tripled keeping the concentration of B constant, the initial rate would
- Increase by a factor of nine
- Increase by a factor of three
- Decrease by a factor of nine
- Increase by a factor of six.
Answer: 1. Increase by a factor of nine
⇒ \(r_1=k[A]^2[B]\)
Keeping concentration of B constant and tripling conc. of A, new rate would be \(r_2=k[3 A]^2[B]=9 k[A]^2[B]\)
∴ \(r_2=9 \times r_1\)
NEET questions on Chemical Kinetics
Question 12. Mechanism of a hypothetical reaction, \(X_2+Y_2 \longrightarrow 2 X Y\), is given below:
- \(X_2 \rightarrow X+X\) (fast)
- \(X+Y_2 \rightleftharpoons X Y+Y\) (slow)
- \(X+Y \longrightarrow X Y\) (fast)
The overall order of the reaction will be
- 2
- 0
- 1.5
- 1
Answer: 3. 1.5
Correct the reactions given in question as \(X_2 \rightleftharpoons{ } X+X\) fast; \(X+Y_2 \longrightarrow X Y+Y\) (slow)
The slow step is the rate-determining step.
Rate \(=k[X]\left[Y_2\right]\)…(1)
Equilibrium constant for fast step, \(K=\frac{[X]^2}{\left[X_2\right]}\)
[X] = \(\sqrt{K\left[X_2\right]}\)
By substituting [X] in equation (1), we get
Rate = \(k \sqrt{K\left[X_2\right]}\left[Y_2\right]=k\left[X_2\right]^{1 / 2}\left[Y_2\right]\)
∴ Order of reaction = \(\frac{1}{2}+1=\frac{3}{2}=1.5\)
Question 13. The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
- The rate is proportional to the surface coverage
- Rate is inversely proportional to the surface coverage
- Rate is independent of the surface coverage
- The rate of decomposition is very slow.
Answer: 1. Rate is proportional to the surface coverage
At low pressure, the rate is proportional to the surface coverage and is of first order while at high pressure, it follows zero-order kinetics due to the complete coverage of the surface area.
Question 14. The rate constant of the reaction A → B is 0. 6 x 10-3 mol L-1 s-1. If the concentration of A is 5 M, then the concentration of B after 20 minutes is
- 3.60 M
- 0.36 M
- 0.72 M
- 1.08 M
Answer: 3. 0.72 M
The rate constant of the reaction A → B is 0. 6 x 10-3 mol L-1 s-1. If the concentration of A is 5 M
The reaction is of zero order as the unit of rate constant is mol L-1 s-1.
Concentration of B =k x t = 0.6 x 10-3 x 20 x 60 =0.72M
Question 15. For a reaction between A and B, the order with respect to A is 2 and the order with respect to B is 3. The concentrations of both A and B are doubled, the rate will increase by a factor of
- 12
- 16
- 32
- 10
Answer: 3. 32
For a reaction between A and B, the order with respect to A is 2 and the order with respect to B is 3. The concentrations of both A and B are doubled
Rate \(_1=k[A]^2[B]^3\)
Rate \(_2=k[2 A]^2[2 B]^3\)
Rate \(_2=32 k[A]^2[B]^3\)
∴ Rate \(_2=32\left(\text { Rate }_1\right)\)
Chemical Kinetics multiple choice NEET
Question 16. In a reaction, A + B → product, the rate is doubled when the concentration of B is doubled, and the rate increases by a factor of 8 when the concentration of both the reactants (A and B) are doubled, the rate law for the reaction can be written as
- Rate = k[A][B]²
- Rate = k[A]²[B]²
- Rate = k[A][B]
- Rate = k[A]²[B]
Answer: 4. Rate = k[A]²[B]
In a reaction, A + B → product, the rate is doubled when the concentration of B is doubled, and the rate increases by a factor of 8 when the concentration of both the reactants (A and B) are doubled
⇒ \(\begin{array}{ccc}
{[A]} & {[B]} & \text { Rate } \\
x & y & R …….(1)\\
x & 2 y & 2 R ……(2)\\
2 x & 2 y & 8 R….(3)
\end{array}\)
Let the rate law; rate \(=k[A]^a[B]^b\)
From data given, \((x)^a(y)^b=R\)….(4)
⇒ \((x)^a(2 y)^b=2 R\)…….(5)
Dividing eqn. (5) by (4), \(\frac{(2 y)^b}{(y)^b}=\frac{2 R}{R} \Rightarrow(2)^b=2=2^1\)
Thus, b=1
From data of (3) experiment, \((2 x)^a(2 y)^b=8 R\)….(6)
From equation (5) and (6), \(\frac{(2 x)^a}{(x)^a}=\frac{8 R}{2 R} \Rightarrow(2)^a=4=2^2\)
Thus, a=2. By replacing the values of a and b in rate law; rate \(=k[A]^2[B]\)
Question 17. Which one of the following statements for the order of a reaction is incorrect?
- Order can be determined only experimentally.
- Order is not influenced by the stoichiometric coefficient of the reactants.
- The order of a reaction is the sum of power to the concentration terms of reactants to express the rate of reaction.
- The order of reaction is always a whole number.
Answer: 4. Order of reaction is always a whole number.
The order of a reaction is not always a whole number. It can be zero, or fractional also.
Question 18. The unit of rate constant for a zero-order reaction is
- mol L-1 s-1
- L mol-1 s-1
- L2 mol-2 s-1
- s-1
Answer: 1. mol L-1 s-1
Rate = k[A]0
mol L-1 s-1 = k
Thus, the unit of rate constant is mol L-1 s-1
Question 19. During the kinetic study of the reaction, 2A + B → C + D, following results were obtained:
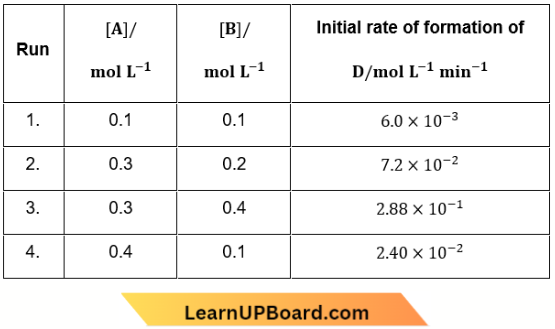
Based on the above data which one of the following is correct?
- Rate = k[A]²[B]
- Rate = k[A][B]
- Rate = k[A]²[B]²
- Rate = k[A][B]²
Answer: 4. Rate = k[A][B]²
Let the rate of reaction be given by rate = \(k[A]^a[B]^b\)
Now consider 2 and 3 where [A] is constant, \(\frac{7.2 \times 10^{-2}}{2.88 \times 10^{-1}}=\frac{[0.3]^a[0.2]^b}{[0.3]^a[0.4]^b}\)
⇒ \(\frac{1}{4}=\left(\frac{1}{2}\right)^b \Rightarrow b=2\)
Now consider 1 and 4, \(\frac{6.0 \times 10^{-3}}{2.4 \times 10^{-2}}=\frac{[0.1]^a[0.1]^b}{[0.4]^a[0.1]^b}\)
⇒ \(\frac{1}{4}=\left(\frac{1}{4}\right)^a \Rightarrow a=1\)
Rate = \(k[A][B]^2\)
Chemical Kinetics multiple choice NEET
Question 20. For the reaction, A + B → products, it is observed that
- On doubling the initial concentration of an only, the rate of reaction is also doubled and
- On doubling the initial concentration of both a and b, there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by
- Rate = k[A] [B]²
- Rate = k[A]²[B]²
- Rate = k[A][B]
- Rate = k[A]²[B]
Answer: 1. Rate = k[A][B]²
R = \(k[A]^m[B]^n\)….(1)
2R = \(k[2 A]^m[B]^n\)…..(2)
from (1), (2) and (3), m=1, n=2….(3)
So, rate \(=k[A][B]^2\)
Question 21. The bromination of acetone that occurs in an acid solution is represented by this equation. \(\mathrm{CH}_3 \mathrm{COCH}_{3(a q)}+\mathrm{Br}_{2(a q)} \longrightarrow \mathrm{CH}_3 \mathrm{COCH}_2 \mathrm{Br}_{(a q)}\) + \(\mathrm{H}_{(a q)}^{+}+\mathrm{Br}_{(a q)}^{-}\)
These kinetic data were obtained for given reaction concentrations. Initial concentrations, M
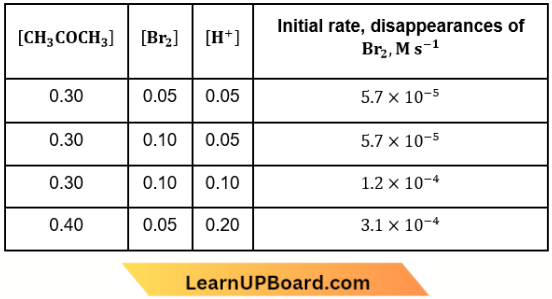
Based on these data, the rate equation is
- Rate \(=k\left[\mathrm{CH}_3 \mathrm{COCH}_3\right]\left[\mathrm{Br}_2\right]\left[\mathrm{H}^{+}\right]^2\)
- Rate \(=k\left[\mathrm{CH}_3 \mathrm{COCH}_3\right]\left[\mathrm{Br}_2\right]\left[\mathrm{H}^{+}\right]\)
- Rate \(=k\left[\mathrm{CH}_3 \mathrm{COCH}_3\right]\left[\mathrm{H}^{+}\right]\)
- Rate \(=k\left[\mathrm{CH}_3 \mathrm{COCH}_3\right]\left[\mathrm{Br}_2\right]\)
Answer: 3. Rate \(=k\left[\mathrm{CH}_3 \mathrm{COCH}_3\right]\left[\mathrm{H}^{+}\right]\)
From the first two experiments, it is clear that when the concentration of Br2 is doubled, the initial rate of disappearance of Br2 remains unaltered. So, the order of reaction with respect to Br2 is zero. Thus, the probable rate law for the reaction will be k[CH3COCH3][H+]
Question 22. The reaction of hydrogen and iodine monochloride is given as: \(\mathrm{H}_{2(g)}+2 \mathrm{ICl}_{(g)} \rightarrow 2 \mathrm{HCl}_{(g)}+\mathrm{I}_{2(g)}\) This reaction is of first order with respect to H2(g) and ICl(g), following mechanisms were proposed.
Mechanism A: \(\mathrm{H}_{2(g)}+2 \mathrm{ICl}_{(g)} \rightarrow 2 \mathrm{HCl}_{(g)}+\mathrm{I}_{2(g)}\)
Mechanism B: \(\mathrm{H}_{2(g)}+\mathrm{ICl}_{(g)} \rightarrow \mathrm{HCl}_{(g)}+\mathrm{HI}_{(g)} \text {; slow } \); \(\mathrm{HI}_{(g)}+\mathrm{ICl}_{(g)} \rightarrow \mathrm{HCl}_{(g)}+\mathrm{I}_{2(g)} \text {; fast }\)
Which of the above mechanism(s) can be consistent with the given information about the reaction?
- A and B both
- Neither A nor B
- A only
- B only
Answer: 4. B only
The slow step is the rate-determining step and it involves 1 molecule of H2(g) and 1 molecule of ICI(g). Hence, the rate will be, r = k[H2(g)] [ICl(g)]
i.e., the reaction is 1st order with respect to H2(g) and ICl(g).
Question 23. The rate of reaction between two reactants A and B decreases by a factor of 4 if the concentration of reactant B is doubled. The order of this reaction with respect to reactant B is
- 2
- -2
- 1
- -1
Answer: 2. -2
The rate of reaction between two reactants A and B decreases by a factor of 4 if the concentration of reactant B is doubled.
Rate of reaction \(=k[A] \alpha[B] \beta\)
α → order of reaction with respect to A
β → order of reaction with respect to B
⇒ \(r_1=k[A]^\alpha[B]^\beta\)
⇒ \(r_2=r_1 / 4=k[A]^\alpha[2 B]^\beta\)
⇒ \(\frac{r_1}{r_2}=\frac{k[A]^\alpha[B]^\beta}{k[A]^\alpha[2 B]^\beta} \Rightarrow 4=\left(\frac{1}{2}\right)^\beta \Rightarrow \beta=-2\)
NEET practice questions Chemical Kinetics
Question 24. If the rate of the reaction is equal to the rate constant, the order of the reaction is
- 0
- 1
- 2
- 3
Answer: 1. 0
A → products
If \(-\frac{d x}{d t}=k\), it means \(-\frac{d x}{d t}=k[A]^0=k\)
Hence, the order of reaction must be zero.
Question 25. 2A → B + C, It would be a zero-order reaction when
- The rate of reaction is proportional to the square of the concentration of A
- The rate of reaction remains the same at any concentration of A
- The rate remains unchanged at any concentration of B and C
- The rate of reaction doubles if the concentration of B is increased to double.
Answer: 2. The rate of reaction remains the same at any concentration of A
2A → B+C
The rate equation of this reaction may be expressed as r = k[A]0, Order= 0, r=k
∴ The rate is independent of the concentration of the reactant A
Question 26. For the reaction; \(2 \mathrm{~N}_2 \mathrm{O}_5 \rightarrow 4 \mathrm{NO}_2+\mathrm{O}_2\) rate and rate constant are 1.02 x 104 and 3.4 x 10-5 sec-1 respectively, then concentration of N2O5 at that time will be
- 1.732
- 3
- 1.02 x 10-4
- 3.4 x 105
Answer: 2. 3
For the reaction; \(2 \mathrm{~N}_2 \mathrm{O}_5 \rightarrow 4 \mathrm{NO}_2+\mathrm{O}_2\) rate and rate constant are 1.02 x 104 and 3.4 x 10-5 sec-1 respectively,
⇒ \(2 \mathrm{~N}_2 \mathrm{O}_5 \rightarrow 4 \mathrm{NO}_2+\mathrm{O}_2\)
This is a first-order reaction.
∴ rate = \(k\left[\mathrm{~N}_2 \mathrm{O}_5\right]\)
⇒ \([\mathrm{N}_2 \mathrm{O}_5]=\text { rate } / k=\frac{1.02 \times 10^{-4}}{3.4 \times 10^{-5}}=3\)
Question 27. The experimental data for the reaction, 2A + B2 →2AB is
⇒ \(\begin{array}{cccl}
\text { Experiment } & {[\boldsymbol{A}]} & {\left[\boldsymbol{B}_2\right]} & \text { Rate }\left(\text { mole s }^{-1}\right) \\
1 & 0.50 & 0.50 & 1.6 \times 10^{-4} \\
2 & 0.50 & 1.00 & 3.2 \times 10^{-4} \\
3 & 1.00 & 1.00 & 3.2 \times 10^{-4}
\end{array}\)
The rate equation for the above data is
- Rate = k [A]²[B]²
- Rate = k [A]²[B]
- Rate = k [B2]
- Rate = k [B2]²
Answer: 3. Rate = k [B2]
For the reaction, \(2 A+B_2 \rightleftharpoons 2 A B\),
Rate \(\propto[A]^x\left[B_2\right]^y\).
From experiment 1, \(1.6 \times 10^{-4} \propto[0.50]^x[0.50]^y\)….(1)
From experiment 2, \(3.2 \times 10^{-4} \propto[0.50]^x[1.00]^y\)…(2)
From experiment 3, \(3.2 \times 10^{-4} \propto[1.00]^x[1.00]^y\)….(3)
On dividing equation (3) by (2), we get, 1 = \(\left[\frac{1.00}{0.50}\right]^x \Rightarrow 1=2^x \Rightarrow 2^0=2^x \Rightarrow x=0\)
Now, divide equation (2) by equation (1) we get, 2 = \(\left[\frac{1.00}{0.50}\right]^y \Rightarrow 2=2^y \Rightarrow y=1\)
Thus, rate equation is : Rate \(=k[A]^0\left[B_2\right]^1=k\left[B_2\right]\)
NEET practice questions Chemical Kinetics
Question 28. The given reaction, \(2 \mathrm{FeCl}_3+\mathrm{SnCl}_2 \rightarrow 2 \mathrm{FeCl}_2+\mathrm{SnCl}_4 \) is an example of
- Third order reaction
- First order reaction
- Second order reaction
- None of these.
Answer: 1. Third-order reaction
For a general reaction, xA + yB + zC → product, the order of the reaction is x + y + z.
Since three molecules undergo a change in concentration, therefore it is a third-order reaction
Question 29. The data for the reaction A + B → C, is
⇒ \(\begin{array}{lccc}
\text { Exp. } & {[A]_0} & {[B]_0} & \text { Initial rate } \\
1 & 0.012 & 0.035 & 0.10 \\
2 & 0.024 & 0.070 & 0.80 \\
3 & 0.024 & 0.035 & 0.10 \\
4 & 0.012 & 0.070 & 0.80
\end{array}\)
The rate law corresponds to the above data is
- Rate = k[A][B]³
- Rate = k[A]²[B]²
- Rate = k[B]³
- Rate = k[B]4.
Answer: 3. Rate = k[B]3
A+B → C
Let rate = k[A]x [B]y
where order ofreaction is (x + y).
Putting the values of exp. 1, 2, and 3, we get the following equations.
0.10 = k [0.012]x [0.035]y ….(1)
0.80=k [0.024]x [0.070]y ……(2)
0.10 = k [0.024]x [0.035]y …..(3)
Dividing (2) by (3), we get \(\frac{0.80}{0.10}=\left(\frac{0.070}{0.035}\right)^y \Rightarrow 2^y=8 \Rightarrow y=3\)
Keeping [A] constant, [B] is doubled, and the rate becomes B times. Dividing eq. (3) by eq. (1), we get \(\frac{0.10}{0.10}=\left(\frac{0.024}{0.012}\right)^x \Rightarrow 2^x=1 \Rightarrow x=0\)
Keeping [B] constant, [A] is doubled, and the rate remains unaffected. Hence, the rate is independent of [A].
rate ∝ [B]³.
Question 30. For a first-order reaction A → Products, the initial concentration of A is 0.1 M, which becomes 0.001 M after 5 minutes. The rate constant for the reaction in min-1 is
- 1.3818
- 0.9212
- 0.4606
- 0.2303
Answer: 2. 0.9212
For a first-order reaction A → Products, the initial concentration of A is 0.1 M, which becomes 0.001 M after 5 minutes.
For a first-order reaction
k = \(\frac{2.303}{t} \log \frac{a}{a-x}\)
k = \(\frac{2.303}{5} \log \frac{0.1}{0.001}\)
k = \(\frac{2.303}{5} \log 10^2=\frac{2.303 \times 2}{5}=0.9212 \mathrm{~min}^{-1}\)
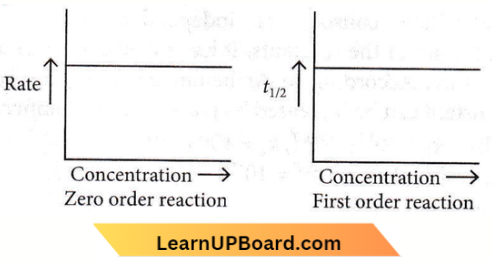
Question 31. The given graph is a representation of the kinetics of a reaction.
The y and x axes for zero and first-order reactions, respectively are
- Zero order (y = concentration and x = time), first order (y = t1/2 and x = concentration)
- Zero order (y = concentration and x = time), first order (y = rate constant and x = concentration)
- Zero order (y = rate and x = concentration), first order (y = t1/2 and x = concentration)
- Zero order (y = rate and x = concentration), first order (y = rate and x = t1/2)
Answer: 3. Zero order (y = rate and x = concentration), first order (y = t1/2 and x = concentration)
Chemistry MCQs Chemical Kinetics NEET
Question 32. The rate constant for a first-order reaction is 4.606 x 10-3 s-1 The time required to reduce 2.0 g of the reactant to 0.2 g is
- 100 s
- 200s
- 500 s
- 1000 s
Answer: 3. 500 s
For a first-order reaction,
k = \(\frac{2.303}{t} \log \frac{[R]_0}{[R]}\)
t = \(\frac{2.303}{4.606 \times 10^{-3} \mathrm{~s}^{-1}} \log \left(\frac{2}{0.2}\right)=\frac{2.303 \times 10^3}{4.606}=500 \mathrm{~s}\)
Question 33. If the rate constant for a first-order reaction is k, the time (t) required for the completion of 99% of the reaction is given by
- t = 2303/k
- t = 0.693/k
- t = 6.909/k
- t = 4.606/k
Answer: 4. t = 4.606/k
For 1 st order reaction,
t = \(\frac{2.303}{k} \log \frac{a}{a-x}=\frac{2.303}{k} \log \frac{100}{100-99}\)
= \(\frac{2.303}{k} \log 10^2=\frac{2.303}{k} \times 2 \times \log 10=\frac{4.606}{k}\)
Question 34. A first-order reaction has a rate constant of 2.303 x 10-3 s-1. The time required for 40 g of this reactant to reduce to 10 g will be [Given that log10 2 = 0.3010]
- 230.3 s
- 301 s
- 2000 s
- 602 s
Answer: 4. 602 s
For a first-order reaction, k = \(\frac{2.303}{t} \log \frac{[A]_0}{[A]_t}\)
⇒ \(2.303 \times 10^{-3}=\frac{2.303}{t} \log \frac{40}{10}\)
t = \(\frac{1}{10^{-3}} \log 2^2=\frac{2}{10^{-3}} \log 2=\frac{2}{10^{-3}} \times 0.3010=602 \mathrm{~s}\)
Question 35. The correct difference between first and second-order reactions is that
- The rate of a first-order reaction does not depend on reactant concentrations; the rate of a second-order reaction does depend on reactant concentrations
- The half-life of a first-order reaction does not depend on [A]02; the half-life of a second-order reaction does depend on [A]0
- A first-order reaction can be catalyzed; a second-order reaction cannot be catalyzed
- The rate of a first-order reaction does depend on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations.
Answer: 2. The half-life of a first-order reaction does not depend on [A]0; the half-life of a second-order reaction does depend on [A]0
For the first order reaction, \(t_{1 / 2}=\frac{0.693}{k}\) independent of initial concentration [A]0
For second order reaction, \(t_{1 / 2}=\frac{1}{k[A]_0}\)
Half-life depends on the initial concentration of the reactant
Chemistry MCQs Chemical Kinetics NEET
Question 36. When the initial concentration of the reactant is doubled, the half-life period of a zero-order reaction
- Is halved
- Is doubled
- Is tripled
- Remains unchanged.
Answer: 2. Is doubled
⇒ \(\left(t_{1 / 2}\right)_{\text {zero }}=\frac{[A]_0}{2 k}\)
The half-life of a zero-order reaction is directly proportional to the initial concentration.
∴ If [A]2 = doubled then, t1/2 = doubled
Question 37. A first-order reaction has a specific reaction rate of 10-2 sec-1. How much time will it take for 20 g of the reactant to reduce to 5 g?
- 138.6 sec
- 346.5 sec
- 693.0 sec
- 238.6 sec
Answer: 1. 138.6 sec
For a first-order reaction, k = \(\frac{2.303}{t} \log \frac{[A]_0}{[A]_t}\) or \(10^{-2}=\frac{2.303}{t} \log \frac{20}{5}\)
⇒ \(10^{-2}=\frac{2.303 \times 0.6020}{t}\) or \(t=138.6 \mathrm{sec}\)
Question 38. The rate of the first-order reaction is 0.04 mol L-1 s-1 at 10 seconds and 0.03 mol L-1 s-1 at 20 seconds after the initiation of the reaction. The half-life period of the reaction is
- 44.1 s
- 54.1s
- 24.1 s
- 34.1 s
Answer: 3. 24.1 s
The rate of the first-order reaction is 0.04 mol L-1 s-1 at 10 seconds and 0.03 mol L-1 s-1 at 20 seconds after the initiation of the reaction.
For a first-order reaction, A → products and for the concentration of the reactant at two different times,
k = \(\frac{2.303}{t_2-t_1} \log \frac{[A]_1}{[A]_2}\)
k = \(\frac{2.303}{t_2-t_1} \log \frac{(\text { rate })_1}{(\text { rate })_2}\) (because rate ∝ [A])
k = \(\frac{2.303}{(20-10)} \log \left(\frac{0.04}{0.03}\right)=0.0287 \mathrm{sec}^{-1}\)
∴ \(t_{1 / 2}= \frac{0.693}{k}=\frac{0.693}{0.0287 \mathrm{sec}^{-1}}=24.14 \mathrm{sec}\)
Question 39. When the initial concentration of a reactant is doubled in a reaction, its half-life period is not affected. The order of the reaction is
- Second
- More than zero but less than the first
- Zero
- First.
Answer: 4. First
The half-life period of a first-order reaction is independent of initial concentration, \(t_{1 / 2}=\frac{0.693}{k}\)
Chemical Kinetics quiz for NEET
Question 40. A reaction is 50% complete in 2 hours and 75% complete in 4 hours. The order of the reaction is
- 1
- 2
- 3
- 0
Answer: 1. 1
As t75% = 2 x t50%, the order of the reaction is one.
Question 41. The half-life of a substance in a certain enzyme-catalyzed reaction is 138 s. The time required for the concentration of the substance to fall from 1.28 mg L-1 to 0.04 mg L-1 is
- 414 s
- 552 s
- 690 s
- 276 s
Answer: 3. 690 s
A fall of concentration from 1.28 mg L-1 to 0.04 mg L-1 requires 5 half-lives.
∴ Time required = 5 x t1/2= 5 x 138 = 690 s
Question 42. The half-life period of a first-order reaction is 1386 seconds. The specific rate constant of the reaction is
- \(0.5 \times 10^{-2} \mathrm{~s}^{-1}\)
- \(0.5 \times 10^{-3} \mathrm{~s}^{-1}\)
- \(5.0 \times 10^{-2} \mathrm{~s}^{-1}\)
- \(5.0 \times 10^{-3} \mathrm{~s}^{-1}\)
Answer: 2. \(0.5 \times 10^{-3} \mathrm{~s}^{-1}\)
Given, \(t_{1 / 2}=1386 \mathrm{~s}\)
For a first-order reaction, \(t_{1 / 2}=\frac{0.693}{k}\) (k = rate constant)
1386 = \(\frac{0.693}{k} \Rightarrow k=5 \times 10^{-4} \mathrm{~s}^{-1}=0.5 \times 10^{-3} \mathrm{~s}^{-1}\)
Question 43. If 60% of a first-order reaction was completed in 60 minutes, 50% of the same reaction would be completed in approximately (log 4 = 0.60, log 5 = 0.69)
- 45 minutes
- 60 minutes
- 40 minutes
- 50 minutes
Answer: 1. 45 minutes
For a first-order reaction, \(k=\frac{2.303}{t} \log \frac{a}{a-x}\)
k = \(\frac{2.303}{60} \log \frac{100}{40}=\frac{2.303}{60} \times \log 2.5=0.0153\)
Again, \(t_{1 / 2}=\frac{2.303}{k} \log \frac{100}{50}=\frac{2.303}{0.0153} \times \log 2=45.31 \mathrm{~min}\)
Chemical Kinetics quiz for NEET
Question 44. In a first-order reaction, A → B, if k is rate constant and the initial concentration of the reactant A is 0.5 M, then the half-life is
- \(\frac{\log 2}{k}\)
- \(\frac{\log 2}{k \sqrt{0.5}}\)
- \(\frac{\ln 2}{k}\)
- \(\frac{0.693}{0.5 k}\)
Answer: 3. \(\frac{\ln 2}{k}\)
For a 1st t order reaction, \(k=\frac{2.303}{t} \log _{10} \frac{a}{a-x}\)
At \(t_{1 / 2}, k=\frac{2.303}{t_{1 / 2}} \log _{10} \frac{a}{a-\frac{a}{2}}\) or \(t_{1 / 2}=\frac{2.303}{k} \log _{10} 2=\frac{\ln 2}{k}\)
Question 45. For a first-order reaction A → B the reaction rate at a reactant concentration of 0.01 M is found to be 2.0 x 10-5 mol L-1 s-1. The half-life period of the reaction is
- 30 s
- 220 s
- 300 s
- 347 s
Answer: 4. 347 s
A → B
For a first-order reaction A → B the reaction rate at a reactant concentration of 0.01 M is found to be 2.0 x 10-5 mol L-1 s-1.
Rate of reaction = 2 x 10-5 mol L-1 s-1
⇒ order of reaction is n = 1, rate = k [A]n = k[A]
⇒ k is the rate constant.
[A] = 0.01M
⇒ k = \(\frac{2 \times 10^{-5}}{0.01}=2 \times 10^{-3}, k=\frac{0.693}{t_{1 / 2}}\)
\(t_{1 / 2}=\frac{0.693}{2 \times 10^{-3}}=346.5 \approx 347 \mathrm{~s}\)Question 46. The rate of a first-order reaction is 1.5 x 10-2 mol L-1 min-1 at 0.5 M concentration of the reactant. The half-life of the reaction is
- 0.383 min
- 23.1 min
- 8.73 min
- 7.53 min
Answer: 2. 23.1 min
The rate of a first-order reaction is 1.5 x 10-2 mol L-1 min-1 at 0.5 M concentration of the reactant.
Rate \(\left(\frac{d x}{d t}\right)=k C\)
i.e., \(1.5 \times 10^{-2}=k \times 0.5$ or, $k=\frac{1.5 \times 10^{-2}}{0.5}\)
For first order reaction, \(t_{1 / 2}=\frac{0.693}{k}=\frac{0.693 \times 0.5}{1.5 \times 10^{-2}}=23.1 \mathrm{~min} \)
Question 47. The reaction A → B follows first-order kinetics. The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 hour. What is the time taken for conversion of 0. 9 mole of A to produce 0.675 mole of B?
- 1 hour
- 0.5 hour
- 0.25 hour
- 2 hours
Answer: 1. 1 hour
The reaction A → B follows first-order kinetics. The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 hour.
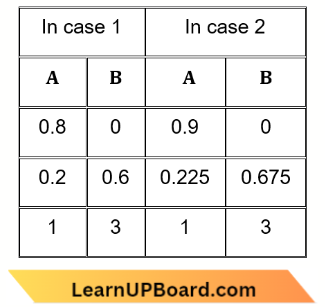
The time taken for the completion of the same fraction of change is independent of initial concentration.
NEET MCQs on Chemical Kinetics
Question 48. For a first-order reaction, the half-life period is independent of
- The first power of final concentration
- Cube root of initial concentration
- Initial concentration
- The square root of the final concentration.
Answer: 3. Initial concentration
For the first order reaction, the rate constant is given by, \(k_1=\frac{1}{t} \ln \frac{a}{a-x}\)
a = initial concentration,(a-x)= concentration at t time
At t = \(t_{1 / 2}, x=a /2\)
⇒ \(k_1=\frac{1}{t_{1 / 2}} \ln \frac{a}{a-a / 2} \Rightarrow k_1=\frac{1}{t_{1 / 2}} \ln 2 \Rightarrow k_1=\frac{0.693}{t_{1 / 2}}\)
Therefore, t1/2 is independent of initial concentration.
Question 49. Given below are two statements: One is labeled as Assertion A and the other is labeled as Reason R:
Assertion A: A reaction can have zero activation energy.
Reason R: The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to threshold value, is called activation energy.
In light of the above statements, choose the correct answer from the options given below.
- A is true but R is false.
- A is false but R is true.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true and R is not the correct
Answer: 2. A is false but R is true.
If \(E_a=0\), then according to Arrhenius equation, \(k=A e^{-E_0 / R T} \Rightarrow k=A e^{0 / R T}=A\)
This implies that every collision results in a chemical reaction which cannot be true. So, a reaction cannot have zero activation energy.
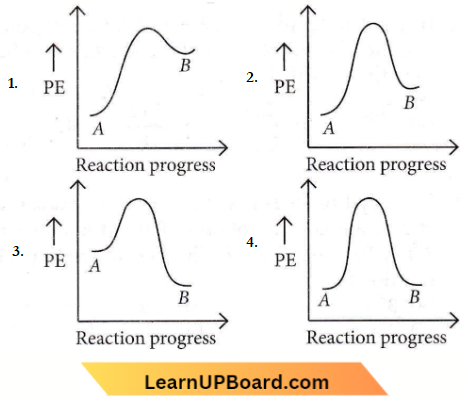
Question 50. For reaction A → B, the enthalpy of the reaction is -4.2 kJ mol-1 and the enthalpy of activation is 9.6 kJ mol-1. The correct potential energy profile for the reaction is shown in the option.
Answer: 3
As the enthalpy of the reaction is negative, hence it is an exothermic reaction.
Question 51. The slope of Arrhenoius (In k vs 1/T) of a first-order reaction is -5 x 10³ K. The value of Ea of the reaction is [Given: R = 8.314 J K-1 mol-1)
- \(-83 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(41.5 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(83.0 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(166 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Answer: 2. \(41.5 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
ln k = \(-\frac{E_a}{R T}+\ln A\)
–\(\frac{E_a}{R}=-5 \times 10^3=-5000\)
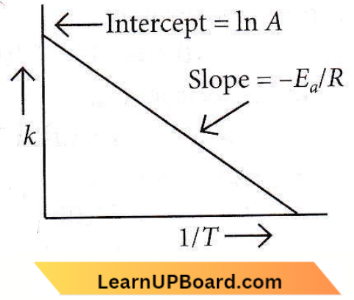
⇒ \(E_a=5000 \times 8.314=41570 \mathrm{~J} \mathrm{~mol}^{-1}\)
= \(41.57 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
NEET MCQs on Chemical Kinetics
Question 52. For a reaction, activation energy Ea = 0, and the rate constant at 200 K is 1.6 x 106 s-1. The rate constant at 400 K will be [Given that gas constant R = 8.314 J K-1 mol-1]
- \(3.2 \times 10^4 \mathrm{~s}^{-1}\)
- \(1.6 \times 10^6 \mathrm{~s}^{-1}\)
- \(1.6 \times 10^3 \mathrm{~s}^{-1}\)
- \(3.2 \times 10^6 \mathrm{~s}^{-1}\)
Answer: 2. \(1.6 \times 10^6 \mathrm{~s}^{-1}\)
According to Arrhenius equation, \(\log \frac{k_2}{k_1}=\frac{E_a}{2.303 R}\left[\frac{T_2-T_1}{T_2 T_1}\right]\)
⇒ \(\log \frac{k_2}{1.6 \times 10^6}=0 ; \frac{k_2}{1.6 \times 10^6}=1\)
⇒ \(k_2=1.6 \times 10^6 \mathrm{~s}^{-1}\)
Question 53. The addition of a catalyst during a chemical reaction alters which of the following quantities?
- Enthalpy
- Activation energy
- Entropy
- Internal energy
Answer: 2. Activation energy
A catalyst provides an alternate path to the reaction which has lower activation energy.
Question 54. The activation energy of a reaction can be determined from the slope of which of the following graphs?
- \(\ln k v s \frac{1}{T}\)
- \(\frac{T}{\ln k} v s \frac{1}{T}\)
- \(\ln k v s T\)
- \(\frac{\ln k}{T} v s T\)
Answer: 1. \(\ln k v s \frac{1}{T}\)
According to Arrhenius equation, \(k=A e^{-E_a / R T}\)
ln k = ln A – \(\frac{E_a}{R T}\)
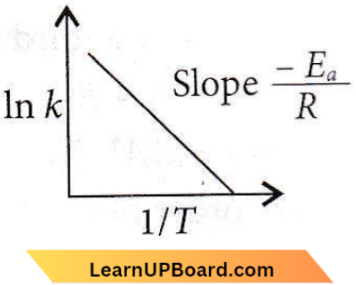
Hence, if ln k is plotted
against 1/T, slope of the line will be \(-\frac{E_a}{R}\)
Question 55. What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20 °C to 35 °C? (R = 8.314 J mol-1 K-1)
- \(34.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(15.1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(342 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(269 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Answer: 1. \(34.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
⇒ \(\log \frac{k_2}{k_1}=\frac{E_a}{2.303 R}\left(\frac{1}{T_1}-\frac{1}{T_2}\right)\)
⇒ \(k_2=2 k_1, T_1=20+273=293 \mathrm{~K}\) or \(T_2=35+273=308 \mathrm{~K}\)
R = \(8.314 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}\)
⇒ \(\log 2=\frac{E_a}{2.303 \times 8.314}\left(\frac{1}{293}-\frac{1}{308}\right)\)
0.3010 = \(\frac{E_a}{19.147} \times \frac{15}{293 \times 308}\)
⇒ \(E_a=34673 \mathrm{~J} \mathrm{~mol}^{-1}=34.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Chemical Kinetics NEET question bank
Question 56. In a zero-order reaction, for every 10 °C rise in temperature, the rate is doubled. If the temperature is increased from 10 °C to 100 °C, the rate of the reaction will become
- 256 times
- 512 times
- 64 times
- 128 times.
Answer: 2. 512 times
In a zero-order reaction, for every 10 °C rise in temperature, the rate is doubled. If the temperature is increased from 10 °C to 100 °C,
At 10°C rise, the rate increases by 2
⇒ \(\frac{r_{100^{\circ} \mathrm{C}}}{r_{10^{\circ} \mathrm{C}}}=2^{\left(\frac{100-10}{10}\right)}=2^9=512 \text { times }\)
Question 57. The activation energy (Ea) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1) and T2) are related by
- \(\ln \frac{k_2}{k_1}=-\frac{E_a}{R}\left(\frac{1}{T_1}-\frac{1}{T_2}\right)\)
- \(\ln \frac{k_2}{k_1}=-\frac{E_a}{R}\left(\frac{1}{T_2}-\frac{1}{T_1}\right)\)
- \(\ln \frac{k_2}{k_1}=-\frac{E_a}{R}\left(\frac{1}{T_2}+\frac{1}{T_1}\right)\)
- \(\ln \frac{k_2}{k_1}=\frac{E_a}{R}\left(\frac{1}{T_1}-\frac{1}{T_2}\right)\)
Answer: 2. \(\ln \frac{k_2}{k_1}=-\frac{E_a}{R}\left(\frac{1}{T_2}-\frac{1}{T_1}\right)\) and 4. \(\ln \frac{k_2}{k_1}=\frac{E_a}{R}\left(\frac{1}{T_1}-\frac{1}{T_2}\right)\)
⇒ \(k_1=A e^{-E_a / R T_1}, k_2=A e^{-E_a / R T_2}\)
ln \(k_1=\ln A-E_a / R T_1\)…(1)
ln \(k_2=\ln A-E_a / R T_2\)…(2)
From eq.(1) and (2), we have ln \(k_2-\ln k_1=\ln A-\frac{E_a}{R T_2}-\ln A+\frac{E_a}{R T_1}\)
ln \(\frac{k_2}{k_1}=\frac{E_a}{R}\left(\frac{1}{T_1}-\frac{1}{T_2}\right) \Rightarrow \ln \frac{k_2}{k_1}=-\frac{E_a}{R}\left(\frac{1}{T_2}-\frac{1}{T_1}\right)\)
Question 58. The rate of the reaction, 2NO + Cl2 → 2NOCl is given by the rate equation, rate = k[NO²[Cl2]. The value of the rate constant can be increased by
- Increasing the temperature
- Increasing the concentration of NO
- Increasing the concentration of the Cl2
- Doing all of these.
Answer: 1. Increasing the temperature
The rate constant is independent of the initial concentration of the reactants. It has a constant value at a fixed temperature. According to the Arrhenius equation, the value of the rate constant can be increased by increasing the temperature.
Question 59. The rate constants k1 and k2 for two different reactions are \(10^{16} \cdot e^{-2000 / T}\) and \(10^{15^2} \cdot e^{-1000 / T}\), respectively. Tire temperature at which k1 = k2 is
- 2000 K
- \(\frac{1000}{2.303}\) K
- 1000 K
- \(\frac{2000}{2.303}\) K
Answer: 2. \(\frac{1000}{2.303}\) K
The rate constants k1 and k2 for two different reactions are \(10^{16} \cdot e^{-2000 / T}\) and \(10^{15^2} \cdot e^{-1000 / T}\), respectively.
⇒ \(k_1=10^{16} e^{-2000 / T}, k_2=10^{15} e^{-1000 / T}\)
When, \(k_1=k_2, 10^{16} e^{-2000 / T}=10^{15} e^{-1000 / T}\)
or \(10 e^{-2000 / T}=e^{-1000 / T}\)
Taking the natural logarithm of both sides, we get ln \(10-\frac{2000}{T}=\frac{-1000}{T}\) or \(2.303-\frac{2000}{T}=\frac{-1000}{T}\)
or, \(\frac{1000}{T}=2.303 \text { or } T=\frac{1000}{2.303} \mathrm{~K}\)
Chemical Kinetics NEET question bank
Question 60. The temperature dependence of the rate constant (k) of a chemical reaction is written in terms of the Arrhenius equation, k = A x e-E*/RT. The activation energy (E*) of the reaction can be calculated by plotting
- \(k v s T\)
- \(k v s \frac{1}{\log T}\)
- \(\log k v s \frac{1}{T}\)
- \(\log k v s \frac{1}{\log T}\)
Answer: 3. \(\log k v s \frac{1}{T}\)
The temperature dependence of the rate constant (k) of a chemical reaction is written in terms of the Arrhenius equation, k = A x e-E*/RT.
On plotting log k vs l/T, we get a straight line, the slope indicates the value of activation energy.
Question 61. The activation energy for a simple chemical reaction A \(\rightleftharpoons\) B is Ea in the forward direction. The activation energy for the reverse reaction
- Is negative of Ea
- Is always less than Ea
- Can be less than or more than Ea
- Is always double of Ea
Answer: 3. Can be less than or more than Ea
The activation energy for a simple chemical reaction A \(\rightleftharpoons\) B is Ea in the forward direction.
Activation energy is the minimum amount of energy required to convert reactant into product. The activation energy for reverse reaction can be less than or more than Ea depending on whether the reaction is exothermic or endothermic.
Question 62. When a biochemical reaction is carried out in the laboratory, outside the human body in the absence of an enzyme, then the rate of reaction obtained is 10-6 times, and the activation energy of the reaction in the presence of an enzyme is
- 6/RT
- P is required
- Different from Ea obtained in the laboratory
- Can’t say anything.
Answer: 3. Different from Ea obtained in the laboratory
According to k = \(A e^{-E a / R T}\) (Arrhenius equation), the activation energy of a reaction in the presence of an enzyme is different from Ea obtained in the laboratory.
Question 63. How do enzymes increase the rate of reactions?
- By lowering the activation energy
- By increasing activation energy
- By changing the equilibrium constant
- By forming enzyme-substrate complex
Answer: 1. By lowering activation energy
Enzymes act like catalysts in biochemical reactions. The presence of an enzyme increases the rate of reaction by lowering the activation energy of the reactant.
Question 64. The activation energy of a chemical reaction can be determined by
- Evaluating rate constants at two different temperatures
- Evaluating velocities of reaction at two different temperatures
- Evaluating rate constant at standard temperature
- Changing concentration of reactants.
Answer: 1. Evaluating rate constants at two different temperatures
Chemical Kinetics NEET question bank
According to Arrhenius equation: \(\log \frac{k_2}{k_1}=\frac{E_a}{2.303 R}\left[\frac{T_2-T_1}{T_2 T_1}\right]\)
where Ea = activation energy
R = gas constant = 8.314 J K-1 mol-1
k1 and k2 are rate constants of the reaction at two different temperatures T1 and T2 respectively.
Question 65. By the action of enzymes, the rate of biochemical reaction
- Does not change
- Increases
- Decreases
- Either (1) or (3).
Answer: 2. Increases
Since the enzymes are regarded as biological catalysts, therefore their action increases the rate of biological reaction.
Question 66. An increase in the concentration of the reactants of a reaction leads to a change in
- Activation energy
- Heat of reaction
- Threshold energy
- Collision frequency.
Answer: 4. Collision frequency.
Collision frequency ∝ number of reacting molecules or atoms
The higher the concentration of reactant molecules, the higher is the probability of collision and so the collision frequency.
