Solutions Chemistry NEET MCQs
NEET Chemistry For Solutions Multiple Choice Questions
Question 1. In one molal solution that contains 0.5 moles of a solute, there is
- 500 mL of solvent
- 500 g of solvent
- 100 mL of solvent
- 1000 g of solvent.
Answer: 2. 500 g of solvent
Molality (m) = \(\frac{\text { Moles of solute }}{\text { Mass of solvent in } \mathrm{kg}}\)
Let the amount of solvent be \(x \mathrm{~g}\).
1 = \(\frac{0.5}{\frac{x}{1000}}\)
x = \(500 \mathrm{~g}\)
Question 2. Which of the following is dependent on temperature?
- Molarity
- Mole fraction
- Weight percentage
- Molality
Answer: 1. Molarity
Molarity is a function of temperature as volume depends on temperature.
Question 3. What is the mole fraction of the solute in a 1.00 m aqueous solution?
- 1.770
- 0.0354
- 0.0177
- 0.177
Answer: 3. 0.0177
1 molal aqueous solution means 1 mole of solute is present in 1000 g of water.
∴ \(x_{\text {solute }}=\frac{1}{1+\frac{1000}{18}}=\frac{1}{56.5}=0.0177\)
Question 4. How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3? The concentrated acid is 70% HNO3.
- 70.0 g cone. HNO3
- 54.0 g cone. HNO3
- 45.0 g cone. HNO3
- 90.0 g cone. HNO3
Answer: 3. 45.0 g cone. HNO3
Molarity = \(\frac{w_{\mathrm{HNO}_3} \times 1000}{M_{\mathrm{HNO}_3} \times V_{\mathrm{sol}(\mathrm{mL})}}\)
or \(2=\frac{w_{\mathrm{HNO}_3}}{63} \times \frac{1000}{250} \Rightarrow w_{\mathrm{HNO}_3}=\frac{63}{2} \mathrm{~g}\)
Mass of acid \(\times \frac{70}{100}=\frac{63}{2}\)
Mass of acid \(=45 \mathrm{~g}\)
Read and Learn More NEET MCQs with Answers
Question 5. Which of the following compounds can be used as antifreeze in automobile radiators?
- Methyl alcohol
- Glycol
- Nitrophenol
- Ethyl alcohol
Answer: 2. Glycol
A 35% (V/V) solution of ethylene glycol is used as an antifreeze in cars for cooling the engine’ At this concentration’ the antifreeze lowers the freezing point of water to 255°4 K (-17.6°C).
Solutions Chemistry NEET MCQs
Question 6. Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mol-1. The volume of acid required to make one litre of 0.1 M H2SO4 solution is
- 16.65 mL
- 22.20 mL
- 5.55 mL
- 11.10 mL
Answer: 3. .55 mL
Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mol-1.
⇒ \(\mathrm{H}_2 \mathrm{SO}_4\) is \(98 \%\) by weight.
Weight of \(\mathrm{H}_2 \mathrm{SO}_4=98 \mathrm{~g}\), Weight of solution \(=100 \mathrm{~g}\)
∴ Volume of solution = \(\frac{\text { mass }}{\text { density }}=\frac{100}{1.80} \mathrm{~mL}\)
= \(55.55 \mathrm{~mL}=0.0555 \mathrm{~L}\)
Molarity of solution = \(\frac{98}{98 \times 0.0555}=18.02 \mathrm{M}\)
Let \(V \mathrm{~mL}\) of this \(\mathrm{H}_2 \mathrm{SO}_4\) is used to prepare 1 litre of 0.1 M \(\mathrm{H}_2 \mathrm{SO}_4\).
∴ mM of concentrated \(\mathrm{H}_2 \mathrm{SO}_4=\mathrm{mM}\) of dilute \(\mathrm{H}_2 \mathrm{SO}_4\)
or, \(V \times 18.02=1000 \times 0.1\)
⇒ V = \(\frac{1000 \times 0.1}{18.02}=5.55 \mathrm{~mL}\)
Question 7. The mole fraction of the solute in one molal aqueous solution is
- 0.009
- 0.018
- 0.027
- 0.036
Answer: 2. 0.018
1 molal aqueous solution means I mole of solute present in 1 kg of H2O.
1 mole of solute present \(\frac{1000}{18}\) mole of H2O
⇒ \(x_{\text {solute }}=\frac{1}{\frac{1000}{18}+1}=\frac{18}{1018}=0.01768=0.018\)
Question 8. 2.5 litres of 1 M NaOH solution is mixed with another 3 litres of 0.5 M NaOH solution. Then find out the arity of the resultant solution.
- 0.80 M
- 1.0 M
- 0.73 M
- 0.50 M
Answer: 3. 0.73 M
2.5 litres of 1 M NaOH solution is mixed with another 3 litres of 0.5 M NaOH solution.
Molecular weight of NaOH =40 g mol-1
2.5 litre of 1 M NaOH solution contain 40 g mol-1 x 1 mol L-1 x 2.5 L = 40 x 2.5 g of NaOH
3 litre of 0.5 M NaOH solution contain
40 g mol-1 x 0.5 mol L-1 x 3 L= 40 x 0.5 x 3 g of NaOH
If these two solutions are mixed, the volume of the resultant solution = (2.5 + 3) = 5.5 litre.
5.51itre of the resultant solution contain = 40(2.5 + 1.5) g of NaOH
1 litre of the resultant solution contain \(\frac{40 \times 4}{5.5} \text { g of } \mathrm{NaOH}=\frac{40 \times 4}{5.5 \times 40} \text { mole of } \mathrm{NaOH}\)
The molarity of the resultant solution = 0.727 = 0.73 M
Question 9. How many grams of dibasic acid (mol. weight 200) should be present in 100 mL of the aqueous solution to give a strength of 0.1 N?
- 10 g
- 2 g
- 1 g
- 20 g
Answer: 3. 1 g
The strength of the solution is 0.1 N.
⇒ \(\frac{w}{E}=\frac{V \times N}{1000}\) (Equivalent weight = \(\frac{200}{2}=100\))
⇒ w = \(\frac{100 \times 0.1 \times 100}{1000}=1 \mathrm{~g}\)
NEET questions on Solutions Chemistry
Question 10. What is the molarity of H2SO4 solution, which has a density 1.84 g/cc at 35°C and contains 98% by weight?
- 18.4 M
- 18 M
- 4.18 M
- 8.14 M
Answer: 1. 18.4 M
We know that 98% H2SO4 by weight means 98 g of H2SO4 is present in 100 g of solution
Therefore, its weight is 98 and moles of \(\mathrm{H}_2 \mathrm{SO}_4\)
= \(\frac{\text { Weight of } \mathrm{H}_2 \mathrm{SO}_4}{\text { Molecular weight }}=\frac{98}{98}=1\)
and volume of solution = \(\frac{\text { Mass }}{\text { Density }}\)
= \(\frac{100}{1.84}=54.35 \mathrm{~mL}=\frac{54.35}{1000} \mathrm{~L}\)
Therefore, molarity of \(\mathrm{H}_2 \mathrm{SO}_4\)
= \(\frac{\text { Moles of } \mathrm{H}_2 \mathrm{SO}_4}{\text { Volume (in litres) }}=\frac{1 \times 1000}{54.35}=18.4 \mathrm{M}\)
Question 11. The concentration unit, independent of temperature, would be
- Normality
- Weight volume per cent
- Molality
- Molarity.
Answer: 3. Molality
The molality involves the weights of the solute and the solvent. Since the weight does not change with the temperature. therefore molality does not depend upon the temperature.
Question 12. How many grams of CH3OH should be added to water to prepare a 150 mL solution of 2 M CH3OH?
- 9.6 x 10³
- 2.4 x 10³
- 9.6
- 2.4
Answer: 3. 9.6
Since the molecular mass of CH3OH is 32 therefore the quantity of CH3OH to prepare 150 mL solution of 2 M CH3OH
= \(\left(\frac{2}{1000}\right) \times 150 \times 32=9.6 \mathrm{~g}\)
Question 13. The correct option for the value of vapour pressure of a solution at 45° C with benzene to octane in a molar ratio 3: 2 is
[At 45° C vapour pressure of benzene is 280 mm Hg and that of octane is 420 mm Hg. Assume ideal gas]
- 350 mm of Hg
- 160 mm of Hg
- 168 mm of Hg
- 336 mm of Hg
Answer: 4. 336 mm of Hg
⇒ \(P_{\text {total }}=P_{\text {bemone }} x_{\text {benzene }}+P_{\text {ocane }} x_{\text {octane }}\)
= \(280 \times \frac{3}{5}+420 \times \frac{2}{5}=168+168\)
= \(336 \mathrm{~mm} \text { of } \mathrm{Hg}\)
NEET questions on Solutions Chemistry
Question 14. In water-saturated air, the mole fraction of water vapour is 0.02. If the total pressure of the saturated air is 1.2 atm, the partial pressure of dry air is
- 1.18 atm
- 1.76 atm
- 1.176 atm
- 0.98 atm.
Answer: 3. 1.176 atm
pwater vapour = xwater vapour x Pwater vapour
= 0.02 x 1.2 = 0.024atm
Ptotal = Pwater vapour+ Pdry air
1.2 = 0.024 + pdry air
Pdry air= 1.2 – 0.024 = 1.176 atm
Partial vapour pressure is directly proportional to mole fraction, p ∝ x.
Question 15. pA and pB are the vapour pressures of pure liquid components, A and B, respectively of an ideal binary solution. If xA represents the mole fraction of component A, the total pressure of the solution will be
- pA + xA(pB – pA)
- pA + xA(pA – pB)
- pB + xA(pB – pA)
- pB + xA(pA– pB)
Answer: 4. pB + xA(pA– pB)
pA and pB are the vapour pressures of pure liquid components, A and B, respectively of an ideal binary solution. If xA represents the mole fraction of component A,
According to Raoult’s law, \(p=x_A p_A+x_B p_B\)….(1)
For binary solutions, \(x_A+x_B=1, x_B=1-x_A\)….(2)
Putting the value of \(x_B\) from eqn. (2) to eqn. (1)
P = \(x_A p_A+\left(1-x_A\right) p_B=x_A p_A+p_B-x_A p_B\)
P = \(p_B+x_A\left(p_A-p_B\right)\)
Solutions multiple choice NEET
Question 16. The vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25°C is 200 mm Hg and 41.5 mm Hg respectively. The vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be
(Molecular mass of CHCl2 = 119.5 u and molecular mass of CH2Cl2 = 85 u)
- 173.9 mm Hg
- 615.0 mm Hg
- 347.9 mm Hg
- 285.5 mm Hg
Answer: None
The vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25°C is 200 mm Hg and 41.5 mm Hg respectively.
⇒ \(p_{\mathrm{CHCl}_5}^{\circ}=200 \mathrm{~mm} \mathrm{Hg}, p^{\mathrm{a}} \mathrm{CH}_2 \mathrm{Cl}_2=41.5 \mathrm{~mm} \mathrm{Hg}\)
Moles of \(\mathrm{CHCl}_3=\frac{\text { Weight }}{\text { Molecular weight }}=\frac{25.5}{119.5}=0.2132111\)
Moles of \(\mathrm{CH}_2 \mathrm{Cl}_2=\frac{40}{85}=0.470\)
⇒ \(x_{\mathrm{CHCl}_3}=\frac{0.213}{0.213+0.470}=0.31\)
⇒ \(x_{\mathrm{CH}_2 \mathrm{Cl}_2}=\frac{0.470}{0.213+0.470}=0.69\)
⇒ \(P_{\text {tocal }}=p^{\circ}{ }_{\mathrm{CHC}_3} x_{\mathrm{CHCl}_3}+p^{\circ}{ }_{\mathrm{CH}_2 \mathrm{Cl}_2} x_{\mathrm{CH}_2 \mathrm{C}_2}\)
= \(200 \times 0.31+41.5 \times 0.69=62+28.63=90.63 \mathrm{~mm} \mathrm{Hg}\)
Question 17. A solution has a 1:4 mole ratio of pentane to hexane. The vapour pressures of the pure hydrocarbons at 20 °C are 440 mm Hg for pentane and 120 mm Hg for hexane. Tire mole fraction of pentane in the vapour phase would be
- 0.200
- 0.549
- 0.786
- 0.478
Answer: 4. 0.478
A solution has a 1:4 mole ratio of pentane to hexane. The vapour pressures of the pure hydrocarbons at 20 °C are 440 mm Hg for pentane and 120 mm Hg for hexane.
⇒ \(\frac{n_{C_6} H_{12}}{n_{C_6} H_{14}}=\frac{1}{4}\)
⇒ \(x_{\mathrm{C}_5 \mathrm{H}_{12}}=\frac{1}{5} \text { and } x_{\mathrm{C}_6 \mathrm{H}_{14}}=\frac{4}{5}\)
⇒ \(p_{\mathrm{C}_5 \mathrm{H}_{12}}^{\circ}=440 \mathrm{~mm} \mathrm{Hg} ; P_{\mathrm{C}_6 \mathrm{H}_{14}}^{\circ}=120 \mathrm{~mm} \mathrm{Hg}\)
⇒ \(P_{\text {total }}=p^{\circ}{ }_{\mathrm{C}_5 \mathrm{H}_{12}} x_{\mathrm{C}_5 \mathrm{H}_{12}}+p^{\circ}{ }_{\mathrm{C}_6 \mathrm{H}_{14}} x_{\mathrm{C}_6 \mathrm{H}_{14}}^5\)
= \(440 \times \frac{1}{5}+120 \times \frac{4}{5}=88+96=184 \mathrm{~mm} \text { of } \mathrm{Hg}\)
By Raoult’s law, \(p_{\mathrm{C}_5 \mathrm{H}_{12}}=p^0 \mathrm{C}_5 \mathrm{H}_{12} x_{\mathrm{C}_5 \mathrm{H}_{12}}\)….(1)
⇒ \(x_{\mathrm{C}_5 \mathrm{H}_{12}} \rightarrow\) mole fraction of pentane in solution.
By Dalton’s law, \(P_{\mathrm{C}_3 \mathrm{H}_{12}}=x_{\mathrm{C}_5 \mathrm{H}_{12}}^{\prime} P_{\text {total }}\)……(2)
⇒ \(x^{\prime}{ }_{\mathrm{C}_5 \mathrm{H}_{12}} \rightarrow\) mole fraction of pentane above the solution.
From (1) and (2), \(P_{\mathrm{C}_5 \mathrm{H}_{12}}=440 \times \frac{1}{5}=88 \mathrm{~mm} \text { of } \mathrm{Hg}\)
⇒ 88 = \(x^{\prime}{ }_{\mathrm{C}_5 \mathrm{H}_{12}} \times 184 \Rightarrow x^{\prime}{ }_{\mathrm{C}_5 \mathrm{H}_{12}}=\frac{88}{184}=0.478\)
Solutions multiple choice NEET
Question 18. The vapour pressure of two liquids P and Q are 80 and 60 torr, respectively. The total vapour pressure of the solution obtained by mixing 3 moles of P and 2 mol of Q would be
- 72 torr
- 140 torr
- 68 torr
- 20 torr
Answer: 1. 72 torr
By Raoult’s Law, \(P_T=p_p^0 x_p^0+p_Q^{\circ} x_Q\) where \(p_P^{\circ}=80\) torr, \(p_Q^{\circ}=60\) torr, \(x_P=\frac{3}{5} ; x_Q=\frac{2}{5}\)
⇒ \(P_T=80 \times \frac{3}{5}+60 \times \frac{2}{5}=48+24=72 \text { torr }\)
⇒ \(P_T=80 \times \frac{3}{5}+60 \times \frac{2}{5}=48+24=72 \text { torr }\)
Question 19. The mixture which shows positive deviation from Raoult’s law is
- Ethanol + acetone
- Benzene + toluene
- Acetone + chloroform
- Chloroethane + bromoethane.
Answer: 1. Ethanol + acetone
A mixture of ethanol and acetone shows a positive deviation from Raoult’s law.
In pure ethanol, molecules are hydrogen bonded. On adding acetone, its molecules get in between the host molecules and break some of, the hydrogen bonds between them. Due to the weakening of interactions, the solution shows a positive deviation from Raoult’s law.
NEET practice questions Solutions
Question 20. For an ideal solution, the correct option is
- Δmix G = 0 at constant T and P
- Δmix S = 0 at constant T and P
- Δmix V ≠ 0 at constant Tand P
- Δmix H = 0 at constant T and P.
Answer: 4. ΔmixH = 0 at constant T and P.
For an ideal solution, Δmix H = 0 and Δmix V = 0 at constant T and P.
Question 21. The mixture that forms maximum boiling azeotrope is
- Heptane + octane
- Water + nitric acid
- Ethanol + water
- Acetone + carbon disulphide.
Answer: 2. Water + nitric acid
Maximum boiling azeotropes are formed by those solutions which show negative deviations from Raoult’s law. H2O and HNO3 mixture show negative deviations.
Question 22. Which of the following statements is correct regarding a solution of two components A and B exhibiting positive deviation from ideal behaviour?
- Intermolecular attractive forces between A-A and B-B are stronger than those between A-B.
- Δmix H = 0 at constant T and p.
- Δmix V= 0 at constant T and P.
- Intermolecular attractive forces between A-A and B-B are equal to those between A-B
Answer: 1. Intermolecular attractive forces between A-A and B-B are stronger than those between A-B.
In case of positive deviation from Raoult’s Law, A-B interactions are weaker than those between A-A or B-B, i.e., in this case, the intermolecular attractive forces between the solute-solvent molecules are weaker than those between the solute-solute and solvent-solvent molecules.
This means that in such a solution, molecules of A (or B) will find it easier to escape than in a pure state. This will increase the vapour pressure and result in positive deviation.
NEET practice questions Solutions
Question 23. Which one of the following is incorrect for an ideal solution?
- ΔHmix= 0
- ΔUmix = 0
- ΔP = Pobs — Pcalculated by Raoults’s law- = 0
- ΔGmix= 0
Answer: 4. ΔHmix= 0
For an ideal solution, \(\Delta H_{\text {mix }}=0, \Delta V_{\mathrm{mix}}=0 \text {, }\)
Now, \(\Delta U_{\text {mix }}=\Delta H_{\text {mix }}-P \Delta V_{\text {mix }}\)
∴ \(\Delta U_{\mathrm{mix}}=0\)
Also, for an ideal solution, \(P_A=x_A P_A^{\circ}, P_B=x_B P_B^{\circ}\)
∴ \(\Delta P=P_{\text {cbs }}-P_{\text {calculaned by Rooult’s law }}=0\)
∴ \(\Delta G_{\text {mix }}= \Delta H_{\text {mix }}-T \Delta S_{\text {mix }}\)
For an ideal solution, \(\Delta S_{\text {mis }} \neq 0\)
∴ \(\Delta G_{\text {mix }} \neq 0\)
Question 24. Which of the following statements about the composition of the vapour over an ideal 1: 1 molar mixture of benzene and toluene is correct?
Assume that the temperature is constant at 25°C. (Given, vapour pressure data at 25°C, benzene = 12.8 kPa, toluene = 3.85 kPa)
- The vapour will contain equal amounts of benzene and toluene.
- Not enough information is given to make a prediction.
- The vapour will contain a higher percentage of benzene
- The vapour will contain a higher percentage of toluene.
Answer: 3. The vapour will contain a higher percentage of benzene
⇒ \(p_{\text {Bewaene }}\) = \(x_{\text {Benzene }}p_{\text {Benaene }}^{\circ}\)
⇒ \(P_{\text {Taluene }}\) = \(x_{\text {Talsene }}p_{\text {Toluene }}^{\circ}\)
For an ideal 1: 1 molar mixture of benzene and toluene, \(x_{\text {Benzene }}=\frac{1}{2} \text { and } x_{\text {Toluene }}=\frac{1}{2}\)
⇒ \(P_{\text {Benzene }}=\frac{1}{2} p_{\text {Benzene }}^{\text {o }}=\frac{1}{2} \times 12.8 \mathrm{kPa}=6.4 \mathrm{kPa}\)
⇒ \(P_{\text {Toluene }}=\frac{1}{2} \rho_{\text {Toluene }}^{\mathrm{a}}=\frac{1}{2} \times 3.85 \mathrm{kPa}=1.925 \mathrm{kPa}\)
Thus, the vapour will contain a high percentage of benzene as the partial vapour pressure of benzene is higher as compared to that of toluene.
Chemistry MCQs Solutions NEET
Question 25. Which condition is not satisfied by an ideal solution?
- ΔmixV = 0
- ΔmixS = 0
- Obeyance to Raoulfs Taw
- ΔmixH = 0
Answer: 2. ΔmixS = 0
For an ideal solution:
- Volume change (ΔV) on mixing should be zero.
- Heat change (ΔH) on mixing should be zero.
- Obeys Raouitt law at every range of concentration.
- Entropy change ((ΔS) on mixing ≠ 0.
Question 26. A solution of acetone in ethanol
- Obeys Raoult’s law
- Shows a negative deviation from Raoult’s law
- Shows a positive deviation from Raoult’s law
- Behaves like a near-ideal solution.
Answer: 3. Shows a positive deviation from Raoult’s law
Both the components escape easily showing higher vapour pressure than the expected value. This is due to the breaking of some hydrogen bonds between ethanol molecules.
Question 27. A solution containing components A and B follows Raoulf’s law
- A – B attraction force is greater than A – A and B-B
- The a-B attraction force is less than A-A and B-B
- A-B attraction force remains the same as A – A and B-B
- The volume of the solution is different from the sum of the volume of the solute and solvent.
Answer: 3. A-B attraction force remains the same as A – A and B-B
Raoultt’s law is valid for ideal solutions only. The element of non-ideality enters into the picture when the molecules of the solute and solvent affect each other’s intermolecular forces. A solution containing components of A and B behaves as an ideal solution when the A – B attraction force remains the same as the A – A and B – B attraction forces.
Chemistry MCQs Solutions NEET
Question 28. All form ideal solutions except
- C6H6 and C6H5CH3
- C2H5Br and C2H5I
- C6H5Cl and C6H5Br
- C2H5I and C2H5OH
Answer: 4. C2H5I and C2H5OH
C2H5OH is associated through H-bonding while C2H5I does not show H-bonding.
Question 29. An ideal solution is formed when its components
- Have no volume change on mixing
- Have no enthalpy change on mixing
- Have both the above characteristics
- Have high solubility.
Answer: 3. Have both of the above characteristics
For the ideal solution, ΔVmixing = 0 and ΔHmixing = 0
Question 30. The following solutions were prepared by dissolving 10 g of glucose (C6H12O6) in 250 ml of water (P1). 10 g of urea (CH4N2O) in 250 ml of water (P2) and 10 g of sucrose (C12H22O11) in 250 ml of water (P3). The right option for the decreasing order of osmotic pressure of these solutions is
- P3 > P1 > P2
- P2 > P1 > P3
- P1 >P2> P3
- P2 > P3 > P1
Answer: 2. P2 > P1 > P3
The following solutions were prepared by dissolving 10 g of glucose (C6H12O6) in 250 ml of water (P1). 10 g of urea (CH4N2O) in 250 ml of water (P2) and 10 g of sucrose (C12H22O11) in 250 ml of water (P3).
Osmotic pressure \((\pi)=C R T\)
∴ \(\pi \propto \mathrm{C}\)
For glucose solution, \(C_1=\frac{10}{180} \times \frac{1000}{250}=0.22 M\)
For urea solution, \(C_2=\frac{10}{60} \times \frac{1000}{250}=0.66 \mathrm{M}\)
For sucrose solution, \(C_3=\frac{10}{342} \times \frac{1000}{250}=0.117 \mathrm{M}\)
Hence, the order of osmotic pressure is \(P_2>P_1>P_3\).
Solutions quiz for NEET
Question 31. The freezing point depression constant (Kf) of benzene is 5.12 K kg mol-1. The freezing point depression for the solution of molality 0.078 m containing a non-electrolyte solute in benzene is (rounded off up to two decimal places)
- 0.20 K
- 0.80 K
- 0.40 K
- 0.60 K
Answer: 3. 0.40 K
Given Kf = 5.12 K kg mol-1, m =0.078m
ΔTf = Kf x m = 5.12 x 0.078 = 0.39936 ≈ 0.40 K
Question 32. If the molality of the dilute solution is doubled, the value of the molal depression constant Kf will be
- Halved
- Tripled
- Unchanged
- Doubled.
Answer: 3. Unchanged
The value of molal depression-constant, Kf is constant for a particular solvent, thus, it will be unchanged when the molality of the dilut. the solution is doubled.
Question 33. At 100°C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732 mm. If Kb = 0.52, the boiling point of this solution will be
- 102 °C
- 103 °C
- 101 °C
- 100 °C
Answer: 3. 101 °C
Given: \(W_B=6.5 \mathrm{~g}, W_A=100 \mathrm{~g}\), \(p_{\mathrm{s}}=732 \mathrm{~mm}, K_b=0.52, T_b^{\circ}=100^{\circ} \mathrm{C}, p^{\circ}=760 \mathrm{~mm}\)
∴ \(\frac{p^{\circ}-p_s}{p^{\circ}}=\frac{n_2}{n_1} \Rightarrow \frac{760-732}{760}=\frac{n_2}{100 / 18}\)
⇒ \(n_2=\frac{28 \times 100}{760 \times 18}=0.2046 \mathrm{~mol}\)
⇒ \(\Delta T_b=K_b \times m\)
⇒ \(T_b-T_b^{\circ}=K_b \times \frac{n_2 \times 1000}{W_A(\mathrm{~g})}\)
⇒ \(T_b-100^{\circ} \mathrm{C}=\frac{0.52 \times 0.2046 \times 1000}{100}=1.06\)
⇒ \(T_b=100+1.06=101.06^{\circ} \mathrm{C}\)
Solutions quiz for NEET
Question 34. 200 mL of an aqueous solution of a protein contains 1.26 g. The osmotic pressure of this solution at 300 K is found to be 2.57 x 10-3 bar. The molar mass of the protein will be (R = 0.083 L bar mol-1 K-1)
- 51022 g mol-1
- 122044 g mol-1
- 31011 g mol-1
- 61038 g mol-1
Answer: 4. 61038 g mol-1
200 mL of an aqueous solution of a protein contains 1.26 g. The osmotic pressure of this solution at 300 K is found to be 2.57 x 10-3 bar.
We know that \(p V=n R T\), where\(n=\frac{w}{M}\)
πV = \(\frac{w}{M} R T\)
M = \(\frac{w R T}{\pi V}=\frac{1.26 \times 0.083 \times 300}{2.57 \times 10^{-3} \times \frac{200}{1000}}\)
= \(\frac{1.26 \times 0.083 \times 300}{2.57 \times 10^{-3} \times 0.2}=61038 \mathrm{~g} \mathrm{~mol}^{-1}\)
Question 35. A solution of sucrose (molar mass = 342 g mol-1) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water. The freezing point of the solution obtained will be (Kf for water = 1.86 K kg mol-1)
- -0.372 °C
- -0.520 °C
- +0.372 °C
- -0.570 °C
Answer: 1. -0.372 °C
A solution of sucrose (molar mass = 342 g mol-1) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water.
We know, \(\Delta T_f=K_f m\)
m = \(\frac{w_B}{M_B} \times \frac{1000}{W_A}=\frac{68.5 \times 1000}{342 \times 1000}=\frac{68.5}{342}\)
⇒ \(\Delta T_f=1.86 \times \frac{68.5}{342}=0.372^{\circ} \mathrm{C}\)
⇒ \(T_f=0-0.372^{\circ} \mathrm{C}=-0.372^{\circ} \mathrm{C}\)
Question 36. During osmosis, the flow of water through a semipermeable membrane is
- From solution having lower concentration only
- From solution having higher concentration only
- From both sides of the semipermeable membrane with equal flow rates
- From both sides of the semipermeable membrane with unequal flow rates.
Answer: 1. From solution having lower concentration only
Question 37. 1.00 g of a non-electrolyte solute (molar mass 250 g mol-1) was dissolved in 51.2 g of benzene. If the freezing point constant, Kf of benzene is 5.12 Kf kg mol-1, the freezing point of benzene will be lowered by
- 0.2 K
- 0.4 K
- 0.3 K
- 0.5 K
Answer: 2. 0.4 K
1.00 g of a non-electrolyte solute (molar mass 250 g mol-1) was dissolved in 51.2 g of benzene. If the freezing point constant, Kf of benzene is 5.12 Kf kg mol-1,
= \(M_B=\frac{1000 \times K_f \times w_B}{W_A \times \Delta T_f}\)
or, \(250=\frac{1000 \times 5.12 \times 1}{51.2 \times \Delta T_f}\)
∴ \(\Delta T_f=\frac{1000 \times 5.12 \times 1}{51.2 \times 250}=0.4 \mathrm{~K}\)
Question 38. A solution containing 10 g per dm³ of urea (molecular mass = 60 g mol-1) is isotonic with a 5% solution of a non-volatile solute. The molecular mass of this non-volatile solute is
- 200 g mol-1
- 250 g mol-1
- 300 g mol-1
- 350 g mol-1
Answer: 3. 300 g mol-1
A solution containing 10 g per dm³ of urea (molecular mass = 60 g mol-1) is isotonic with a 5% solution of a non-volatile solute.
Molar concentration of urea = \(\frac{10}{60}\) dm3
Molar concentration of non-volatile solution = \(\frac{50}{M_B} \mathrm{~L}^{-1}=\frac{50}{M_B} \mathrm{dm}^{-3}\)
For isotonic solutions, \(\frac{10}{60}=\frac{50}{M_B}\)
MB =300 g mol-1
Question 39. A solution of urea (mol. mass 56 g mol-1) boils at 100.18°C at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1 respectively, the above solution will freeze at
- 0.654°C
- – 0.654°C
- 6.54°C
- -6.54°C
Answer: 2. – 0.654°C
A solution of urea (mol. mass 56 g mol-1) boils at 100.18°C at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1 respectively,
⇒ \(\Delta T_f=K_f m\)….(1)
⇒ \(\Delta T_b=K_b m\)….(2)
⇒ \(\frac{\Delta T_f}{\Delta T_b}=\frac{K_f}{K_b}\)….(3)
⇒ \(\Delta T_f\) depression in freezing point
⇒ \(\Delta T_b \rightarrow\) elevation in boiling point
⇒ \(K_f=1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\)
⇒ \(K_b=0.512 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}, \Delta T_b=100.18-100=0.18\)
From eq. (3), \(\frac{\Delta T_f}{0.18}=\frac{1.86}{0.512}\)
⇒ \(\Delta T_f=0.654=T_f^{\circ}-T_f=0-T_f \Rightarrow T_f=-0.654^{\circ} \mathrm{C}\)
Freezing point of urea in water \(=-0.654^{\circ} \mathrm{C}\)
NEET MCQs on Solutions
Question 40. A solution contains a non-volatile solute of molecular mass M2. Which of the following can be used to calculate the molecular mass of solute in terms of osmotic pressure? Pure water can be obtained from seawater by
- \(M_2=\left(\frac{m_2}{\pi}\right) V R T\)
- \(M_2=\left(\frac{m_2}{V}\right) \frac{R T}{\pi}\)
- \(M_2=\left(\frac{m_2}{V}\right) \pi R T\)
- \(M_2=\left(\frac{m_2}{V}\right) \frac{\pi}{R T}\)
Answer: 2. \(M_2=\left(\frac{m_2}{V}\right) \frac{R T}{\pi}\)
For dilute solution, \(\pi=\frac{n}{V} R T\)
⇒ \(\pi V=\frac{m_2}{M_2} R T \Rightarrow M_2=\frac{m_2 R T}{\pi V}\)
Question 41. Pure water can be obtained from seawater by
- Centrifugation
- Plasmolysis
- Reverse osmosis
- Sedimentation
Answer: 3. Reverse osmosis
Question 42. From the colligative properties of the solution, which one is the best method for the determination of the molecular weight of proteins and polymers?
- Osmotic pressure
- Lowering in vapour pressure
- Lowering in freezing point
- Elevation in boiling point
Answer: 1. Osmotic pressure
Question 43. The vapour pressure of benzene at a certain temperature is 640 mm of Hg. A non-volatile and non-electrolyte solid, weighing 2.175 g is added to 39.08 of benzene. The vapour pressure of the solution is 600 mm of Hg. What is the molecular weight of a solid substance?
- 69.5
- 59.6
- 49.50
- 79.8
Answer: 1. 69.5
The vapour pressure of benzene at a certain temperature is 640 mm of Hg. A non-volatile and non-electrolyte solid, weighing 2.175 g is added to 39.08 of benzene. The vapour pressure of the solution is 600 mm of Hg.
p°benzene = 640 mm Hg, ps = 600 mm Hg
wB = 2.175 g, Wbenzene = 39.08 g
From Raoultt law \(\frac{p^{\circ}-p_8}{p^{\circ}}=\frac{w_B \times M_{\text {benzene }}}{W_{\text {benzene }} \times M_B} \Rightarrow \frac{640-600}{640}=\frac{2.175 \times 78}{39.08 \times M_B}\)
⇒ MB = 69.5
Question 44. If 0.15 g of a solute, dissolved in 15 g of solvent, is boiled at a temperature higher by 0.216°C, than that of the pure solvent. The molecular weight of the substance (Molal elevation constant for the solvent is 2.16°C) is
- 10.1
- 100
- 1.01
- 1000
Answer: 1. 10.1
⇒ \(w_B=0.15 \mathrm{~g}, W_A=15 \mathrm{~g}, \Delta T_b=0.216^{\circ} \mathrm{C}\)
⇒ \(K_b=2.16, m=? \)
As \(\Delta T_b=\frac{1000 \times K_b \times w_B}{M_B \times W_A}\)
⇒ \(M_B=\frac{1000 \times 2.16 \times 0.15}{0.216 \times 15}=100\)
Solutions NEET question bank
Question 45. A 5% solution of cane sugar (mol. wt. = 342) is isotonic with 1% solution of a substance X. The molecular weight of X is
- 68.4
- 171.2
- 34.2
- 136.8
Answer: 1. 68.4
For isotonic solutions, \(C_1=C_2\) \(\frac{W_1}{M_1 V_1}=\frac{W_2}{M_2 V_2} \Rightarrow \frac{5}{342 \times 0.1}=\frac{1}{M_2 \times 0.1}\)
⇒ \(M_2=\frac{342}{5}=68.4\)
Question 46. The vapour pressure of a solvent decreased by 10 mm of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2. What should be the mole fraction of the solvent if the decrease in the vapour pressure is to be 20 mm of mercury?
- 0.4
- 0.6
- 0.8
- 0.2
Answer: 2. 0.6
The vapour pressure of a solvent decreased by 10 mm of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2.
x2 (mole fraction of solute) = 0.2
From Raoults law \(\frac{p^a-p_8}{p^{\circ}}=x_2 \Rightarrow \frac{10}{p^{\circ}}=0.2 \Rightarrow p^{\circ}=50 \mathrm{~mm} \mathrm{Hg}\)
Again, when \(p^{\circ}-p_s=20 \mathrm{~mm} \mathrm{Hg}\), then \(\frac{p^{\circ}-p_S}{p^{\circ}}=\) mole fraction of solute \(=\frac{20}{50}=0.4\)
⇒ mole fraction of solvent \(=1-0.4=0.6\)
Question 47. The vapour pressure of CCl4 at 25°C is 143 mm Hg If 0.5 g of a non-volatile solute (mol. weight = 65) is dissolved in 100 g CCl4, the vapour pressure of the solution will be
- 199.34 mm Hg
- 143.99 mm Hg
- 141.43 mm Hg
- 94.39 mm Hg.
Answer: 3. 141.43 mm Hg
Vapour pressure of pure solvent (p°A) = 143 mm Hg, weight of solute (wB) = 0.5 g, weight of solvent (WA) = 100 g, the molecular weight of solute MB = 65 and molecular weight of solvent (MA) = 154.
⇒ \(\frac{p_A^{\circ}-p_s}{p_A^a}=\frac{w_B M_A}{M_B W_A} \text { or } \frac{143-p_s}{143}=\frac{0.5 \times 154}{65 \times 100}\)
or \(p_s=141.31 \mathrm{~mm} \mathrm{Hg}\)
Question 48. The relationship between osmotic pressure at 273 K when 10 g glucose (p1), 10 g urea (p2), and 10 g sucrose (p3) are dissolved in 250 mL of water is
- p2>p1 > p3
- p2>p3>p1
- p1>p2>p3
- p3>p1>p2
Answer: 1. p2>p1> p3
Weight of glucose = 10 g,
Weight of urea = 10 g and weight of sucrose = 10 g
The number of moles of glucose, \(\left(n_1\right)=\frac{\text { Weight }}{\text { Molecular weight }}=\frac{10}{180}=0.05\)
Similarly, number of moles of urea \(\left(n_2\right)=\frac{10}{60}=0.16\) and the number of moles of sucrose \(\left(n_3\right)=\frac{10}{342}=0.03\)
Solutions NEET question bank
Question 49. According to Raoult’s law, the relative lowering of vapour pressure for a solution is equal to
- Mole fraction of solute
- Mole fraction of solvent
- Moles of solute
- Moles of solvent.
Answer: 1. Mole fraction of solute
Question 50. If 0.1 M solution of glucose and 0.1 M solution of urea are placed on two sides of the semipermeable membrane to equal heights, then it will be correct to say that
- There will be no net movement across the membrane
- Glucose will flow towards the glucose solution
- Urea will flow towards the glucose solution
- Water will flow from the urea solution to glucose.
Answer: 1. There will be no net movement across the membrane
There is no net movement of the solvent through the semipermeable membrane between two solutions of equal concentration
Question 51. Which one is a colligative property?
- Boiling point
- Vapour pressure
- Osmotic pressure
- Freezing point
Answer: 3. Osmotic pressure
The properties which depend only upon the number of solute particles present in the solution irrespective of their nature are called colligative properties. Lowering in vapour pressure, elevation in boiling point, depression in freezing point and osmotic pressure are colligative properties.
Question 52. Blood cells retain their normal shape in solution which are
- Hypotonic to blood
- Isotonic to blood
- Hypertonic to blood
- Equinormal to blood.
Answer: 2. Isotonic to blood
Blood cells neither swell nor shrink in isotonic solution. The solutions having the same osmotic pressure are called isotonic solutions.
Question 53. The relative lowering of the vapour pressure is equal to the ratio between the number of
- Solute molecules to the solvent molecules
- Solute molecules to the total molecules in the solution
- Solvent molecules to the total molecules in the solution
- Solvent molecules to the total number of ions of the solute.
Answer: 2. Solute molecules to the total molecules in the solution
Relative lowering of vapour pressure is equal to the mole fraction of solute which is the ratio of solute molecules to the total molecules in the solution
Solutions NEET question bank
Question 54. The van’t Hoff factor (1) for a dilute aqueous solution of the strong electrolyte barium hydroxide is
- 0
- 1
- 2
- 3
Answer: 4. 3
Being a strong electrolyte, Ba(OH)2 undergoes 100% dissociation in a dilute aqueous solution, \(\mathrm{Ba}(\mathrm{OH})_{2(a q)} \rightarrow \mathrm{Ba}^{2+}{ }_{\langle(q)}+2 \mathrm{OH}^{-}{ }_{(a q)}\)
Thus, vant Hoff factor i = 3
Question 55. The boiling point of 0.2 mol kg-1 solution of X in water is greater than the equimolal solution of Y in water. Which one of the following statements is true in this case?
- The molecular mass of X is less than the molecular mass of Y.
- Y is undergoing dissociation in water while X undergoes no change.
- X is undergoing dissociation in water.
- The molecular mass of X is greater than the molecular mass of Y.
Answer: 3. X is undergoing dissociation in water.
ΔTb = i x Kb m
For equimolal solutions, elevation in boiling point will be higher if the solution undergoes dissociation i.e., i > l.
Question 56. Of the following 0.10 m aqueous solutions, which one will exhibit the largest freezing point depression?
- KCl
- C6H12O6
- AI2(SO4)3
- K2SO4
Answer: 3. AI2(SO4)3
⇒ \(\Delta T_f=i \times K_f \times m\)
So, \(\Delta T_f \propto i\) (van’t Hoff factor)
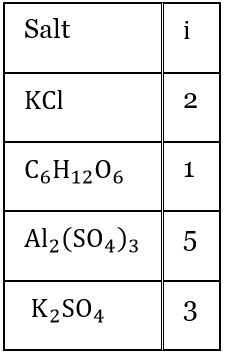
Hence, I am the maximum i.e., 5 for AI2(SO4)3
Question 57. The van’t Hoff factor i for a compound which undergoes dissociation in one solvent and association in another solvent is respectively
- Less than one and greater than one
- Less than one and less than one
- Greater than one and less than one
- Greater than one and greater than one.
Answer: 3. Greater than one and less than one
From the value of van t Hoff factor I it is possible to determine the degree of dissociation or association. In the case of dissociation, i is greater than I and in the case of an association, i is less than 1.
Question 58. The freezing point depression constant for water is -1.86 °Cm-1, If 5.00 g Na2SO4 is dissolved in 45.0 g H2O, the freezing point is changed by -3.82 °C. Calculate the van’t Hoff factor for Na2SO4.
- 2.05
- 2.63
- 3.11
- 0.381
Answer: 2. 2.63
We know that, \(\Delta T_f=i \times K_f \times \frac{w_B \times 1000}{M_B \times W_A}\)
Given: \(\Delta T_f=3.82, K_f=1.86,\)
⇒ \(w_B=5, M_B=142, W_A=45\)
i = \(\frac{\Delta T_f \times M_B \times W_A}{K_f \times w_B \times 1000}=\frac{3.82 \times 142 \times 45}{1.86 \times 5 \times 1000}=2.63\)
Solutions NEET question bank
Question 59. A 0.1 molal aqueous solution of a weak acid is 30% ionized. If Kf for water is 1.86°C/m, the freezing point of the solution will be
- – 0.18 °C
- – 0.54 °C
- – 0.36 °C
- – 0.24 °C
Answer: 4. – 0.24 °C
We know that ΔTf =i x Kf x m
Here i is vant Hoff factor.
i for weak acid is 1 + α.
Here α is the degree of dissociation i,e., 30/100 = 0.3
∴ i = 1 + α =1+0.3=1.3
ΔTf = i x Kf x m= 1.3 x 1.86 x 0.1 = 0.24
∴ Freezing point of the solution, Tf= T°f – ΔTf =0-0.24=-0.24°C
Question 60. An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase?
- Addition of NaCl
- Addition of Na2SO4 m
- Addition of 1.00 molal KI
- Addition of water
Answer: 4. Addition of water
The addition of water to an aqueous solution of KI causes the concentration of the solution to decrease thereby increasing the vapour pressure. In the other three options, the electrolytes undergo ionization, which leads to a lowering of vapour pressure.
Question 61. A 0.0020 m aqueous solution of an ionic compound [CO(NH3)5(NO2)]Cl freezes at -0.00732 °C. The number of moles of ions that 1 mol of ionic compound produces on being dissolved in water will be (Kf = -1.86 °C/m)
- 3
- 4
- 1
- 2
Answer: 4. 2
A 0.0020 m aqueous solution of an ionic compound [CO(NH3)5(NO2)]Cl freezes at -0.00732 °C.
The number of moles of ions produced by 1 mol of ionic compound = i
Applying, ΔTf =i x Kf x m
0.00732 = i x 1.86 x 0.002
⇒ i = \(\frac{0.00732}{1.86 \times 0.002}=1.96 \div 2\)
Question 62. 0. 5 molal aqueous solution of a weak acid (HX) is 20% ionised. If Kf for water is 1.86 K kg mol-1, the lowering in freezing point of the solution is
- 0.56 K
- 1.12 K
- -0.56 K
- -1.12 K
Answer: 2. 1.12 K
HX ⇔ H+ + x–
Total=1+α
∴ i=1 + α = 1+0.2 = 1.2
ΔTf = i x Kf x m = 1.2 x 1.86 x 0.5 = 1.116 K≈ 1.12 K
NEET questions on Solutions Chemistry
Question 63. Which of the following 0.10m aqueous solution will have the lowest freezing point?
- KI
- C12H22O11
- Al2(SO4)3
- C5H10O5
Answer: 3. Al2(SO4)3
Since Al2 (SO4)3 gives a maximum number of ions on dissociation, therefore it will have the lowest freezing point.
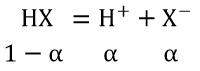
ΔTf = i Kf m
Question 64. Which of the following salts has the same value of van’t Hoff factor (1) as that of K3[Fe(CN)6]?
- Na2SO4
- Al(NO3)3
- Al2(SO4)4
- NaCl
Answer: 2. Al(NO3)3
⇒ \(\mathrm{K}_3\left[\mathrm{Fe}(\mathrm{CN})_6\right] \rightleftharpoons 3 \mathrm{~K}^{+}+\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\) and \(\mathrm{Al}\left(\mathrm{NO}_3\right)_3 \rightleftharpoons \mathrm{Al}^{3+}+3 \mathrm{NO}_3^{-}\)
Since both AI(NO3)3 and K3[Fe(CN)6] give the same number of ions, therefore they have the same van t Hoff factor.
Question 65. At 25°C, the highest osmotic pressure is exhibited by 0.1 M solution of
- Glucose
- Urea
- CaCl2
- KCl.
Answer: 3. CaCl2
In solution, CaClf gives three ions, KCl gives two ions while glucose and urea are covalent molecules so they do not undergo ionisation. Since osmotic pressure is a colligative property and depends upon the number of solute particles (ions), therefore, 0.1 M solution of CaCI2 exhibits the highest osmotic pressure.
NEET questions on Solutions Chemistry
Question 66. Which of the following aqueous solutions has a minimum freezing point?
- 0.01 m NaCl
- 0.005 m C2H5OH
- 0.005 mMgI2
- 0.005 mMgSO4
Answer: 1. 0.01 m NaCl
Here, ΔTf = i x Kf x m
vant Hoff factor, i = 2 for NaCl, so conc. = 0.02, which is the maximum in the present case.
Hence, ΔTf is the maximum or freezing point is minimum in 0.01 m NaCI.
