Electrochemistry NEET MCQs
NEET Chemistry For Electrochemistry Multiple Choice Questions
Question 1. The standard electrode potential (E°) values of Al3+/Al, Ag+/Ag, K+/K, and Cr3+/Cr are -1.66 V, 0.80 V, -2.93 V and -0.74 V, respectively. The correct decreasing order of reducing power of the metal is
- Ag > Cr > Al > K
- K > Al > Cr > Ag
- K > Al > Ag > Cr
- Al > K > Ag > Cr
Answer: 2. K > Al > Cr > Ag
Given
The standard electrode potential (E°) values of Al3+/Al, Ag+/Ag, K+/K, and Cr3+/Cr are -1.66 V, 0.80 V, -2.93 V and -0.74 V, respectively.
The higher the value of E°red, the stronger is the oxidizing power. Thus, the decreasing order of reducing the power of the metals K>AI>Cr>Ag.
Question 2. A button cell used in watches function as following: \(\mathrm{Zn}_{(s)}+\mathrm{Ag}_2 \mathrm{O}_{(s)}+\mathrm{H}_2 \mathrm{O}_{(l)} \rightleftharpoons 2 \mathrm{Ag}_{(s)}+\mathrm{Zn}_{(a q)}^{2+}+2 \mathrm{OH}_{(a q)}^{-}\) If half cell potentials are \(\mathrm{Zn}^{2+}(a q)+2 e^{-} \rightarrow \mathrm{Zn}_{(s)} ; E^{\circ}=-0.76 \mathrm{~V}\); \(\mathrm{Ag}_2 \mathrm{O}_{(s)}+\mathrm{H}_2 \mathrm{O}_{(l)}+2 e^{-} \rightarrow 2 \mathrm{Ag}_{(s)}+2 \mathrm{OH}_{(a q)}^{-} ; E^{\circ}=0.34 \mathrm{~V}\)
The cell potential will be
- 0.84 V
- 1.34 V
- 1.10 V
- 0.42 V
Answer: 3. 1.10 V
⇒ \(E_{\text {cell }}^{\circ}=E_{\text {O.P. }}^{\circ}+E_{\text {R.P. }}^{\circ}=0.76+0.34=1.10 \mathrm{~V}\)
Question 3. The standard reduction potentials of the half-reactions are given below:
- \(\mathrm{F}_{2(g)}+2 e^{-} \rightarrow 2 \mathrm{~F}_{(a q)}^{-} ; E^{\circ}=+2.85 \mathrm{~V}\)
- \(\mathrm{Cl}_{2(g)}+2 e^{-} \rightarrow 2 \mathrm{Cl}_{(a q)}^{-} ; E^{\circ}=+1.36 \mathrm{~V}\)
- \(\mathrm{Br}_{2(l)}+2 e^{-} \rightarrow 2 \mathrm{Br}_{(a q)}^{-} ; E^{\circ}=+1.06 \mathrm{~V}\)
- \(\mathrm{I}_{2(s)}+2 e^{-} \rightarrow 2 \mathrm{I}_{(a q)}^{-} ; E^{\circ}=+0.53 \mathrm{~V}\)
The strongest oxidizing and reducing agents respectively are
- F2 and I–
- Br2 and Cl–
- Cl2 and Br–
- Cl2 and I2
Answer: 1. F2 and I–
Less lower the value of reduction potential, the stronger the reducing agent thus, I am the strongest reducing agent. More positive, the value of reduction potential shows good oxidizing properties thus, the strongest oxidizing agent is F2.
Read and Learn More NEET MCQs with Answers
Electrochemistry NEET MCQs
Question 4. Standard electrode potentials of three metals X, Y, and Z are -1.2 V, + 0.5 V, and – 3.0 V respectively. The reducing power of these metals will be
- Y>Z>X
- Y> X> Z
- Z>X>Y
- X> Y> Z
Answer: 3. Z>X>Y
The more negative the value of reduction potential, the stronger will be the reducing agent.
So, Z (-3.0 V) > X (-1.2 V) > Y (+ 0.5 V)
Question 5. The standard electrode potential for Sn4++/ Sn2+ couple is +0.15 V and that for the Cr3+/Cr couple is -0.74 V. These two couples in their standard state are connected to make a cell. The cell potential will be
- + 1.19 V
- + 0.89 V
- + 0.18 V
- + 1.83 V
Answer: 2. + 0.89 V
Given
The standard electrode potential for Sn4++/ Sn2+ couple is +0.15 V and that for the Cr3+/Cr couple is -0.74 V. These two couples in their standard state are connected to make a cell.
⇒ \(E_{\text {cell }}^{\circ}=E_{\text {cathode }}^{\circ}-E_{\text {anode }}^{\circ}\) = 0.15-(-0.74)=0.15+0.74=0.89 V
Question 6. A solution contains Fe2+, Fe3+, and I– ions. This solution was treated with iodine at 35°C. E° for Fe3+/Fe2+ is + 0.77 V and E° for I2/2I– = 0.536 V. The favorable redox reaction is
- I2 will be reduced to I–
- There will be no redox reaction
- I– will be oxidized to I2
- Fe2+ will be oxidized to Fe3+.
Answer: 3. I will be oxidized to I2
Given
A solution contains Fe2+, Fe3+, and I– ions. This solution was treated with iodine at 35°C. E° for Fe3+/Fe2+ is + 0.77 V and E° for I2/2I– = 0.536 V.
Since the reduction potential of \(\mathrm{Fe}^{3+} / \mathrm{Fe}^{2+}\) is greater than that of \(\mathrm{I}_{2} / \mathrm{I}^{-}\) will be reduced and I– will be oxidised.
⇒ \(2 \mathrm{Fe}^{3+}+2 \mathrm{I}^{-} \longrightarrow 2 \mathrm{Fe}^{2+}+\mathrm{I}_2\)
NEET questions on Electrochemistry
Question 7. Consider the following relations for emf of an electrochemical cell
- EMF of cell = (Oxidation potential of anode) – (Reduction potential of cathode)
- EMF of cell = (Oxidation potential of anode) + (Reduction potential of cathode)
- EMF of cell = (Reductional potential of anode) + (Reduction potential of cathode)
- EMF of cell = (Oxidation potential of anode) – (Oxidation potential of cathode)
Which of the above relations is correct?
- (3) and (1)
- (1) and (2)
- (3) and (4)
- (2) and (4)
Answer: 4. (2) and (4)
EMF of a cell = Reduction potential of cathode – Reduction potential of anode
= Reduction potential of cathode + Oxidation potential of anode
= Oxidation potential of the anode Oxidation potential of the cathode.
Question 8. On the basis of the following E° values, the strongest oxidizing agent is \({\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}+e^{-} ; E^{\circ}=-0.35 \mathrm{~V}}\); \(\mathrm{Fe}^{2+} \rightarrow \mathrm{Fe}^{3+}+e^{-} ; E^{\circ}=-0.77 \mathrm{~V}\)
- \(\mathrm{Fe}^{3+}\)
- \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\)
- \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}\)
- \(\mathrm{Fe}^{2+}\)
Answer: 1. \(\mathrm{Fe}^{3+}\)
⇒ \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-} \rightarrow\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}, E^{\circ}\) = \(+0.35 \mathrm{~V}\); \(\mathrm{Fe}^{3+} \rightarrow \mathrm{Fe}^{2+} ; E^{\circ}=+0.77 \mathrm{~V}\)
The higher the +ve reduction potential stronger the oxidising agent. Oxidizing agent oxidizes other compounds and gets themselves reduced easily. Thus, Fe3+ is the strongest oxidizing agent.
Question 9. A hypothetical electrochemical cell is shown below: A/A+ (x M)||B+ (y M)| B The emf measured is + 0.20 V. The cell reaction is
- \(A+B^{+} \rightarrow A^{+}+B\)
- \(A^{+}+B \rightarrow A+B^{+}\)
- \(A^{+}+e^{-} \rightarrow A ; B^{+}+e^{-} \rightarrow B\)
- The cell reaction cannot be predicted.
Answer: 1. \(A+B^{+} \rightarrow A^{+}+B\)
From the given expression:
At anode A → A+ + e– (oxidation)
At cathode B+ + e– → B (reduction)
Overall reaction is A + B+ → A+ + B
NEET questions on Electrochemistry
Question 10. \(E_{\mathrm{Fe}^{2+} / \mathrm{Fe}}^{\circ}=-0.441 \mathrm{~V} \text { and } E_{\mathrm{Fe}^{3+} / \mathrm{Fe}^{2+}}^{\circ}=0.771 \mathrm{~V}\), the standard EMF of the reaction \(\mathrm{Fe}+2 \mathrm{Fe}^{3+} \rightarrow 3 \mathrm{Fe}^{2+}\) will be
- 0.111 V
- 0.330 V
- 1.653 V
- 1.212 V
Answer: 4. 1.212 V
⇒ \(\mathrm{Fe}^{2+}+2 e^{-} \longrightarrow \mathrm{Fe} ; E^{\circ}=-0.441 \mathrm{~V}\)…..(1)
⇒ \(\mathrm{Fe}^{3+}+e^{-} \longrightarrow \mathrm{Fe}^{2+} ; E^{\circ}=0.771 \mathrm{~V}\)….(2)
⇒ \(\mathrm{Fe}+2 \mathrm{Fe}^{3+} \longrightarrow 3 \mathrm{Fe}^{2+} ; E^{\circ}=?\)
To get the above equation, (2) x 2 – (1)
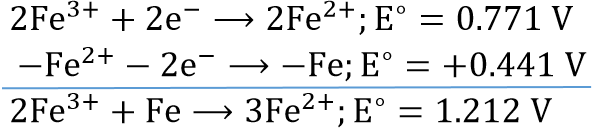
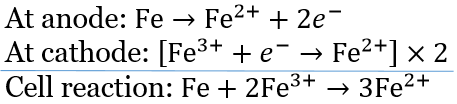
Question 11. Standard electrode potentials are Fe2+/Fe; E° = -0.44 and Fe3+/Fe2+; E° = 0.77. Fe2+, Fe3+, and Fe blocks are kept together, then
- Fe3+ increases
- Fe3+ decreases
- Fe2+/Fe3+ remains unchanged
- Fe2+ decreases.
Answer: 2. Fe3+ decreases
⇒ \(E_{\mathrm{Fe}^{2+} / \mathrm{Fe}}^{\circ}=-0.44 \mathrm{~V}\)
⇒ \(E_{\mathrm{Fe}^{3+}}^{\circ} / \mathrm{Fe}^{2+}=+0.77 \mathrm{~V}\)
If a cell is constructed by combining these two electrodes oxidation occurs at Fe2+ /Fe electrode.
If Fe2+, Fe3+, and Fe blocks are kept together then Fe3+ reacts with Fe to yield Fe2+ i.e., the concentration of Fe3+ is decreased and that of Fe2+ is increased.
Electrochemistry multiple choice NEET
Question 12. Electrode potential for the following half-cell reactions are
- \(\mathrm{Zn} \rightarrow \mathrm{Zn}^{2+}+2 e^{-}; E^{\circ}=+0.76 \mathrm{~V}\)
- \(\mathrm{Fe} \rightarrow \mathrm{Fe}^{2+}+2 e^{-} ; E^{\circ}=+0.44 \mathrm{~V}\)
The EMF for the cell reaction \(\mathrm{Fe}^{2+}+\mathrm{Zn} \rightarrow \mathrm{Zn}^{2+}+\mathrm{Fe}\) will be
- -0.32 V
- + 1.20 V
- -1.20 V
- + 0.32 V
Answer: 4. + 0.32 V
⇒ \(E_{\mathrm{Zn} / \mathrm{Zn}^{2+}}^{\circ}=+0.76 \mathrm{~V}\)
⇒ \(E_{\mathrm{Fe} / \mathrm{Fe}}{ }^{2+}=0.44 \mathrm{~V} \Rightarrow E_{\mathrm{Fe}}^{\circ}{ }^{2+} / \mathrm{Re}=-0.44 \mathrm{~V}\)
⇒ \(E_{\text {cell }}^{\circ}=E_{\text {O.P. }}^{\circ}+E_{\text {R.P. }}^{\circ}=+0.76-0.44=+0.32 \mathrm{~V}\)
Question 13. An electrochemical cell is set up of: Pt;H2 (1 atm) | HCl(0.1M) || CH3COOH(0.1M) |H2( 1 atm); Pt. The e.m.f. of this cell will not be zero, because
- Acids used in two compartments are different
- e.m.f. depends on the molarities of acids used
- The temperature is constant
- pH of 0.1 M HCl and 0.1 M CH3COOH is not the same.
Answer: 4. pH of 0.1 M HCl and 0.1 M CH3COOH is not the same
Since it is a concentration cell and the concentration of H+ ions in two electrolyte solutions (HCl and CH33COOH) are different i.e., pH of 0.1 M HCl and 0.1 M CH3COOH is not the same, therefore e.m.f. of this cell will not be zero
Question 14. Standard reduction potentials at 25°C of Li+|Li, Ba2+|Ba, Na+|Na, and Mg2+|Mg are -3.05, -2.90, -2.71, and -2.37 volt respectively. Which one of the following is the strongest oxidizing agent?
- Ba2+
- Mg2+
- Na+
- Li+
Answer: 2. Mg2+
Given
Standard reduction potentials at 25°C of Li+|Li, Ba2+|Ba, Na+|Na, and Mg2+|Mg are -3.05, -2.90, -2.71, and -2.37 volt respectively.
The more positive or less negative the reduction potential value, the stronger is the oxidizing agent.
Electrochemistry multiple choice NEET
Question 15. A solution of potassium bromide is treated with each of the following. Which one would liberate bromine?
- Hydrogen iodide
- Sulfur dioxide
- Chlorine
- Iodine
Answer: 3. Chlorine
A stronger oxidizing agent (Cl2) displaces a weaker oxidizing agent (Br2) from its salt solution.
⇒ \(2 \mathrm{KBr}+\mathrm{Cl}_2 \rightarrow 2 \mathrm{KCl}+\mathrm{Br}_2\)
Question 16. Given below are two statements: One is labelled as Assertion A and the other is labeled as Reason R;
Assertion A: In equation ΔrG = -nFEcell, the value of ΔrG depends on n.
Reason R: cell is an intensive property and ArG is an extensive property.
In light of the above statements, choose the correct answer from the options given below.
- A is true but R is false.
- A is false but R is true.
- Both A and R are true and R is the correct explanation of A.
- Both A and R are true and R is not the correct explanation of A.
Answer: 4. Both A and R are true and R is not the correct explanation of A.
ΔrG =-nFEcell
Ecell, is an intensive parameter but ΔrG is an extensive thermodynamic property and the value of ΔrG depends on n.
NEET practice questions Electrochemistry
Question 17. Given below are half-cell reactions: \(\mathrm{MnO}_4^{-}+8 \mathrm{H}^{+}+5 e^{-} \longrightarrow \mathrm{Mn}^{2+}+4 \mathrm{H}_2 \mathrm{O}\); \(E_{\mathrm{Mn}^{2+} / \mathrm{MnO}_4^{-}}^{\circ}=-1.510 \mathrm{~V}\); \(\frac{1}{2} \mathrm{O}_2+2 \mathrm{H}^{+}+2 e^{-} \longrightarrow \mathrm{H}_2 \mathrm{O}; E_{\mathrm{O}_2 / \mathrm{H}_2 \mathrm{O}}^{\circ}=+1.223 \mathrm{~V}\) Will the permanganate ion, MnO4 water in the presence of an acid?
- Yes, because E°cell= +0.287 V
- No, because E°cell = -0.287 v
- Yes, because E°cell = +2.733 V
- No, because E°cell = -2.733 V
Answer: 1. Yes, because E°cell= +0.287 V
⇒ \(E_{\text {cell }}^{\circ}=E_{\text {cathode }}^{\circ}-E_{\text {anode }}^{\circ}=+1.510-1.223=+0.287 \mathrm{~V}\)
As \(E_{\text {cell }}^{\circ}\) is positive, hence the reaction is feasible.
Question 18. At 298 K the standard electrode potentials of Cu2+ / Cu, Zn2+/ Zn, Fe2+/ Fe, and Ag+/Ag are 0.34 V, -0.76 V, -0.44 V, and 0.80 V respectively. On the basis of standard electrode potential, predict which of the following reactions cannot occur.
- \(\mathrm{CuSO}_{4(a q)}+\mathrm{Zn}_{(s)} \longrightarrow \mathrm{ZnSO}_{4(a q)}+\mathrm{Cu}_{(s)}\)
- \(\mathrm{CuSO}_{4(a q)}+\mathrm{Fe}_{(s)} \longrightarrow \mathrm{FeSO}_{4(a q)}+\mathrm{Cu}_{(s)}\)
- \(\mathrm{FeSO}_{4(a q)}^{4(a q)}+\mathrm{Zn}_{(s)} \longrightarrow \mathrm{ZnSO}_{4(a q)}+\mathrm{Fe}_{(s)}\)
- \(2 \mathrm{CuSO}_{4(a q)}+2 \mathrm{Ag} g_{(s)} \longrightarrow 2 \mathrm{Cu}_{(s)}+\mathrm{Ag}_2 \mathrm{SO}_{4(a q)}\)
Answer: 4. \(2 \mathrm{CuSO}_{4(a q)}+2 \mathrm{Ag} g_{(s)} \longrightarrow 2 \mathrm{Cu}_{(s)}+\mathrm{Ag}_2 \mathrm{SO}_{4(a q)}\)
The values of the standard reduction potential of Cu and Ag suggest that Cu would undergo oxidation (lower reduction potential) and Ag would undergo reduction (higher reduction potential).
Hence, the cell reaction will be \(\mathrm{Cu}+2 \mathrm{Ag}^{+} \longrightarrow \mathrm{Cu}^{2+}+2 \mathrm{Ag}\)
NEET practice questions Electrochemistry
Question 19. Find the emf of the cell in which the following reaction takes place at \(298 K \mathrm{Ni}_{(s)}+2 \mathrm{Ag}^{+}(0.001 \mathrm{M}) \longrightarrow \mathrm{Ni}^{2+}(0.001 \mathrm{M})+2 \mathrm{Ag}_{(s)}\)
Given that \(E_{\text {cell }}^{\circ}=10.5 \mathrm{~V}, \frac{2.303 R T}{F}=0.059 \text { at } 298 \mathrm{~K}\)
- 1.0385 V
- 1.385 V
- 0.9615 V
- 1.05 V
Answer: 3. 0.9615 V
According to the Nernst equation,
E = \(E_{\text {cell }}^{\circ}-\frac{0.059}{n} \log \frac{\left[\mathrm{Ni}^{2+}\right]}{\left[\mathrm{Ag}^{+}\right]^2}\)
⇒ \(E_{\text {cell }}^{\circ}=1.05(\text { Given) }\)
E = \(1.05-\frac{0.059}{2} \log \frac{(0.001)}{(0.001)^2}\)
= \(1.05-\frac{0.059}{2} \log 10^3=1.05-\frac{0.059 \times 3}{2}\)
= \(1.05-0.0885=0.9615 \mathrm{~V}\)
Question 20. For the cell reaction: \(2 \mathrm{Fe}_{(a q)}^{3+}+2 \mathrm{I}_{(a q)}^{-} \rightarrow 2 \mathrm{Fe}_{(a q)}^{2+}+\mathrm{I}_{2(a q)}\) E°cell = 0.24 V at 298 K. The standard Gibbs’ energy (ΔrG°) of the cell reaction is [Given that Faraday constant, F = 96500 C mol-1]
- 23.16 kJ mol-1
- -46.32 kJ mol-1
- -23.16 kJ mol-1
- 46.32 kJ mol-1
Answer: 2. -46.32 kJ mol-1
The standard Gibbs energy, \(\left(\Delta G^{\circ}\right)=-n F E_{\text {cell }}^{\circ}\) Value of n=2
⇒ \(\Delta G^{\circ}=-2 \times 96500 \times 0.24=-46320 \mathrm{~J}\)
= \(-46.32 \mathrm{~kJ} / \mathrm{mol}\)
Chemistry MCQs Electrochemistry NEET
Question 21. For a cell involving one electron, E°cell= 0.59 V at 298 K, the equilibrium constant for the cell reaction is [Given that \(\frac{2.303 R T}{F}\)= 0.059 V at T = 298 K]
- 1.0 x 1030
- 1.0 x 102
- 1.0 x 105
- 1.0 x 1010
Answer: 4. 1.0 x 1010
According to Nernst equation, \(E_{\text {cell }}=E_{\text {cell }}^{\circ}-\frac{0.059}{n} \log Q_c\)
At equilibrium \(E_{\text {cell }}=0\), \(Q_c=K_c\)
⇒ \(E_{\text {cell }}^{\mathrm{a}}=\frac{0.059}{n} \log K_c \Rightarrow 0.59=\frac{0.059}{1} \log K_c\)
⇒ \(K_c=\text { antilog } 10 \Rightarrow K_c=1 \times 10^{10}\)
Question 22. In the electrochemical cell: Zn|ZnSO4(0.01 M)||CuSO4(1.0 M)|Cu, the emf of this Daniell cell is E1 When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E1 From the followings, which one is the relationship between E1 and E2? (Given, RT/F = 0.059)
- E1 < E2
- E1 > E2
- E2 = 0 ≠ E1
- E1 = E2
Answer: 2. E1 > E2
Given
In the electrochemical cell: Zn|ZnSO4(0.01 M)||CuSO4(1.0 M)|Cu, the emf of this Daniell cell is E1 When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E1
⇒ \(E_{\text {cell }}=E_{\text {cell }}^o-\frac{0.059}{n} \log \frac{\left[\mathrm{Zn}^{2+}\right]}{\left[\mathrm{Cu}^{2+}\right]}\)
⇒ \(E_1=E^{\circ}-\frac{0.059}{2} \log \frac{0.01}{1}\)
⇒ \(E_1=E^{\circ}-\frac{0.059}{2}(-2)=E^{\circ}+0.059\)
⇒ \(E_2=E^{\circ}-\frac{0.059}{2} \log \frac{1}{0.01}=E^{\circ}-0.059\)
Hence, \(E_1>E_2\).
Chemistry MCQs Electrochemistry NEET
Question 23. If the E°cell for a given reaction has a negative value, which of the following gives the correct relationships for the values of ΔG° and Keq?
- \(\Delta G^{\circ}>0 ; K_{\text {eq }}<1\)
- \(\Delta G^{\circ}>0 ; K_{\text {eq }}>1\)
- \(\Delta G^0<0 ; K_{\text {eq }}>1\)
- \(\Delta G^{\circ}<0 ; K_{\text {eq }}<1\)
Answer: 1. \(\Delta G^{\circ}>0 ; K_{\text {eq }}<1\)
⇒ \(\Delta G^{\circ}=-n F E_{\text {cell }}^{\circ}\)
If \(E_{\text {cell }}^{\circ}=- ve then \Delta G^{\circ}=+ ve\)
i.e; \(\Delta G^{\circ}>0\).
⇒ \(\Delta G^{\mathrm{a}}=-n R T \log K_{\mathrm{eq}}\)
For \(\Delta G^{\circ}=+ \text { ve, } K_{\text {eq }}=- \text { ve i.e., } K_{e q}<1 \text {. }\)
Question 24. The pressure of H2 required to make the potential of H2 electrode zero in pure water at 298 K is
- 10-10 atm
- 10-4 atm
- 10-14 atm
- 10-12 atm.
Answer: 3. 10-14 atm
pH = 7 for water.
⇒ \(-\log \left[\mathrm{H}^{+}\right]=7 \Rightarrow\left[\mathrm{H}^{+}\right]=10^{-7}\)
⇒ \(2 \mathrm{H}^{+}{ }_{(a q)}^{+}+2 e^{-} \longrightarrow \mathrm{H}_{2(g)}\)
⇒ \(E_{\text {cell }}=E_{\text {cell }}^{\circ}-\frac{0.0591}{2} \log \frac{P_{\mathrm{H}_2}}{\left[\mathrm{H}^{+}\right]^2}\)
0 = \(0-\frac{0.0591}{2} \log \frac{P_{\mathrm{H}_2}}{\left(10^{-7}\right)^2}\)
⇒ \(\log \frac{P_{\mathrm{H}_2}}{\left(10^{-7}\right)^2}=0 \Rightarrow \frac{P_{\mathrm{H}_2}}{\left(10^{-7}\right)^2}=1\) (because log 1=0)
⇒ \(p_{\mathrm{H}_2}=10^{-14} \mathrm{~atm}\).
Electrochemistry quiz for NEET
Question 25. A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH =10 and by passing hydrogen gas around the platinum wire at one atm pressure. The oxidation potential of the electrode would be
- 0.118 V
- 1.18 V
- 0.059 V
- 0.59 V
Answer: 4. 0.59 V
Given
A hydrogen gas electrode is made by dipping platinum wire in a solution of HCl of pH =10 and by passing hydrogen gas around the platinum wire at one atm pressure.
⇒ \(\underset{1 \mathrm{~atm}}{\mathrm{H}_2} \longrightarrow \underset{10^{-10}}{2 \mathrm{H}^{+}}+2 e^{-}\)
⇒ \(E_{\mathrm{H}_2 / \mathrm{H}^{+}}=0-\frac{0.059}{2} \log \frac{\left(10^{-10}\right)^2}{1}\)
⇒ \(E_{\mathrm{H}_2 / \mathrm{H}^{+}}=+0.59 \mathrm{~V}\)
Question 26. Consider the half-cell reduction reaction \(\mathrm{Mn}^{2+}+2 e^{-} \rightarrow \mathrm{Mn}, E^{\circ}=-1.18 \mathrm{~V}\); \(\mathrm{Mn}^{2+} \rightarrow \mathrm{Mn}^{3+}+e^{-}, E^{\circ}=-1.51 \mathrm{~V}\) The \(E^{\circ}\) for the reaction, \(3 \mathrm{Mn}^{2+} \rightarrow \mathrm{Mn}^0+2 \mathrm{Mn}^{3+}\) and possibility of the forward reaction are respectively
- -4.18 V and yes
- + 0.33 V and yes
- + 2.69 V and no
- – 2.69 V and no.
Answer: 4. – 2.69 V and no
⇒ \(\mathrm{Mn}^{2+}+2 e^{-} \longrightarrow \mathrm{Mn} ; E^{\circ}=-1.18 \mathrm{~V}\)
⇒ \(2 \mathrm{Mn}^{2+} \longrightarrow 2 \mathrm{Mn}^{3+}+2 e^{-} ; E^{\circ}=-1.51 \mathrm{~V}\)
By adding equations (1) and (2), we get an equation for the cell, \(3 \mathrm{Mn}^{2+} \longrightarrow \mathrm{Mn}+2 \mathrm{Mn}^{3+}; E^{\circ}=-2.69 \mathrm{~V}\)
Since the E value is negative, so the process is non-spontaneous as ΔG° is positive.
Electrochemistry quiz for NEET
Question 27. The Gibbs’ energy for the decomposition of Al2O3 at 500 °C is as follows \(\frac{2}{3} \mathrm{Al}_2 \mathrm{O}_3 \rightarrow \frac{4}{3} \mathrm{Al}+\mathrm{O}_2, \Delta_{\mathrm{r}} G=+960 \mathrm{~kJ} \mathrm{~mol}^{-1}\) The potential difference needed for the electrolytic reduction of aluminum oxide (Al2O3) at 500 °C is at least
- 4.5 V
- 3.0 V
- 2.5 V
- 5.0 V
Answer: 3. 2.5 V
The Gibbs’ energy for the decomposition of Al2O3 at 500 °C is as follows \(\frac{2}{3} \mathrm{Al}_2 \mathrm{O}_3 \rightarrow \frac{4}{3} \mathrm{Al}+\mathrm{O}_2, \Delta_{\mathrm{r}} G=+960 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
⇒ \(\Delta G^{\circ}=-n F E^{\circ}\)
F = \(96500, \Delta G^{\circ}=+960 \times 10^3 \mathrm{~J} / \mathrm{mol}\)
⇒ \(\frac{2}{3} \mathrm{Al}_2 \mathrm{O}_3 \rightarrow \frac{4}{3} \mathrm{Al}+\mathrm{O}_2\)
Total number of \(\mathrm{Al}\) atoms in \(\mathrm{Al}_2 \mathrm{O}_3=\frac{2}{3} \times 2=\frac{4}{3}\)
⇒ \(\mathrm{Al}^{3+}+3 e^{-} \longrightarrow \mathrm{Al}\)
As \(3 e^{-}\) change occur for each Al-atom
total n = \(\frac{4}{3} \times 3=4\)
⇒ \(E^{\circ}=-\frac{\Delta G^{\circ}}{n F}=-\frac{960 \times 1000}{4 \times 96500} \Rightarrow E^{\circ}=-2.48 \approx-2.5 \mathrm{~V}\)
Question 28. The electrode potentials for, \(\mathrm{Cu}^{2+}{ }_{(a q)}+e^{-} \rightarrow \mathrm{Cu}_{(a q)}^{+} \text {and } \mathrm{Cu}^{+}{ }_{(a q)}+e^{-} \rightarrow \mathrm{Cu}_{(s)}\) are + 0.15 V and + 0.50 V respectively. The value of \(E^{\circ}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}\) will be
- 0.500 V
- 0.325 V
- 0.650 V
- 0.150 V
Answer: 2. 0.325 V
Given
The electrode potentials for, \(\mathrm{Cu}^{2+}{ }_{(a q)}+e^{-} \rightarrow \mathrm{Cu}_{(a q)}^{+} \text {and } \mathrm{Cu}^{+}{ }_{(a q)}+e^{-} \rightarrow \mathrm{Cu}_{(s)}\) are + 0.15 V and + 0.50 V respectively.
⇒ \(\mathrm{Cu}_{(\mathrm{aq})}^{2+}+e^{–} \longrightarrow \mathrm{Cu}_{(\mathrm{aq})}^{+} ; E_1^0=0.15 \mathrm{~V}\)
⇒ \(\mathrm{Cu}^{+}{ }_{(\mathrm{aq})}+e^{-} \longrightarrow \mathrm{Cu}_{(s)} ; E_2{ }^{\circ}=0.50 \mathrm{~V}\)
⇒ \(\mathrm{Cu}^{2+}+2 e^{-} \longrightarrow \mathrm{Cu} ; E^{\circ}=?\)
Now, \(\Delta G^a=\Delta G_1{ }^{\circ}+\Delta G_2{ }^{\circ}\)
or, \(-n F E^{\circ}=-n_1 F E_1{ }^{\circ}-n_2 F E_2{ }^{\circ}\)
or, \(E^{\circ}=\frac{n_1 E_1^{\circ}+n_2 E_2^{\circ}}{n}=\frac{1 \times 0.15+1 \times 0.50}{2}=0.325 \mathrm{~V}\)
NEET MCQs on Electrochemistry
Question 29. For the reduction of silver ions with copper metal, the standard cell potential was found to be + 0.46 V at 25 °C. The value of standard Gibbs energy, ΔG° will be (F = 96500 C mol-1)
- – 89.0 kJ
- – 89.0 J
- -44.5 kJ
- – 98.0 kJ
Answer: 1. – 89.0 kJ
For the reduction of silver ions with copper metal, the standard cell potential was found to be + 0.46 V at 25 °C.
The cell reaction can be written as \(\mathrm{Cu}+2 \mathrm{Ag}^{+} \longrightarrow \mathrm{Cu}^{2+}+2 \mathrm{Ag}\)
We know, \(\Delta G^{\circ}=-n F E^{\circ}{ }_{\text {cell }}\)
= – 2 x 96500 x 0.46 = – 88780 J
= – 88.78 kJ ≈ -89 kJ
Question 30. Given:
- \(\mathrm{Cu}^{2+}+2 e^{-} \rightarrow \mathrm{Cu}, E^{\circ}=0.337 \mathrm{~V}\)
- \(\mathrm{Cu}^{2+}+e^{-} \rightarrow \mathrm{Cu}^{+}, E^{\circ}=0.153 \mathrm{~V}\)
Electrode potential, E° for the reaction, \(\mathrm{Cu}^{+}+e^{-} \rightarrow \mathrm{Cu}\), will be
- 0.90 V
- 0.30 V
- 0.38 V
- 0.52 V
Answer: 4. 0.52 V
Given, \(\mathrm{Cu}^{2+}+2 e^{-} \longrightarrow \mathrm{Cu} ; E_1^0=0.337 \mathrm{~V}\); \(\mathrm{Cu}^{2+}+e^{-} \longrightarrow \mathrm{Cu}^{+} ; E_2^{\circ}=0.153 \mathrm{~V}\)
The required reaction is \(\mathrm{Cu}^{+}+e^{-} \longrightarrow \mathrm{Cu} ; E_3^{\circ}=?\)
Applying, \(\Delta G^{\circ}=-n F E^{\circ}, \Delta G_3^{\circ}=\Delta G_1^{\circ}-\Delta G_2^{\circ}\)
⇒ \(-\left(n_3 F E_3^{\circ}\right)=-\left(n_1 F E_1^{\circ}\right)-\left(-n_2 F E_2^{\circ}\right)\)
or \(E_3^{\circ}=2 \times E_1^{\circ}-E_2^{\circ}\)
or \(E_3^{\circ}=(2 \times 0.337)-0.153=0.52 \mathrm{~V}\).
NEET MCQs on Electrochemistry
Question 31. Standard free energies of formation (in kJ/mol) at 298 K are -237.2, -394.4, and -8.2 for H2O(l), CO2(g), and pentane(g), respectively. The value of £°cell for the pentane-oxygen fuel cell is
- 1.0968 V
- 0.0968 V
- 1.968 V
- 2.0968 V
Answer: 1. 1.0968 V
Given
Standard free energies of formation (in kJ/mol) at 298 K are -237.2, -394.4, and -8.2 for H2O(l), CO2(g), and pentane(g), respectively.
⇒ \(\mathrm{C}_5 \mathrm{H}_{12(g)}+8 \mathrm{O}_{2(g)} \longrightarrow 5 \mathrm{CO}_{2(\mathrm{~g})}+6 \mathrm{H}_2 \mathrm{O}_{(l)}\)
⇒ \(\Delta G^{\circ}=[(-394.4 \times 5)+(-237.2 \times 6)]-[(-8.2)+(8 \times 0)]\) =-3387 kJ
Note that the standard free energy change of elementary substances is taken as zero.
For the fuel cell, the complete cell reaction is: \(\mathrm{C}_5 \mathrm{H}_{12(\mathrm{~g})}+8 \mathrm{O}_{2(\mathrm{~g})} \longrightarrow 5 \mathrm{CO}_{2(\mathrm{~g})}+6 \mathrm{H}_2 \mathrm{O}_{(0)}\)
which is the combination of the following two half-reactions: \(\mathrm{C}_5 \mathrm{H}_{12(g)}+10 \mathrm{H}_2 \mathrm{O}_{(\mathrm{l})} \longrightarrow 5 \mathrm{CO}_{2(g)}+32 \mathrm{H}^{+}+32 e^{-}\)
and \(8 \mathrm{O}_{2(\mathrm{~g})}+32 \mathrm{H}^{+}+32 e^{-} \longrightarrow 16 \mathrm{H}_2 \mathrm{O}_{(l)}\)
Therefore, the number of electrons exchanged is 32 here, i.e., n=32.
⇒ \(\Delta G^{\circ}=-n F E^{\circ}=-3387 \times 10^3 \mathrm{~J}\)
= \(-32 \times 96500 \mathrm{~J} / \text { Volt } \times E^0\)
Thus, \(E^{\circ}=1.0968 \mathrm{~V}\)
Question 32. The equilibrium constant of the reaction: \(\mathrm{Cu}_{(s)}+2 \mathrm{Ag}_{(a q)}^{+} \rightarrow \mathrm{Cu}^{2+}{ }_{(a q)}+2 \mathrm{Ag}_{(s)}\); \(E^{\circ}=0.46 \mathrm{~V}\) at \(298 \mathrm{~K}\) is
- \(2.0 \times 10^{10}\)
- \(4.0 \times 10^{10}\)
- \(4.0 \times 10^{15}\)
- \(2.4 \times 10^{10}\)
Answer: 3. \(4.0 \times 10^{15}\)
For a cell reaction in equilibrium at 298 K, \(E_{\text {cell }}^{\circ}=\frac{0.0591}{n} \log K_c\)
where, \(K_c=\) equilibrium constant, n= number of electrons involved in the electrochemical cell reaction.
Given, \(E_{\text {cell }}^0=0.46 \mathrm{~V}, n=2\)
0.46 = \(\frac{0.0591}{2} \times \log K_c or, \log K_c=\frac{2 \times 0.46}{0.0591}=15.57\)
or, \(K_c=3.7 \times 10^{15} \approx 4 \times 10^{15}\)
Electrochemistry NEET question bank
Question 33. The standard e.m.f. of a galvanic cell involving cell reaction with n = 2 is found to be 0.295 V at 25°C. The equilibrium constant of the reaction would be
- 2.0 x 1011
- 4.0 x 1012
- 1.0 x 102
- 1.0 x 1010
(Given F = 96500 C mol-1, R = 8.314 J K-1 mol-1)
Answer: 4. 1.0 x 1010
E = \(E^{\circ}-\frac{0,0591}{n} \log _{10} \mathrm{Q}\) at \(25^{\circ} \mathrm{C}\)
At equilibrium, E=0, Q=K
0 = \(E^{\circ}-\frac{0.0591}{n} \log _{10} K\)
or, \(K=\mathrm{antilog}\left[\frac{n E^{\circ}}{0.0591}\right]\)
K = \(\mathrm{antilog}\left[\frac{2 \times 0.295}{0.0591}\right]=\mathrm{antilog}\left[\frac{0.590}{0.0591}\right]\)
= \(\mathrm{antilog} 10=1 \times 10^{10}\)
Question 34. On the basis of the information available from the reaction, \(4 / 3 \mathrm{Al}+\mathrm{O}_2 \rightarrow 2 / 3 \mathrm{Al}_2 \mathrm{O}_3, \Delta G=-827 \mathrm{~kJ} \mathrm{~mol}^{-1} \text { of } \mathrm{O}_2\), the minimum e.m.f. required to carry out an electrolysis of Al2O3 is (F = 96500 C mol-1)
- 2.14 V
- 4.28 V
- 6.42 V
- 8.56 V
Answer: 1. 2.14 V
⇒ \(\Delta G^{\circ}=-n F E^{\circ}\)
⇒ \(E^{\circ}=\frac{\Delta G^{\circ}}{-n F}=\frac{-827000}{-4 \times 96500}=2.14 \mathrm{~V}\)
⇒ \(\left(1 \mathrm{Al} \equiv 3 e^{-}, \frac{4}{3} \mathrm{Al}=\frac{4}{3} \times 3 e^{-}=4 e^{-}\right)\)
Question 35. For the disproportionation of copper \(2 \mathrm{Cu}^{+} \rightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}, E^{\circ}\) is (Given : \(E^{\circ}\) for \(\mathrm{Cu}^{2+} / \mathrm{Cu}\) is \(0.34 \mathrm{~V}\) and \(E^{\circ}\) for \(\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}\) is \(0.15 \mathrm{~V}\)
- 0.49 V
- -0.19 V
- 0.38 V
- -0.38 V
Answer: 3. 0.38 V
For the reaction, \(2 \mathrm{Cu}^{+} \longrightarrow \mathrm{Cu}^{2+}+\mathrm{Cu}\) the cathode is \(\mathrm{Cu}^{+} / \mathrm{Cu}\) and anode is \(\mathrm{Cu}^{+} / \mathrm{Cu}^{2+}\).
Given, \(\mathrm{Cu}^{2+}+2 e^{-} \longrightarrow \mathrm{Cu}_2 E_1^{\circ}=0.34 \mathrm{~V}\)…..(1)
\(\mathrm{Cu}^{2+}+e^{-} \longrightarrow \mathrm{Cu}^{+} ; E_2^{\circ}=0.15 \mathrm{~V}\)….(2)
\(\mathrm{Cu}^{+}+e^{-} \longrightarrow \mathrm{Cu} ; E_3^{\circ}=?\)….(3)
Now, \(\Delta G_1^{\circ}=-n F E_1^{\circ}=-2 \times 0.34 \times F=-0.68 F\)
⇒ \(\Delta G_2^{\circ}=-1 \times 0.15 \times F, \Delta G_3^{\circ}=-1 \times E_3^{\mathrm{a}} \times F\)
Again \(\Delta G_1^{\circ}=\Delta G_2^{\circ}+\Delta G_3^{\circ}\)
-0.68 F = \(-0.15 F-E_3^0 \times F\)
⇒ \(E_3^{\circ}=0.68-0.15=0.53 \mathrm{~V}\)
⇒ \(E_{\text {cell }}^{\circ}=E_{\text {cathode }\left(\mathrm{Cu}^{+} / \mathrm{Cu}\right)}^{\circ}-E_{\text {anode }\left(\mathrm{Cu}^{2+} / \mathrm{Cu}^{+}\right)}^{\circ}\)
= \(0.53-0.15=0.38 \mathrm{~V}\)
Electrochemistry NEET question bank
Question 36. E° for the cell, \(\mathrm{Zn}\left|\mathrm{Zn}^{2+}{ }_{(a q)}\right|\left|\mathrm{Cu}^{2+}{ }_{(a q)}\right| \mathrm{Cu}\) is \(1.10 \mathrm{~V}\) at \(25^{\circ} \mathrm{C}\), the equilibrium constant for the reaction \(\mathrm{Zn}+\mathrm{Cu}_{(\text {aq) }}^{2+} \rightarrow \mathrm{Cu}+\mathrm{Zn}_{(\text {aq })}^{2+}\) is of the order
- \(10^{+18}\)
- \(10^{+17}\)
- \(10^{-28}\)
- \(10^{+37}\)
Answer: 4. \(10^{+37}\)
Nernst equation is E = \(E^{\circ}-\frac{0.059}{2} \log K\)
⇒ \(E^{\circ}=\frac{0.059}{2} \log K\) (E=0 at equilibrium condition)
⇒ 1.1 = \(\frac{0.059}{2} \log K \Rightarrow K=1.9 \times 10^{+37}\)
Question 37. The conductivity of a centriolar solution of KCI at 25°C is 0.0210 ohm-1 cm-1 and the resistance of the cell containing the solution at 25°C is 60 ohm. The value of the cell constant is
- 1.26 cm-1
- 3.34 cm-1
- 1.34 cm-1
- 3.28 cm-1
Answer: 1. 1.26 cm-1
The conductivity of a centriolar solution of KCI at 25°C is 0.0210 ohm-1 cm-1 and the resistance of the cell containing the solution at 25°C is 60 ohm.
Given, \(\mathrm{K}=0.0210 \mathrm{ohm}^{-1} \mathrm{~cm}^{-1}, R=60 \mathrm{ohm}\)
k = \(\frac{1}{R} \times \frac{l}{a}\) (where \(\frac{l}{a}=\) cell constant) or \(0.0210=\frac{1}{60} \times \frac{l}{a}\)
or \(\frac{l}{a}=60 \times 0.0210=1.26 \mathrm{~cm}^{-1}\)
Question 38. The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol-1 respectively. The molar conductance of CH3COOH at infinite dilution is.
Choose the right option for your answer.
- 540.48 S cm2 mol-1
- 201.28 S cm2 mol-1
- 390.71 S cm2 mol-1
- 698.28 S cm2 mol-1
Answer: 3. 390.71 S cm2 mol-1
The molar conductance of NaCl, HCl, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm2 mol-1 respectively.
⇒ \(\Lambda_{\mathrm{CH}_3 \mathrm{COOH}}^{\infty}=\Lambda_{\mathrm{CH}_3 \mathrm{COONa}}^{\circ}+\Lambda_{\mathrm{HCl}}^{\circ}-\Lambda_{\mathrm{NaCl}}^{\circ}\)
= \(91.0+426.16-126.45\)
= \(390.71 \mathrm{Scm}^2 \mathrm{~mol}^{-1}\)
Electrochemistry NEET question bank
Question 39. The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol-1. What is the dissociation constant of acetic acid? Choose the correct option.
⇒ \(\left[\begin{array}{c}
\Lambda_{\mathrm{H}^{+}}^{\circ}=350 \mathrm{Scm}^2 \mathrm{~mol}^{-1} \\
\Lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}=50 \mathrm{Scm}^2 \mathrm{~mol}^{-1}
\end{array}\right]\)
- \(2.50 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}\)
- \(1.75 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1}\)
- \(2.50 \times 10^{-4} \mathrm{~mol} \mathrm{~L}^{-1}\)
- \(1.75 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}\)
Answer: 4. \(1.75 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}\)
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol-1.
⇒ \(\Lambda_{m\left(\mathrm{CH}_3 \mathrm{COOH}\right)}^{\circ}=\lambda_{\mathrm{H}^{+}}^{\circ}+\lambda_{\mathrm{CH}_3 \mathrm{COO}^{-}}^{\circ}\)
= \(350+50=400 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
Degree of dissociation, \(\alpha=\frac{\Lambda_m^c}{\Lambda_m^{\circ}}=\frac{20}{400}=0.05\)
So, dissociation constant, \(K_a=c \alpha^2\) (for weak electrolytes)
= \(0.007(0.05)^2=1.75 \times 10^{-5} \mathrm{~mol} \mathrm{~L}^{-1}\)
