Basic Concepts Of Chemistry NEET MCQs
NEET Chemistry For Some Basic Concepts Of Chemistry Multiple Choice Questions
Question 1. The dimensions of pressure are the same as that of
- Force per unit volume
- Energy per unit volume
- Force
- Energy
Answer: 2. Energy per unit volume
Pressure = \(\frac{\text { Force }}{\text { Area }}\)
Therefore, dimensions of pressure = \(\frac{\mathrm{MLT}^{-2}}{\mathrm{~L}^2}=\mathrm{ML}^{-1} \mathrm{~T}^{-2}\)
and dimensions of energy per unit volume = \(\frac{\text { Energy }}{\text { Volume }}=\frac{\mathrm{ML}^2 \mathrm{~T}^{-2}}{\mathrm{~L}^3}=\mathrm{ML}^{-1} \mathrm{~T}^{-2}\)
Question 2. Given the numbers: 161 cm, 0.161 cm, 0.0161 cm. The number of significant figures for the three numbers is
- 3, 3, and 4 respectively
- 3, 4 and 4 respectively
- 3,4 and 5 respectively
- 3, 3, and 3 respectively.
Answer: 4. 3, 3, and 3 respectively.
Given the numbers: 161 cm, 0.161 cm, 0.0161 cm.
Zeros placed left to the number are never significant, therefore the no. of significant figures for the numbers 161 cm, 0.161 cm, and 0.0161 cm are the same, i.e., 3.
Question 3. Equal masses of H2, O2, and methane have been taken in a container of volume V at a temperature of 27 °C in identical conditions. The ratio of the volumes of gases H2, O2 methane would be
- 8:16:1
- 16:8:1
- 16:1:2
- 8:1:2
Answer: 3. 16:1:2
Equal masses of H2, O2, and methane have been taken in a container of volume V at a temperature of 27 °C in identical conditions.
According to Avogadrot’s hypothesis, the ratio of the volumes of gases will be equal to the ratio of their no. of moles.
So, number og molecules = \(\frac{\text{Mass}}{\text{Molecule mass}}\)
∴ \(n_{\mathrm{H}_2}=\frac{w}{2} ; n_{\mathrm{O}_2}=\frac{w}{32} ; n_{\mathrm{CH}_4}=\frac{w}{16}\)
So, the ratio is \(\frac{w}{2}: \frac{w}{32}: \frac{w}{16}\) or 16: 1: 2.
Read and Learn More NEET MCQs with Answers
Basic Concepts of Chemistry NEET MCQs
Question 4. What volume of oxygen gas (O2) measured at 0°C and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions?
- 5 L
- 10 L
- 7 L
- 6 L
Answer: 1. 5 L
∴ \(\mathrm{C}_3\mathrm{H}_8+\underset{5\mathrm{vol}} 5\mathrm{O}_2 \rightarrow \underset{3\mathrm{vol}} 3\mathrm{CO}_2+\underset{4\mathrm{vol}} 4 \mathrm{H}_2\mathrm{O}\)
According to the above equation, 1 volume or 1 liter of propane requires 5 volume or 5 liters of O2 to burn completely.
Question 5. 0. 24 g of a volatile gas, upon vaporization, gives 45 mL vapor at NTP. What will be the vapor density of the substance? (Density of H2 = 0.089 g/L)
- 95.93
- 59.93
- 95.39
- 5.993
Answer: 2. 59.93
Weight of gas = 0.24 g,
Volume of gas = 45 mL = 0.045 litre and density of H2 = 0.089 8/L
Weight of 45 mL of H2 = density x volume = 0.089 x 0.045 = 4.005 x 10-3 g
Therefore, Yapour density
= \(\frac{\text { Weight of certain volume of substance }}{\text { Weight of same volume of hydrogen }}=\frac{0.24}{4.005 \times 10^{-3}}=59.93\)
NEET questions on Basic Concepts of Chemistry
Question 6. The molecular weights of O2 and SO2 are 32 and 64 respectively. At 15°C and 150 mmHg pressure, one liter of O2 contains ‘N’ molecules. The number of molecules in two liters of SO2 under the same conditions of temperature and pressure will be
- N/2
- N
- 2 N
- 4 N
Answer: 3. 2 N
The molecular weights of O2 and SO2 are 32 and 64 respectively. At 15°C and 150 mmHg pressure, one liter of O2 contains ‘N’ molecules.
If 1L of one gas contains N molecules, 2L of any gas under the same conditions will contain 2N molecules.
Question 7. What is the weight of oxygen required for the complete combustion of 2.8 kg of ethylene?
- 2.8 kg
- 6.4 kg
- 9.6 kg
- 96 kg
Answer: 3. 9.6 kg
∴ \({\mathrm{C}_2 \mathrm{H}_4}+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}\)
For complete combustion, \(2.8 \mathrm{~kg} \text { of } \mathrm{C}_2 \mathrm{H}_4\) requires = \(\frac{96}{28} \times 2.8 \times 10^3 \mathrm{~g}\)
= \(9.6 \times 10^3 \mathrm{~g}=9.6 \mathrm{~kg}\) of \(\mathrm{O}_2\)
Question 8. An element, X has the following isotopic composition:
- 200X: 90%
- 199X: 8.0%
- 202X: 2.0%
The weighted average atomic mass of the naturally occurring element X is closest to
- 201 amu
- 202 amu
- 199 amu
- 200amu
Answer: 4. 200amu
Average isotopic mass of X = \(\frac{200 \times 90+199 \times 8+202 \times 2}{90+8+2}\)
= \(\frac{18000+1592+404}{100}=199.96 \mathrm{amu}=200 \mathrm{amu}\)
Question 9. Boron has two stable isotopes, 10B(19%) and 11B(81%). Calculate the average weight of boron in the periodic table.
- 10.8
- 10.2
- 11.2
- 10.0
Answer: 1. 10.8
Average atomic mass = \(\frac{19 \times 10+81 \times 11}{100}=10.81\)
NEET questions on Basic Concepts of Chemistry
Question 10. Which one of the following has a maximum number of atoms?
- 1 g of Ag(s) [Atomic mass of Ag = 108]
- 1 g of Mg(s) [Atomic mass of Mg = 24]
- 1 g of O(2)(g) [Atomic mass of O = 16]
- 1 g of Li(s) [Atomic mass of Li = 7]
Answer: 4. 1 g of Li(s) [Atomic mass of Li = 7]
1 mole of substance = \(N_A\) atoms
108 g of \(\mathrm{Ag}=N_A\) atoms \(\Rightarrow 1 \mathrm{~g}\) of \(\mathrm{Ag}=\frac{N_A}{108}\) atoms
24 g of \(\mathrm{Mg}=N_A\) atoms \(\Rightarrow 1 \mathrm{~g}\) of \(\mathrm{Mg}=\frac{N_A}{24}\) atoms
32 g of \(\mathrm{O}_2=N_A\) molecules \(=2 N_A\) atoms
⇒ \(1 \mathrm{~g}\) of \(\mathrm{O}_2=\frac{N_A}{16}\) atoms
7 g of \(\mathrm{Li}=N_A\) atoms \(\Rightarrow 1 \mathrm{~g}\) of \(\mathrm{Li}=\frac{N_A}{7}\) atoms
Therefore, 1 g of Li(s) has a maximum number of atoms.
Question 11. In which case is a number of molecules of water maximum?
- 18 mL of water
- 0.18 g of water
- 0.00224 L of water vapors at 1 atm and 273 K
- 1-3 mol of water
Answer: 1. 18 mL of water
1. Mass of water= V x d = 18 x 1 = 18g
Molecules of water = mole x \(N_A\) = \(\frac{18}{18} N_A=N_A\)
2. Molecules of water = mole x \(\mathrm{N}_{\mathrm{A}}=\frac{0.18}{18} \mathrm{~N}_{\mathrm{A}}\)
3. Moles of water = \(\frac{0.00224}{22.4}=10^{-4}\)
Molecules of water = mole x NA = 10-4 NA
4. Molecules of water = mole x NA = 10-3 NA
Basic Chemistry multiple choice NEET
Question 12. Suppose the elements X and Y combine to form two compounds XY2 and X3Y2– When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
- 40,30
- 60,40
- 20, 30
- 30, 20
Answer: 1. 40,30
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2– When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g,
Let the atomic weight of element X is x and that of element Y is y.
For \(X Y_2, n=\frac{w}{\text { Molecule weight}}\)
0.1 = \(\frac{10}{x+2 y} \Rightarrow x+2 y=\frac{10}{0.1}\) =100……(1)
For \(X_3 Y_2\), n= \(\frac{w}{\text { Molecule weight}}\)
0.05 = \(\frac{9}{3 x+2 y} \Rightarrow 3 x+2 y=\frac{9}{0.05}=180\)….(2)
On solving equations (1) and (2), we get x = 40
40 + 2y = 100
⇒ = 2y = 60
∴ y = 30
Question 13. If Avogadro number NA, is changed from 6.022 x 1023 mol-1 to 6.022 x 1020 mol-1, this would change
- The mass of one mole of carbon
- The ratio of chemical species to each other in a balanced equation
- The ratio of elements to each other in a compound
- The definition of mass in units of grams.
Answer: 1. The mass of one mole of carbon
Mass of 1 mo1 (6.022x 1023 atoms)of carbon =12g
If Avogadro number is changed to 6.022 x 1020 atoms then the mass of 1 mol of carbon
= \(\frac{12 \times 6.022 \times 10^{20}}{6.022 \times 10^{23}}=12 \times 10^{-3} \mathrm{~g}\)
Basic Chemistry multiple choice NEET
Question 14. The number of water molecules is maximum in
- 1.8 grams of water
- 18 grams of water
- 18 moles of water
- 18 molecules of water.
Answer: 3. 18 molecules of water.
1.8 gram of water = \(\frac{6.023 \times 10^{23}}{18} \times 1.8\)
= \(6.023 \times 10^{27}\) molecules
= 6.023 x 1022 molecules
18 gram of water = 6.023 x 1023 molecules
18 moles of water = 18 x 6.023 x 1023 molecules
Question 15. A mixture of gases contains H2 and O2 gases in the ratio of 1: 4 (w/w). What is the molar ratio of the two gases in the mixture?
- 16:1
- 2: 1
- 1: 4
- 4: 1
Answer: 4. 4: 1
Number of moles of \(\mathrm{H}_2=1 / 2\)
Number of moles of \(\mathrm{O}_2=\frac{4}{32}\)
Hence, molar ratio = \(\frac{1}{2}: \frac{4}{32}=4: 1\)
Question 16. Which has the maximum number of molecules among the following?
- 44 g CO2
- 48 g O3
- 8 g H2
- 64 g SO2
Answer: 3. 64 g SO2
8 g H2 has 4 moles while the others have 1 mole each
NEET practice questions Basic Concepts of Chemistry
Question 17. The number of atoms in 0.1 mol of a triatomic gas is (NA = 6.02 x 1023 mol-1)
- 6.026 x 1022
- 1.806 x 1023
- 3.600 x 1023
- 1.800 x1022
Answer: 2. 1.806 x 1023
No. of atoms = NA x No. of moles x 3 = 6.023 x 1023 x 0.1 x 3 = 1.806 x 1023
Question 18. The maximum number of molecules is present in
- 15 L of H2 gas at STP
- 5 L of N2 gas at STP
- 0.5g of H2 gas
- 10g of O2 gas.
Answer: 1. 15 L of H2 gas at STP
AT STP, 22.4L=6.023x 1023 molecules
15 L \(\mathrm{H}_2=\frac{6.023 \times 10^{23} \times 15}{22.4}=4.033 \times 10^{23}\) molecules
5 L \(\mathrm{~N}_2=\frac{6.023 \times 10^{23} \times 5}{22.4}=1.344 \times 10^{23}\) molecules
2 g \(\mathrm{H}_2=6.023 \times 10^{23}\) molecules
0.5 g \(\mathrm{H}_2=\frac{6.023 \times 10^{23} \times 0.5}{2}=1.505 \times 10^{23}\) molecules
32 g \(\mathrm{O}_2=6.023 \times 10^{23}\)
10 g of \(\mathrm{O}_2=\frac{6.023 \times 10^{23} \times 10}{32}=1.882 \times 10^{23}\) molecules
Question 19. Which has the maximum molecules?
- 7g N2
- 2g H2
- 16g NO2
- 16g O2
Answer: 2. 2g H2
number of molecules = moles \(\times N_A\)
Molecules of \(\mathrm{N}_2=\frac{7}{14} N_A=0.5 N_A\), Molecules of \(\mathrm{H}_2=N_A\)
Molecules of \(\mathrm{NO}_2=\frac{16}{46} N_A=0.35 N_A\)
Molecules of \(\mathrm{O}_2=\frac{16}{32} N_A=0.5 N_A\)
∴ \(2 \mathrm{~g} \mathrm{H}_2\left(1\right. mole \left.\mathrm{H}_2\right)\) contains maximum molecules.
Question 20. The specific volume of cylindrical virus particle is 6.2 x 10-2 cc/g whose radius and length are 7 A and 10 A respectively. If NA = 6.02 x 1022, find the molecular weight of the virus.
- 15.4 kg/mol
- 1.54 x 104 kg/mol
- 3.08 x 104 kg/mol
- 3.08 x 103 kg/mol
Answer: 1. 15.4 kg/mol
The specific volume of cylindrical virus particle is 6.2 x 10-2 cc/g whose radius and length are 7 A and 10 A respectively. If NA = 6.02 x 1022,
Specific volume (volume of 1 g) of cylindrical virus
particle = 6.02 x 10-2 cc/g
Radius of virus, r = 7Å = 7x 10-8 cm
Volume of virus = πr²l
= \(\frac{22}{7} \times\left(7 \times 10^{-8}\right)^2 \times 10 \times 10^{-8}=154 \times 10^{-23} c c\)
weight of one virus particle = \(\frac{\text { Volume }(\mathrm{cc})}{\text { Specific volume }(\mathrm{cc} / \mathrm{g})}\)
= \(\frac{154 \times 10^{-23}}{6.02 \times 10^{-2}} \mathrm{~g}\)
∴ Molecular weight of virus = weight of \(N_A\) particles
= \(\frac{154 \times 10^{-23}}{6.02 \times 10^{-2}} \times 6.02 \times 10^{23} \mathrm{~g} / \mathrm{mol}\)
= \(15400 \mathrm{~g} / \mathrm{mol}=15.4 \mathrm{~kg} / \mathrm{mol}\)
NEET practice questions Basic Concepts of Chemistry
Question 21. The number of atoms in 4.25 g of NH3 is approximately
- 4 x 1023
- 2 x 1023
- 1 x 1023
- 6 x 1023
Answer: 4. 6 x 1023
17 g of \(\mathrm{NH}_3=4 N_A\) atoms
4.25 g of \(\mathrm{NH}_3=\frac{4 N_A}{17} \times 4.25\) atoms \(=N_A\) atoms \(=6 \times 10^{23}\) atoms
Question 22. Haemoglobin contains 0.334% of iron by weight. The molecular weight of hemoglobin is approximately 67200. The number of iron atoms (Atomic weight of Fe is 56) present in one molecule of hemoglobin is
- 4
- 6
- 3
- 2
Answer: 1. 4
Haemoglobin contains 0.334% of iron by weight. The molecular weight of hemoglobin is approximately 67200.
Quantity of iron in one molecule = \(\frac{67200}{100} \times 0.334=224.45 \mathrm{amu}\)
No. of iron atoms in one molecule of haemoglobin = \(\frac{224.45}{56}\) = 1
Question 23. The number of moles of oxygen in one liter of air containing 21% oxygen by volume, under standard conditions, is
- 0.0093 mol
- 2.10 mol
- 0.186 mol
- 0.21 mol
Answer: 1. 0.0093 mol
Volume of oxygen in one litre of air = \(\frac{21}{100}\) x 1000 = 210 mL.
Therefore, no. of moles = \(\frac{210}{224000}\) = 0.0093 mol
Question 24. The total number of valence electrons in 4.2 g of N3– ion is (NA is the Avogadro’s number)
- 2.1 NA
- 4.2 NA
- 1.6 NA
- 3.2 NA
Answer: 3. 1.6 NA
Each nitrogen atom has 5 valence electrons, therefore the total number of valence electrons in N3– ion is 16.
Since the molecular mass of N3– is 42, therefore the total number of valence electrons in 4.2 g of \(\mathrm{N}_3^{-} \text {ion }=\frac{4.2}{42} \times 16 \times N_A=1.6 N_A\)
Chemistry MCQs Basic Concepts NEET
Question 25. The number of gram molecules of oxygen in 6.2 x 1024 CO molecules is
- 10 g molecules
- 5 g molecules
- lg molecule
- 0.5 g molecules.
Answer: 2. 5 g molecules
Avogadro’s number, NA = 6.02 x 1023 molecules = 1 mole
∴ 6.02 x 1024 CO molecules = 10 moles CO
= 10 g atoms of O = 5 g molecules of O2
Question 26. The ratio of Cp and Cv of a gas ‘X’ is 1.4. The number of atoms of the gas ‘X’ present in 11.2 liters of it at NTP will be
- 6.02 x 1023
- 1.2 x 1023
- 3.01 x 1023
- 2.01 x 1023
Answer: 1. 6.02 x 1023
Here, Cp/Cv = 1.4, which shows that the gas is diatomic.
22.4L at NTP = 6.02 x 1023 molecules
∴ 11.2 L at NTP = 3.01 x 1023 molecules
Since gas is diatomic
∴ 11.2 L at NTP = 2 x 3.01 x 1023 atoms =6.02 x 1023 atoms
Question 27. The number of oxygen atoms in 4.4 g of CO2 is
- 1.2 x 1023
- 6 x 1022
- 6 x 1023
- 12 x 1023
Answer: 1. 1.2 x 1023
1 mol of CO2 = 44gof CO2
∴ 4.4 g CO2 = 0.1 mol CO2= 6 x 1022 molecules
[Since, 1 mole CO2 = 6 x 1023 molecules]
=2 x 6 x 1022 atoms of O2 = 1.2 x 1023 atoms of O
Chemistry MCQs Basic Concepts NEET
Question 28. 1 cc N2O at NTP contains
- \(\frac{1.8}{224} \times 10^{22}\) atoms
- \(\frac{6.02}{22400} \times 10^{23}\) molecules
- \(\frac{1.32}{224} \times 10^{23}\) electrons
- All of the above.
Answer: 4. All of the above.
22400 cc of \(\mathrm{N}_2{O}\) contain \(6.02 \times 10^{23}\) molecules
∴ 1 cc of \(\mathrm{N}_2 \mathrm{O}\) contain \(\frac{6.02 \times 10^{23}}{22400}\) molecules
Since in \(\mathrm{N}_2 \mathrm{O}\) molecule there are 3 atoms.
∴ \(1 \mathrm{cc} \mathrm{N}_2 \mathrm{O}=\frac{3 \times 6.02 \times 10^{23}}{22400}\) atoms \(=\frac{1.8 \times 10^{22}}{224}\) atoms
No. of electrons in a molecule of \(\mathrm{N}_2 \mathrm{O}=7+7+8=22\)
Hence, no. of electrons in \(1 \mathrm{cc}\) of \(\mathrm{N}_2 \mathrm{O}\)
= \(\frac{6.02 \times 10^{23}}{22400} \times 22\) electrons \(=\frac{1.32}{224} \times 10^{23}\) electrons
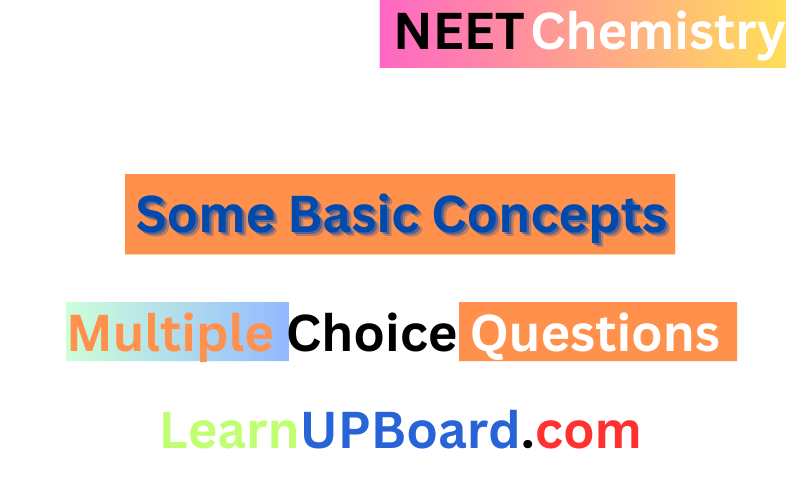
Question 29. An organic compound contains 78% (by wt) carbon and the remaining percentage of hydrogen. The right option for the empirical formula of this compound is [Atomic weight of C is 12, H is 1]
- CH4
- CH
- CH2
- CH3
Answer: 4. CH3
Given the percentage of carbon – 78% and hence the percentage of hydrogen – 22%
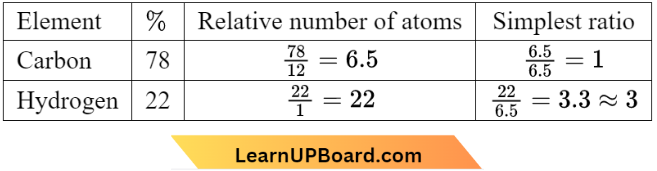
∴ Empirical formula = CH3
Basic Chemistry quiz for NEET
Question 30. An organic compound contains carbon, hydrogen, and oxygen. Its elemental analysis gave C, 38.71%, and H, 9.67%. The empirical formula of the compound would be
- CHO
- CH4O
- CH3O
- CH2O
Answer: 3. CH3O
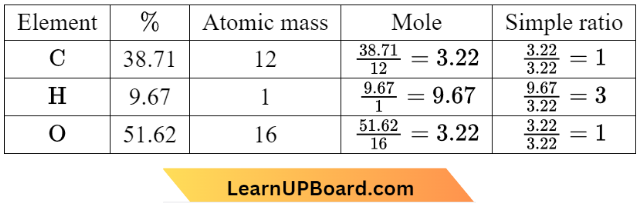
Hence, the empirical formula of the compound would be CH3O
Question 31. The percentage of Se in peroxidase anhydrous enzyme is 0.5% by weight (atomic weight = 78.4) then the minimum molecular weight of peroxidase anhydrous enzyme is
- 1.568 x 104
- 1.568 x 103
- 15.68
- 2.136 x 104
Answer: 1. 1.568 x 104
In peroxidase anhydrous enzyme, 0.5% Se is present means, 0.5 g Se is present in 100 g of the enzyme.
In a molecule of enzyme one Se atom must be present.
Hence, 78.4 g Se will be present in \(\frac{100}{0.5}\) x 78.4 =1.568 x 104
∴ The minimum molecular weight of the enzyme is 1.568 x 104
Question 32. Which of the following fertilizers has the highest nitrogen percentage?
- Ammonium sulphate
- Calcium cyanamide
- Urea
- Ammonium nitrate
Answer: 3. Urea
Urea(NH2CONH2), %of N = \(\frac{28}{60}\) x 100= 46.66%
Similarly, % of N in other compounds are:
(NH4)2SO4 = 21.2%; CaCN2 = 35.0% and NH4NO3 = 35.0%
Basic Chemistry quiz for NEET
Question 33. The right option for the mass of CO2 produced by heating 20 g of 20% pure limestone is (Atomic mass of Ca = 40) \(\mathrm{CaCO}_3\) \(\underrightarrow{1200 \mathrm{~K}}\) CaO + CO2
- 2.64 g
- 1.32 g
- 1.12 g
- 1.76 g
Answer: 4. 1.76 g
∴ \(\underset{40+12+16×3=100 \mathrm{~g}}{\mathrm{CaCO_3}}\) \(\underrightarrow{1200 K}\) \(\underset{40+16=56 \mathrm{~g}}{\mathrm{CaO}}+\underset{44 \mathrm{~g}}{\mathrm{CO}_2}\)
100 g CaCO2 produced CO2 gas = 44 g
20 gCaCO3 will produce CO2 gas = \(\frac{44}{100}\) = 8.8 g
If sample is 100% pure, CO2 produced = 8.8 g
If sample is 20% pure, CO2 produced = \(\frac{8.8}{100}\) x 20 = 17.6 g
Question 34. What mass of 95% pure CaCO3 will be required to neutralize 50 mL of 0.5 M HCl solution according to the following reaction \(\mathrm{CaCO}_{3(s)}+2 \mathrm{HCl}_{(a q)} \rightarrow \mathrm{CaCl}_{2(a q)}+\mathrm{CO}_{2(g)}+2 \mathrm{H}_2 \mathrm{O}_{(l)}\)
- 1.25 g
- 1.32 g
- 3.65 g
- 9.50 g
Answer: 2. 1.32 g
Volume of HCl = \(50 \mathrm{~mL}=0.05 \mathrm{~L}\)
Molarity of HCl= 0.5M
∴ Moles of HCl = \(0.05 \times 0.5=0.025\) moles
∴ \(\mathrm{CaCO}_3+2 \mathrm{HCl} \longrightarrow \mathrm{CaCl}_2+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}\)
∴ For 2 moles of \(\mathrm{HCl}, \mathrm{CaCO}_3\) required =1 mole
For 0.025 moles of \(\mathrm{HCl}, \mathrm{CaCO}_3\) required = \(\frac{0.025}{2}\) moles
Mass of \(\mathrm{CaCO}_3\) required = \(100 \times \frac{0.025}{2}=1.25 \mathrm{~g}\)
For 95% pure \(\mathrm{CaCO}_3\), mass of \(\mathrm{CaCO}_3\) required \(=\frac{1.25}{95} \times 100 \mathrm{~g}\)
= \(1.315 \mathrm{~g} \approx 1.32 \mathrm{~g}\)
NEET MCQs on Basic Chemistry Concepts
Question 35. The number of moles of hydrogen molecules required to produce 20 moles of ammonia through the Habers process is
- 40
- 10
- 20
- 30
Answer: 4. 30
Habert process, N2 + 3H2 → 2NH3
2 moles of NH3 are formed by 3 moles of H2
∴ 20 moles of NH3 will be formed by 30 moles of H2
Question 36. The density of 2 M aqueous solution of NaOH is 1. 28 g/cm³. The molality of the solution is [Given that molecular mass of NaOH = 40 g mol-1]
- 1.20 m
- 1.56 m
- 1.67 m
- 1.32 m
Answer: 3. 1.67 m
Density = 1.28 g/cc Concentration of solution = 2 M
Molar mass of NaOH = 40 g mol-1
Volume of solution = 1 L = 1000 mL
Massof solution=d x V=1.28 x 1000= 1280g
Mass of solute = n x Molar mass = 2 x 40 = 80 g
Mass of solvenl = (1280 – 80) C = 1200 g
Number of moles of solute = \(\frac{80}{40}\) = 2
∴ Molarity = \(\frac{2 \times 1000}{1200}\) = 1.67 m
Question 37. A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with a cone. H2SO4. The evolved gaseous mixture is passed through KOH pellets. The weight (in g) of the remaining product at STP will be
- 1.4
- 3.0
- 2.8
- 4.4
Answer: 3. 2.8
A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with a cone. H2SO4. The evolved gaseous mixture is passed through KOH pellets.
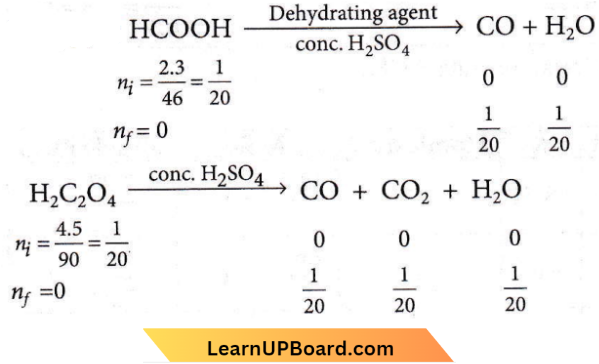
H2O gets absorbed by conc. H2SO4 Gaseous mixture (containing CO and CO2) when passed through KOH pellets, CO2 gets absorbed.
Moles of CO left (unabsorbed)= \(\frac{1}{20}\) + \(\frac{1}{20}\) = \(\frac{1}{10}\)
Mass of CO = moles x molar mass = \(\frac{1}{10}\) x 28 = 2.8 g
NEET MCQs on Basic Chemistry Concepts
Question 38. What is the mass of the precipitate formed when 50 mL of 16.9% solution of AgNO3 is mixed with 50 mL of 5.8% NaCl solution? (Ag = 107.8, N = 14,0 = 16, Na = 23, Cl = 35.5)
- 3.5 g
- 7 g
- 14 g
- 28 g
Answer: 2. 7 g
16.9% solution of AgNO3 means 16.9 g of AgNO3 in 100 mL of solution.
= 8.45 g of AgNO3 in 50 mL solution.
Similarly, 5.8 g of NaCI in 100 mL solution
= 2.9 g of NaCl in 50 mL solution.
The reaction can be represented as
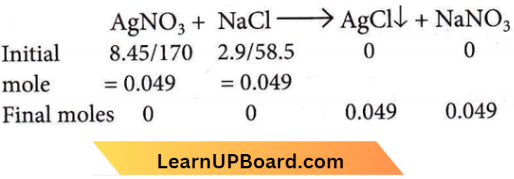
∴ Mass of AgCl precipitated = 0.049 x 143.3 = 7.02 ≈7 g
Question 39. 20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g of magnesium oxide. What will be the percentage purity of magnesium carbonate in the sample? (Atomic weight of Mg = 24)
- 96
- 60
- 84
- 75
Answer: 3. 84
20.0 g of a magnesium carbonate sample decomposes on heating to give carbon dioxide and 8.0 g of magnesium oxide.
∴ \(\underset{84 \mathrm{~g}}{\mathrm{MgCO}_{3(s)}}\) \(\underrightarrow{\Delta}\) \(\underset{\mathrm{40 g}}{\mathrm{MgO}}{(s)}+\mathrm{CO}_{2(g)}\)
84 g of \(\mathrm{MgCO}_3 \equiv 40 \mathrm{~g}\) of \(\mathrm{MgO}\)
∴ 20 g of \(\mathrm{MgCO}_3 \equiv \frac{40}{84} \times 20=9.52 \mathrm{~g}\) of MgO
Actual yield =8 g of MgO
∴ % purity = \(\frac{8}{9.52} \times 100=84 \%\)
Question 40. When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2(g), each at STP, the moles of HCl(g) formed is equal to
- l mol of HCl(g)
- 2 mol of HCl(g)
- 0.5 mol of HCl(g)
- 1.5 mol of HCl(g)
Answer: 1. l mol of HCl(g)
∴ \(n_{\mathrm{H}_2}=\frac{22.4}{22.4}=1 \mathrm{~mol} ; n_{\mathrm{Cl}_2}=\frac{11.2}{22.4}=0.5 \mathrm{~mol}\)
The reaction is as,
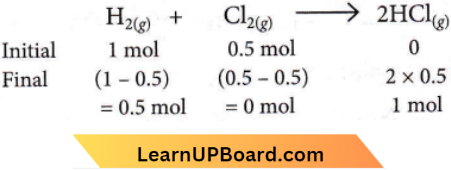
Here, Cl2 is the limiting reagent. So, 1 mole of HCl(g) is formed
NEET MCQs on Basic Chemistry Concepts
Question 41. 1.0 g of magnesium is burnt with 0.56 g O2 in a closed vessel. Which reactant is left in excess and how much? (Atomic weight Mg = 24, O = 16)
- Mg, 0.16 g
- 02, 0.16 g
- Mg, 0.44 g
- 02, 0.28 g
Answer: 1. Mg, 0.16 g
∴ \(n_{\mathrm{Mg}}=\frac{1}{24}=0.0416\) moles
∴ \(n_{\mathrm{O}_2}=\frac{0.56}{32}=0.0175\) mole
The balanced equation is
⇔ \(\begin{array}{lccc}
& 2 \mathrm{Mg}+ & \mathrm{O}_2 \longrightarrow & 2 \mathrm{MgO} \\
\text { Initial } & 0.0416 \text { mole } & 0.0175 \text { mole } & 0 \\
\text { Final } & (0.0416-2 \times 0.0175) & 0 & 2 \times 0.0175 \\
& =0.0066 \text { mole } & &
\end{array}\)
Here, O2 is limiting the reagent
∴ Mass of Mg left in excess = 0.0066 x 24 = 0.16 g
Question 42. 6.02 x 1020 molecules of urea are present in 100 mL of its solution. The concentration of the solution is
- 0.001 M
- 0.1 M
- 0.02 M
- 0.01 M
Answer: 4. 0.01 M
Moles of urea = \(\frac{6.02 \times 10^{20}}{6.02 \times 10^{23}}=0.001\)
Concentration of solution = \(\frac{0.001}{100} \times 1000=0.01 \mathrm{M}\)
Question 43. An experiment, it showed that 10 mL of 0.05 M solution of chloride required 10 mL of 0.1 M solution of AgNO3, which of the following will be the formula of the chloride (X stands for the symbol of the element other than chlorine)?
- X2Cl2
- XCl2
- XCl4
- X2Cl
Answer: 2. XCl2
Miltimoles of solution of chloride = 0’05 x 10 = 0.5
Millimoles of AgNO3 solution = 10 x 0.1 = 1
So, the millimoles of AgNO3 are double that of the chloride solution
∴ XCl2 + 2AgNO3 → 2AgCl + X(NO3)2
Question 44. 25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, the molar concentration of sodium ion, Na+ and carbonate ions, CO–2 are respectively (Molar mass of Na2CO3 = 106 g mo-1)
- 0.955 M and 1.910 M
- 1.910 M and 0.955 M
- 1.90 M and 1.910 M
- 0.477 M and 0.477 M
Answer: 2. 1.910 M and 0.955 M
Given that the molar mass of Na2CO3= 106 g
Molarity of solution = \(\frac{25.3 \times 1000}{106 \times 250}=0.955 \mathrm{M}\)
⇒ \(\mathrm{Na}_2 \mathrm{CO}_3 \rightarrow 2 \mathrm{Na}^{+}+\mathrm{CO}_3^{2-}\)
∴ \({\left[\mathrm{Na}^{+}\right]=2\left[\mathrm{Na}_2 \mathrm{CO}_3\right]=2 \times 0.955=1.910 \mathrm{M}}\)
∴ \({\left[\mathrm{CO}_3^{2-}\right]=\left[\mathrm{Na}_2 \mathrm{CO}_3\right]=0.955 \mathrm{M}}\)
NEET MCQs on Basic Chemistry Concepts
Question 45. 10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. The amount of water produced in this reaction will be
- 3 mol
- 4 mol
- 1 mol
- 2 mol
Answer: 2. 4 mol

10 g of H2 = 5 mol and 64 g of O2= 2 mol
∴ In this reaction, oxygen is the limiting reagent so the amount of H2O produced depends on the amount of O2.
Since 0.5 mol of O2 gives I mol of H2O
∴ 2 mol of O2 will give 4 mol of H2O
Question 46. How many moles of lead(2) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g HCl?
- 0.011
- 0.029
- 0.044
- 0.333
Answer: 2. 0.029
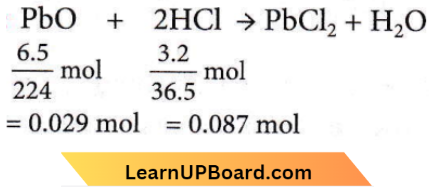
The formation of moles of lead(2) chloride depends upon the no. of moles of PbO which acts as a limiting reagent here. So, a number of moles of PbCl2 formed will be equal to the no. of moles of PbO i.e.,0.029.
Question 47. The mass of carbon anode consumed (giving only carbon dioxide) in the production of 270 kg of aluminum metal from bauxite by the Hall process is
- 270 kg
- 540 kg
- 90 kg
- 180 kg
(Atomic mass: AI = 27)
Answer: 3. 90 kg
∴ \(\underset{\text { (From bauxite) }}{3 \mathrm{C}+2 \mathrm{Al}_2 \mathrm{O}_3 \longrightarrow 4 \mathrm{Al}+3 \mathrm{CO}_2}\)
4 moles of AI is produced by 3 moles of C.
1 mole of Al is produced by \(\frac{3}{4}\) mole of C
∴ \(\frac{270 \times 1000}{27}=10^4\) moles of Al is produced by \(\frac{3}{4} \times 10^4\) moles of C
Amount of carbon used = \(\frac{3}{4} \times 10^4 \times 12 \mathrm{~g}\)
= \(\frac{3}{4} \times 10 \times 12 \mathrm{~kg}=90 \mathrm{~kg}\)
Basic Chemistry NEET question bank
Question 48. The molarity of liquid HCl, if the density of the solution is 1.17 g/cc is
- 36.5
- 18.25
- 32.05
- 42.10
Answer: 3. 32.05
Density= 1.17 g/cc.
⇒ 1 cc. the solution contains 1.17 g of HCl
∴ Molarity = \(\frac{1.17 \times 1000}{36.5 \times 1}=32.05\)
Question 49. The volume of CO2 obtained by the complete decomposition of 9.85 g of BaCO3 is
- 2.24 L
- 1.12 L
- 0.84 L
- 0.56 L
Answer: 2. 1.12 L
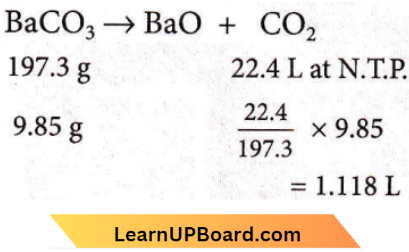
9.85 g of BaCO3 will produce 1.118 L of CO2 at N.T.p, on the complete decomposition.
Question 50. In the reaction \(4 \mathrm{NH}_{3(g)}+5 \mathrm{O}_{2(g)} \rightarrow 4 \mathrm{NO}_{(g)}+6 \mathrm{H}_2 \mathrm{O}_{(l)}\), when 1 mole of ammonia and 1 mole of O2 are made to react to completion
- All the oxygen will be consumed
- 1.0 mole of NO will be produced
- 1.0 mole of H2O is produced
- All the ammonia will be consumed.
Answer: 1. All the oxygen will be consumed
In the reaction \(4 \mathrm{NH}_{3(g)}+5 \mathrm{O}_{2(g)} \rightarrow 4 \mathrm{NO}_{(g)}+6 \mathrm{H}_2 \mathrm{O}_{(l)}\)
∴ \(\underset{4 moles}4 \mathrm{NH}_{3(g)}+\underset{5 moles}5 \mathrm{O}_{2(g)} \rightarrow \underset{4 moles}4 \mathrm{NO}_{(g)} + \underset{6 moles} 6 \mathrm{H}_2 \mathrm{O}_{(l)}\)
⇒ 1 mole of NH3 requires = 514 = 1.25 mole of oxygen while
I mole of O2 requires =4/5 = 0.8 mole of NH3
Therefore, all oxygen will be consumed.
Basic Chemistry NEET question bank
Question 51. The amount of zinc required to produce 224 mL of H2 at STP on treatment with dilute H2SO4 will be
- 65 g
- 0.065 g
- 0.65 g
- 6.5 g
Answer: 3. 0.65 g

Since 65 g of zinc reacts to liberate 22400 mL of H2 at STP, therefore the amount of zinc needed to produce 224 mL of H, at STP = \(\frac{65}{224000}\) x 224 = 0.65 g
Question 52. At STP the density of CCl4 vapor in g/L will be nearest to
- 6.87
- 3.42
- 10.26
- 4.57
Answer: 1. 6.87
Weight of 1 mol of CCl4 vapour = 12 + 4 x 35.5 = 154 g
∴ Density of CCl4 vapour = \(\frac{154}{22.4}\) gL-1 = 6.875 g L-1
