NEET Questions On States Of Matter
NEET Chemistry For States Of Matter Multiple Choice Question
Question 1. Intermolecular forces are forces of attraction and repulsion between interacting particles that will include
- Dipole-dipole forces
- Dipole-induced dipole forces
- Hydrogen bonding
- Covalent bonding
- Dispersion forces.
Choose the most appropriate answer from the options given below:
- 1, 2, 3, 5 are correct.
- 1, 3, 4, 5 are correct.
- 2, 3, 4, and 5 are correct.
- 1, 2, 3, 4 are correct.
Answer: 1. 1, 2, 3, 5 are correct.
Intermolecular forces are the forces of attraction and repulsion between interacting particles (atoms and molecules).
This term does not include the electrostatic forces that exist between the two oppositely charged ions and the forces that hold atoms of a molecule together, i.e., covalent bonds.
Question 2. Dipole-induced dipole interactions are present in which of the following pairs?
- HCl and He atoms
- SiF4 and He atoms
- H2O and alcohol
- Cl2 and CCl4
Answer: 1. HCl and He atoms
HCI is polar (μ ≠ 0) and He is non-polar (μ = 0) giving dipole-induced dipole interactions.
Question 3. Which one of the following is the correct order of interactions?
- Covalent < hydrogen bonding < van der Waals < dipole-dipole
- van der Waals < hydrogen bonding < dipole- dipole < covalent
- van der Waals < dipole-dipole < hydrogen bonding < covalent
- Dipole-dipole < van der Waals’ < hydrogen bonding < covalent.
Answer: 2. van der Waals < hydrogen bonding < dipole- dipole < covalent
The strength of interaction follows the order: van der Waals < hydrogen-bonding < dipole-dipole < covalent. It is so because the bond length of the H-bond is larger than that of a covalent bond.
Also covaient bond is strongest because, tire greater the extent of overlapping, the stronger the bond formed.
Read and Learn More NEET MCQs with Answers
NEET questions on States of Matter
Question 4. Which of the following statements is wrong for gases?
- Confined gas exerts uniform pressure on the walls of its container in all directions.
- The volume of the gas is equal to the volume of the container confining the gas.
- Gases do not have a definite shape and volume.
- The mass of a gas cannot be determined by weighing a container in which it is enclosed.
Answer: 4. Mass of a gas cannot be determined by weighing a container in which it is enclosed.
Mass of the gas = Mass of the cylinder including gas – Mass of empty cylinder.
So, the mass of a gas can be determined by weighing the container in which it is enclosed.
Thus, the statement (4) is wrong or gases
Question 5. Which of the following options is the correct graphical representation of Boyle Law?
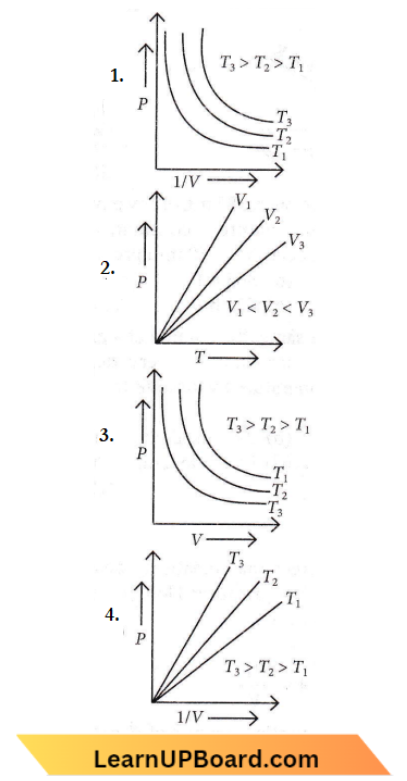
Answer: 4
The graph in option (4) correctly the Boyle’s law.
States of Matter NEET MCQs
Question 6. Choose the correct option for a graphical representation of Boyle’s law, which shows a graph of pressure vs volume of a gas at different temperatures.
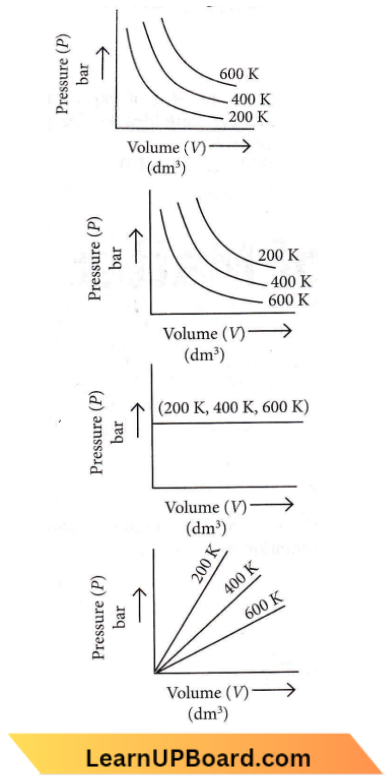
Answer: 1
According to Boyle’s law, \(P \propto \frac{1}{V}\) (at constan T)
The graph between P and V is a hyperbola
Question 7. At 25°C and 730 mm pressure, 380 mL of dry oxygen was collected. If the temperature is constant, what volume will the oxygen occupy at 760 mm pressure?
- 569 mL
- 365 mL
- 265 mL
- 621 mL
Answer: 2. 365 mL
At 25°C and 730 mm pressure, 380 mL of dry oxygen was collected. If the temperature is constant
V1 = 380 mL, P1 =730 mm, V2 = ? P2= 760 mm.
From Boyle’s Iaw, P1V1 = P2V2
⇒ \(V_2=\frac{P_1 V_1}{P_2}=\frac{730 \times 380}{760}=365 \mathrm{~mL}\)
Question 8. Pressure remaining the same, the volume of a given mass of an ideal gas increases for every degree centigrade rise in temperature by a definite fraction of its volume at
- 0°C
- Its critical temperature
- Absolute zero
- It’s Boyle temperature.
Answer: 1. 0°C
According to Charles’ law which states that the volume of the given mass of a gas increases or decreases by 1/27.3 of its volume at 0°C for a degree rise or fall of temperature at constant pressure.
∴ \(V_t=V_0\left(1+\frac{t}{273}\right) \text { at constant } P \text { and } n \text {. }\)
Question 9. Which one is not the correct mathematical equation for Daltons’ Law of partial pressure? Here p = total pressure of the gaseous mixture
- \(p=p_1+p_2+p_3\)
- \(p=n_1 \frac{R T}{V}+n_2 \frac{R T}{V}+n_3 \frac{R T}{V}\)
- \(p_i=x_i p\), where \(p_i=\) partial pressure of \(i^{\text {th }}\) gas \(x_i=\) mole fraction of \(i^{\text {th }}\) gas in gaseous mixture
- \(p_i=x_i p_i^{\circ}\), where \(x_i=\) mole fraction of \(i^{\text {th }}\) gas in gaseous mixture, \(p_i^{\circ}=\) pressure of \(i^{\text {th }}\) gas in pure state
Answer: 4. \(p_i=x_i p_i^{\circ}\), where \(x_i=\) mole fraction of \(i^{\text {th }}\) gas in gaseous mixture, \(p_i^{\circ}=\) pressure of \(i^{\text {th }}\) gas in pure state
States of Matter NEET MCQs
Question 10. A 10.0 L flask contains 64 g of oxygen at 27°C. (Assume O1 gas is behaving ideally). The pressure inside the flask in the bar is (Given R = 0.0831 L bar K-1 mol-1)
- 2.5
- 498.6
- 49.8
- 4.9
Answer: 4. 4.9
A 10.0 L flask contains 64 g of oxygen at 27°C. (Assume O1 gas is behaving ideally).
V = 10 L, Mass of O1 = 64 g, T = 300 K
According to the ideal gas equation, PV = nRT
P = \(\frac{n R T}{V}=\frac{64}{32} \times \frac{0.0831 \times 300}{10} \Rightarrow P=4.986 \mathrm{bar}\)
Question 11. Choose the correct option for the total pressure (in atm) in a mixture of 4 g O2 and 2 g H2 confined in a total volume of one litre at 0° C is [Given R = 0.082 L atm mol-1 K-1, T = 273 K]
- 26.02
- 2.518
- 2.602
- 25.18
Answer: 4. 25.18
Applying PV = \(\frac{w}{M} R T\)
∴ \(P_{\mathrm{O}_2}=\frac{4}{32} \times \frac{0.0821 \times 273}{1}=2.80 \mathrm{~atm}\)
∴ \(P_{\mathrm{H}_2}=\frac{2}{2} \times \frac{0.0821 \times 273}{1}=22.4 \mathrm{~atm}\)
Now, according to Dalton’s law, \(P_{\text {total }}=P_{\mathrm{O}_2}+P_{\mathrm{H}_2}=2.80+22.4=25.2 \mathrm{~atm}\)
Question 12. A mixture of N2 and Ar gases in a cylinder contains 7 g of N2 and 8 g of Ar. If the total pressure of the mixture of the gases in the cylinder is 27 bar, the partial pressure of N2 is [Use atomic masses (in g mol-1): N2= 14, Ar = 40]
- 9 bar
- 12 bar
- 15 bar
- 18 bar.
Answer: 3. 15 bar
A mixture of N2 and Ar gases in a cylinder contains 7 g of N2 and 8 g of Ar. If the total pressure of the mixture of the gases in the cylinder is 27 bar
Number of moles of \(\mathrm{N}_2=\frac{7}{28}\) = 0.25 mol
Number of moles of Ar = \(\frac{8}{40}\) = 0.2 mol
Mole fraction of \(\mathrm{N}_2=\frac{0.25}{0.25+0.2}=\frac{0.25}{0.45}=0.55\)
Partial pressure of N2 gas = mole fraction x total pressure = 0.55 x 27 = 14.85 ≈ 15 bar
States of Matter multiple choice NEET
Question 13. The volume occupied by 1.8 g of water vapour at 374°C and 1 bar pressure will be [Use R = 0.083 bar L K-1 mol-1]
- 96.66 L
- 55.87 L
- 3.10 L
- 5.37 L
Answer: 4. 5.37 L
m = \(1.8 \mathrm{~g} \Rightarrow n=\frac{m}{M}=\frac{1.8}{18}=0.1 \mathrm{~mol}\)
T = \(374^{\circ} \mathrm{C}=647 \mathrm{~K}, P=1\) bar, R=\(0.083 \mathrm{bar} \mathrm{L} \mathrm{K}^{-1} \mathrm{~mol}^{-1}\)
V = \(\frac{n R T}{P}=\frac{0.1 \times 0.083 \times 647}{1}=5.37 \mathrm{~L}\)
Question 14. Equal moles of hydrogen and oxygen gases are placed in a container with a pinhole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape?
- 3/8
- 1/2
- 1/8
- 1/4
Answer: 3. 1/8
Equal moles of hydrogen and oxygen gases are placed in a container with a pinhole through which both can escape.
Let the number of moles of each gas = x
Fraction of hydrogen escaped \(=\frac{1}{2} x\)
⇒ \(\frac{r_{\mathrm{O}_2}}{r_{\mathrm{H}_2}}=\sqrt{\frac{M_{\mathrm{H}_2}}{M_{\mathrm{O}_2}}} \Rightarrow \frac{n_{\mathrm{O}_2} / t}{\frac{x}{2} / t}=\sqrt{\frac{2}{32}}=\sqrt{\frac{1}{16}}=\frac{1}{4}\)
⇒ \(\frac{n_{\mathrm{O}_2} / t}{\frac{x}{2} / t}=\frac{1}{4} \Rightarrow n_{\mathrm{O}_2}=\frac{1}{8} x\)
Hence, the fraction of oxygen escaped = 1/8
Question 15. What is the density of N2 gas at 227°C and 5.00 atm pressure? (R = 0.082 L atm K-1 mol-1)
- 1.40 g/mL
- 2.81 g/mL
- 3.41 g/mL
- 0.29 g/mL
Answer: 3. 3.41 g/mL
PV=nRT
PV = \(\frac{w}{M} R T\left[n=\frac{\text { Weight of the gas taken }(W)}{\text { Mol. mass of gas }(M)}\right]\)
P = \(\frac{w}{M} \times \frac{R T}{V} ; P=\frac{d R T}{M} \quad\left[\text { Density }=\frac{\text { Mass }}{\text { Volume }}\right]\)
d = \(\frac{P M}{R T}=\frac{5 \times 28}{0.0821 \times 500}=3.41 \mathrm{~g} / \mathrm{mL}\)
States of Matter multiple choice NEET
Question 16. 50 mL of each gas A and of gas B takes 150 and 200 seconds respectively to effuse through a pinhole under similar conditions. If the molecular mass of gas B is 36, the molecular mass of gas A will be
- 96
- 128
- 32
- 64
Answer: None
50 mL of each gas A and of gas B takes 150 and 200 seconds respectively to effuse through a pinhole under similar conditions.
According to Graham’s law of diffusion, \(\frac{r_1}{r_2}=\sqrt{\frac{d_2}{d_1}}=\sqrt{\frac{M_2}{M_1}}, r_A=\frac{V_A}{T_A}, \quad r_B=\frac{V_B}{T_B}\)
⇒ \(\frac{V_A / T_A}{V_B / T_B}=\sqrt{\frac{M_B}{M_A}}\)
⇒ \(V_A=V_B, T_A=150 \mathrm{sec}, T_B=200 \mathrm{sec}, M_B=36, M_A=?\)
⇒ \(\frac{T_B}{T_A}=\sqrt{\frac{M_B}{M_A}} \Rightarrow \frac{200}{150}=\sqrt{\frac{36}{M_A}}\)
⇒ \(\frac{4}{3}=\sqrt{\frac{36}{M_A}} \text { or } \frac{4 \times 4}{3 \times 3}=\frac{36}{M_A} \text { or } M_A=\frac{36}{4 \times 4} \times 3 \times 3=20.25\)
Question 17. A certain gas takes three times as long to effuse out as helium. Its molecular mass will be
- 27 u
- 36 u
- 64 u
- 9u
Answer: 2. 36 u
According to Graham’s law of diffusion, \(r \propto \frac{1}{\sqrt{d}} \propto \frac{1}{\sqrt{M}} \Rightarrow \frac{r_1}{r_2}=\sqrt{\frac{M_2}{M_1}}\)
Rate of diffusion = \(\frac{\text { Volume of gas diffused }(V)}{\text { Time taken }(t)}\)
∴ \(\frac{V_1 / t_1}{V_2 / t_2}=\sqrt{\frac{M_2}{M_1}}\)
If the same volume of two gases diffuses, then \(V_1=V_2\)
∴ \(\frac{t_2}{t_1}=\sqrt{\frac{M_2}{M_1}}\)
Here \(t_2=3 t_1, M_1=4 \mathrm{u}, M_2=\)?
∴ \(\frac{3 t_1}{t_1}=\sqrt{\frac{M_2}{4}} \Rightarrow 3=\sqrt{\frac{M_2}{4}} \Rightarrow 9=\frac{M_2}{4} \Rightarrow M_2=36 \mathrm{u}\)
Question 18. Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively. The molecular mass of A is 49 u. The molecular mass of B will be
- 50.00 u
- 12.25 u
- 6.50 u
- 25.00 u
Answer: 2. 12.25 u
Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively.
We know that \(\frac{r_A}{r_B}=\frac{V / t_A}{V / t_B}=\sqrt{\frac{M_B}{M_A}}\)
⇒ \(\frac{t_B}{t_A}=\sqrt{\frac{M_B}{M_A}} \Rightarrow \frac{10}{20}=\sqrt{\frac{M_B}{49}}\)
⇒ \(\left(\frac{10}{20}\right)^2=\frac{M_B}{49} \Rightarrow \frac{100}{400}=\frac{M_B}{49}\)
⇒ \(M_B=\frac{49 \times 100}{400}=12.25 \mathrm{u}\)
Question 19. A gaseous mixture was prepared by taking equal moles of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is
- 0.5 atm
- 0.8 atm
- 0.9 atm
- 1 atm
Answer: 1. 0.5 atm
A gaseous mixture was prepared by taking equal moles of CO and N2. If the total pressure of the mixture was found 1 atmosphere
⇒ \(p_{\mathrm{CO}}+p_{\mathrm{N}_2}=1 \mathrm{~atm}\)
⇒ \(2 p_{\mathrm{N}_2}=1\)
⇒ \(p_{\mathrm{N}_2}=\frac{1}{2}=0.5 \mathrm{~atm}\)
NEET practice questions States of Matter
Question 20. A bubble of air is underwater at a temperature 15°C and a pressure of 1.5 bar. If the bubble rises to the surface where the temperature is 25°C and the pressure is 1.0 bar, what will happen to the volume of the bubble?
- The volume will become greater by a factor of 1.6.
- The volume will become greater by a factor of 1.1.
- The volume will become smaller by a factor of 0.70.
- The volume will become greater by a factor of 2.5
Answer: 1. Volume will become greater by a factor of 1.6.
A bubble of air is underwater at a temperature 15°C and a pressure of 1.5 bar. If the bubble rises to the surface where the temperature is 25°C and the pressure is 1.0 bar,
From ideal gas equation, \(V \propto \frac{T}{P}\)
Given \(T_1=15+273=288 \mathrm{~K}, P_1=1.5\) bar \(T_2=25+273=298 \mathrm{~K}, P_2=1\) bar \(V_1 \propto \frac{288}{1.5}\)
i.e., \(V_1 \propto 192\) and \(V_2 \propto \frac{298}{1}\)
∴ \(\frac{V_2}{V_1}=\frac{298}{192}=1.55 \approx 1.6\)
Question 21. The pressure exerted by 6.0 g of methane gas in a 0.03 m³ vessel at 129°C is (Atomic masses: C = 12.01, H = 1.01 and R = 8.314 J K-1 mol-1)
- 275216 Pa
- 13409 Pa
- 41648 Pa
- 31684 Pa
Answer: 3. 41648 Pa
Given, the mass of CH4, w = 6g
Volume of CH4, V= 0.03 m³
T = 129°C = 129 +273 = 402 K, R = 8.314 J K-1 mol-1
Molecular mass of CHr, M = 12.01 + 4 x 1.01 = 16.05
PV = nRT = \(\frac{w}{M} R T\)
∴ P = \(\frac{w}{M} \frac{R T}{V}=\frac{6}{16.05} \times \frac{8.314 \times 402}{0.03}\)
= \(41647.7 \mathrm{~Pa} \approx 41648 \mathrm{~Pa}\)
Question 22. Which of the following mixtures of gases does not obey the Daltorx law of partial pressure?
- Cl2 and SO2
- CO2 and He
- O2 and CO2
- N2 and O2
Asnwer: 1. Cl2 and SO2
∴ \(\mathrm{Cl}_2+\mathrm{SO}_2\) \(\underrightarrow{\text { Sunlight }}\) \(\underset{\mathrm{Sulphuryl chloride}}{\mathrm{SO}_2 \mathrm{Cl}_2}\)
Dalton’s law of partial pressure is applicable only in those cases where gases are non-reacting. As Cl2 and SO2 react to form SO2Cl2 this law is not obeyed in a given case.
Question 23. At what temperature, the rate of effusion of N2 would be 1.625 times the rate of SO2 at 50°C?
- 373°C
- 620°C
- 100°C
- 173°C
Answer: 3. 10°C
∴ \(r_1=1.625 r_2\) and \(T_2=50^{\circ} \mathrm{C}=323 \mathrm{~K}\)
We know that \(\frac{r_1}{r_2}=\sqrt{\frac{M_2}{M_1} \times \frac{T_1}{T_2}}\)
or \(1.625=\sqrt{\frac{64}{28} \times \frac{T_1}{323}}\) or \(T_1=\frac{(1.625)^2 \times 28 \times 323}{64}=373.15 \mathrm{~K}=100.15^{\circ} \mathrm{C}\)
NEET practice questions States of Matter
Question 24. 50 mL of hydrogen diffuses out through a small hole of a vessel, in 20 minutes. The time taken by 40 mL of oxygen to diffuse out is
- 32 minutes
- 64 minutes
- 8 minutes
- 12 minutes
Answer: 2. 64 minutes
Volume of hydrogen = 50 mI; Time for diffusion (t) = 20 min and volume of oxygen = 40 mI.
Rate of diffusion of hydrogen (r1) = 50/20 =2.5 mL/min
Rate of diffusion of oxygen (r2) = 40/t mL/min
Since the molecular mass of hydrogen (M1) = 2 and that of oxygen (M2) = 32
Therefore \(\frac{r_1}{r_2}=\sqrt{\frac{M_2}{M_1}} \Rightarrow \frac{2.5}{40 / t}=\sqrt{\frac{32}{2}} \Rightarrow \frac{t}{16}=4 \Rightarrow t=64 \text { minutes }\)
Question 25. Under what conditions will a pure sample of an ideal gas not only exhibit a pressure of I atm but also a concentration of 1-mole litre-1? (R = 0.082 litre atm mol-1 deg-1)
- At STP
- When V = 22.4 litres
- When T=12K
- Impossible under any conditions
Answer: 3. When T= 12K
PV = nRT or \(P=\frac{n}{V} R T=C R T\)
Hence, \(1=1 \times 0.082 \times T \Rightarrow T=\frac{1}{0.082}=12 \mathrm{~K}\)
Question 26. The correct value of the gas constant ‘R’ is close to
- 0.082 litre-atmosphere K
- 0.082 litre-atmosphere K-1 mol-1
- 0.082 litre-atmosphere-1 K mol-1
- 0.082 litre-1 atmosphere-1 K mol.
Answer: 2. 0.082 litre-atmosphere K-1 mol-1
Question 27. Select one correct statement. In the gas equation, PV = nRT
- n is the number of molecules of a gas
- V denotes the volume of one mole of the gas
- n moles of the gas have a volume of V
- P is the pressure of the gas when only one mole of gas is present.
Answer: 3. n moles of the gas have a volume of V
In the ideal gas equation, PV=nRT
n moles of the gas have volume V
Chemistry MCQs States of Matter NEET
Question 28. At constant temperature, in a given mass of an ideal gas
- The ratio of pressure and volume always remains constant
- Volume always remains constant
- Pressure always remains constant
- The product of pressure and volume always remains constant.
Answer: 4. The product of pressure and volume always remains constant.
According to Boyle’s law at a constant temperature, \(P \propto \frac{1}{V} \text { or } P V=\text { constant }\)
Question 29. If P, V M, T and R are pressure, volume, molar mass, temperature and gas constant respectively, then for an ideal gas, the density is given by
- \(\frac{R T}{P M}\)
- \(\frac{P}{R T}\)
- \(\frac{M}{V}\)
- \(\frac{P M}{R T}\)
Answer: 4. \(\frac{P M}{R T}\)
Ideal gas equation is, \(P V=n R T=\frac{m}{M} R T\)
or \(P M=\frac{m}{V} R T=d R T\) [here d= density]
⇒ d = \(\frac{P M}{R T}\)
Question 30. The correct gas equation is
- \(\frac{V_1 T_2}{P_1}=\frac{V_2 T_1}{P_2}\)
- \(\frac{P_1 V_1}{P_2 V_2}=\frac{T_1}{T_2}\)
- \(\frac{P_1 T_1}{V_1}=\frac{P_2 V_2}{T_2}\)
- \(\frac{V_1 V_2}{T_1 T_2}=P_1 P_2\)
Answer: 2. \(\frac{P_1 V_1}{P_2 V_2}=\frac{T_1}{T_2}\)
∴ \(\frac{P V}{T}=\text { constant or } \frac{P_1 V_1}{T_1}=\frac{P_2 V_2}{T_2} \Rightarrow \frac{P_1 V_1}{P_2 V_2}=\frac{T_1}{T_2}\)
Chemistry MCQs States of Matter NEET
Question 31. By what factor does the average velocity of a gaseous molecule increase when the temperature (in Kelvin) is doubled?
- 2.0
- 2.8
- 4.0
- 1.4
Answer: 4. 1.4
Average velocity = \(\sqrt{\frac{8 R T}{\pi M}}\)
When T becomes 2T then average velocity = \(\sqrt{\frac{8 R(2 T)}{\pi M}}\)
i.e., \(\sqrt{2}\) or 1.41 times increase.
Question 32. The temperature of a gas is raised from 27°C to 927°C. The root mean square speed of the gas
- Remains same
- \(\text { gets } \sqrt{\frac{927}{27}} \text { times }\)
- Gets halved
- Gets doubled.
Answer: 4. Gets doubled.
T1 =27°C = 300 K and T2 = 927°C= 1200 K
We know that root means square speed (v) ∝ √T.
Therefore root mean square speed of the gas, when its temperature is raised = \(\sqrt{\frac{T_2}{T_1}}=\sqrt{\frac{1200}{300}}=2 \text { times }\)
Question 33. The ratio among most probable velocity, mean velocity and root mean square velocity is given by
- \(1: 2: 3\)
- \(1: \sqrt{2}: \sqrt{3}\)
- \(\sqrt{2}: \sqrt{3}: \sqrt{8 / \pi}\)
- \(\sqrt{2}: \sqrt{8 / \pi}: \sqrt{3}\)
Answer: 4. \(\sqrt{2}: \sqrt{8 / \pi}: \sqrt{3}\)
Most probable velocity, \(\left(u_{m p}\right)=\sqrt{\frac{2 R T}{M}}\)
Mean velocity, \((\bar{v})=\sqrt{\frac{8 R T}{\pi M}}\)
Root mean square velocity, \(\left(u_{\text {r.m.s }}\right)=\sqrt{\frac{3 R T}{M}}\)
∴ \(u_{m p}: \bar{v}: u_{r, m, s}=\sqrt{\frac{2 R T}{M}}: \sqrt{\frac{8 R T}{\pi M}}: \sqrt{\frac{3 R T}{M}}=\sqrt{2}: \sqrt{\frac{8}{\pi}}: \sqrt{3}\)
States of Matter quiz for NEET
Question 34. The root mean square velocity at STP for the gases H2, N2, O2 and HBr are in the order
- \(\mathrm{H}_2<\mathrm{N}_2<\mathrm{O}_2<\mathrm{HBr}\)
- \(\mathrm{HBr}<\mathrm{O}_2<\mathrm{N}_2<\mathrm{H}_2\)
- \(\mathrm{H}_2<\mathrm{N}_2=\mathrm{O}_2<\mathrm{HBr}\)
- \(\mathrm{HBr}<\mathrm{O}_2<\mathrm{H}_2<\mathrm{N}_2\)
Answer: 2. \(\mathrm{HBr}<\mathrm{O}_2<\mathrm{N}_2<\mathrm{H}_2\)
PV = \(\frac{1}{3} m n u^2=\frac{1}{3} M u^2\) or \(u=\sqrt{3 P V / M}\),
At STP, \(u \propto \sqrt{\frac{1}{M}}\) and molecular masses of \(\mathrm{H}_2, \mathrm{~N}_2, \mathrm{O}_2\) and \(\mathrm{HBr}\) are 2, 28, 32 and 81 .
Question 35. The root mean square velocity of a gas molecule is proportional to
- \(m^{1 / 2}\)
- \(m^0\)
- \(m^{-1 / 2}\)
- m
Answer: 3. \(m^{-1 / 2}\)
PV = \(\frac{1}{3} m N u^2\), here u= root mean square velocity.
Now \(u^2=\frac{3 P V}{m N}\) or \(u \propto \frac{1}{\sqrt{m}}\)
Question 36. The energy absorbed by each molecule (A2) of a substance is 4.4 x 10-19 J and bond energy per molecule is 4.0 x 10-19 J. The kinetic energy of the molecule per atom will be
- \(2.2 \times 10^{-19} \mathrm{~J}\)
- \(2.0 \times 10^{-19} \mathrm{~J}\)
- \(4.0 \times 10^{-20} \mathrm{~J}\)
- \(2.0 \times 10^{-20} \mathrm{~J}\)
Answer: 4. \(2.0 \times 10^{-20} \mathrm{~J}\)
The energy absorbed by each molecule (A2) of a substance is 4.4 x 10-19 J and bond energy per molecule is 4.0 x 10-19 J.
Energy absorbed by each molecule = 4.4 x 10-19 J
The energy required to break the bond = 4.0 x 10-19 J
The remaining energy is converted to kinetic energy
= (4.4 x 10-19 – 4.0 x 10-19 )J = 0.4 x 10-19 J per molecule
∴ Kinetic energy per atom = 0.2 x 10-19 J or 2 x 10-20 J
States of Matter quiz for NEET
Question 37. If a gas expands at a constant temperature, it indicates that
- The kinetic energy of molecules remains the same
- Number of the molecules of gas increases
- The kinetic energy of molecules decreases
- The pressure of the gas increases.
Answer: 1. Kinetic energy of molecules remains the same
The average translationalK’E. of one molecule of an ideal gas will be given by \(E_t=\frac{K. E .}{N_A}=\frac{3 / 2 R T}{N_A}=\frac{3}{2} k T\)
where \(R / N_A=\) Boltzmann constant
i.e. \(E_t \propto T\)
Question 38. The average molar kinetic energy of CO and N2 at the same temperature is
- KE1 = KE2
- KE1 > KE2
- KE1 < KE2
- Can’t say anything. Both volumes are not given.
Answer: 1. KE1 = KE2
K.E = \(\frac{3}{2}\)RT (for one mole of a gas)
As temperatures are the same and KE is independent of molecular mass KE1 = KE2.
Question 39. The average kinetic energy of an ideal gas, per molecule in S.I. units, at 25°C will be
- \(6.17 \times 10^{-20} \mathrm{~J}\)
- \(7.16 \times 10^{-20} \mathrm{~J}\)
- \(61.7 \times 10^{-20} \mathrm{~J}\)
- \(6.17 \times 10^{-21} \mathrm{~J}\)
Answer: 4. \(6.17 \times 10^{-21} \mathrm{~J}\)
Temperature (T) = 25°C=298K.
Therefore, K.E. per molecule = \(\frac{3 R T}{2 N_A}=\frac{3 \times 8.314 \times 298}{2 \times\left(6.02 \times 10^{23}\right)}=6.17 \times 10^{-21} \mathrm{~J}\)
Question 40. At STP, 0.50 mol H2 gas and 1.0 mol He gas
- Have equal average kinetic energies
- Have equal molecular speeds
- Occupy equal volumes
- Have equal effusion rates.
Answer: 1. Have equal average kinetic energies
Because average kinetic energy depends only on temperature K.E = \(\frac{3}{2}\) K T
NEET MCQs on States of Matter
Question 41. Internal energy and pressure of a gas per unit volume are related as
- \(P=\frac{2}{3} E\)
- \(P=\frac{3}{2} E\)
- \(P=\frac{1}{2} E\)
- \(P=2 E\)
Answer: 1. \(P=\frac{2}{3} E\)
PV = \(\frac{1}{3} m n u^2=\frac{1}{3} M u^2\)
= \(\frac{2}{3} \cdot \frac{1}{2} M u^2=\frac{2}{3} E\) (because \(\frac{1}{2} M u^2=E\))
or P = \(\frac{2}{2} E\) per unit volume.
Question 42. A closed flask contains water in all its three states solid, liquid and vapour at 0°C. In this situation, the average kinetic energy of water molecules will be
- The greatest in all the three states
- The greatest in vapour state
- The greatest in the liquid state
- The greatest in the solid state.
Answer: 2. The greatest vapour state
Velocity and hence average KE. of water molecules is maximum in the gaseous state.
Question 43. Which is not true in the case of an ideal gas?
- It cannot be converted into a liquid.
- There is no interaction between the molecules.
- All molecules of the gas move at the same speed.
- At a given temperature, PV is proportional to the amount of the gas.
Answer: 3. All molecules of the gas move at the same speed.
Molecules in an ideal gas range with different speeds. Due to collision between the particles their speed changes.
Question 44. A gas at 350 K and 15 bar has a molar volume 20 per cent smaller than that of an ideal gas under the same conditions. Tire correct option about the gas and its compressibility factor (Z) is
- Z < 1 and repulsive forces are dominant
- Z > 1 and attractive forces are dominant
- Z > 1 and repulsive forces are dominant
- Z < 1 and attractive forces are dominant.
Answer: 4. Z < 1 and attractive forces are dominant.
A gas at 350 K and 15 bar has a molar volume 20 per cent smaller than that of an ideal gas under the same conditions.
∴ \(V_{\text {ideal }}=V, V_{\text {real }}=V-0.2 \mathrm{~V}=0.8 \mathrm{~V}\)
Z = \(\frac{V_{\text {real }}}{V_{\text {ideal }}}=0.8\)
NEET MCQs on States of Matter
Question 45. A gas such as carbon monoxide would be most likely to obey the ideal gas law at
- Low temperatures and high pressures
- High temperatures and high pressures
- Low temperatures and low pressures
- High temperatures and low pressures.
Answer: 4. High temperatures and low pressures
Real gases show ideal gas behaviour at high temperatures and low pressures.
Question 46. Maximum deviation from ideal gas is expected from
- \(\mathrm{CH}_{4(g)}\)
- \(\mathrm{NH}_{3(g)}\)
- \(\mathrm{H}_{2(g)}\)
- \(\mathrm{N}_{2(g)}\)
Answer: 2. \(\mathrm{NH}_{3(g)}\)
NH3 is a polar molecule, thus more attractive forces between NH3 molecules.
Question 47. For real gases, van der Waals’ equation is written as \(\left(p+\frac{a n^2}{V^2}\right)(V-n b)=n R T \) where a and b are van der Waals’ constants. Two sets of gases are
- \(\mathrm{O}_2, \mathrm{CO}_2, \mathrm{H}_2\) and \(\mathrm{He}\)
- \(\mathrm{CH}_4, \mathrm{O}_2\) and \(\mathrm{H}_2\)
The gases given in set-1 in increasing order of 2 and gases given in set-2 in decreasing order of 1 are arranged below. Select the correct order from the following:
- \(\mathrm{He}<\mathrm{H}_2<\mathrm{CO}_2<\mathrm{O}_2\)
- \(\mathrm{CH}_4>\mathrm{H}_2>\mathrm{O}_2\)
- \(\mathrm{O}_2<\mathrm{He}<\mathrm{H}_2<\mathrm{CO}_2\)
- \(\mathrm{H}_2>\mathrm{O}_2>\mathrm{CH}_4\)
- \(\mathrm{H}_2<\mathrm{He}<\mathrm{O}_2<\mathrm{CO}_2\)
- \(\mathrm{CH}_4>\mathrm{O}_2>\mathrm{H}_2\)
- \(\mathrm{H}_2<\mathrm{O}_2<\mathrm{He}<\mathrm{CO}_2\)
- \(\mathrm{O}_2>\mathrm{CH}_4>\mathrm{H}_2\)
Answer: 3. \(\mathrm{H}_2<\mathrm{He}<\mathrm{O}_2<\mathrm{CO}_2\) and \(\mathrm{CH}_4>\mathrm{O}_2>\mathrm{H}_2\)
States of Matter NEET question bank
Question 48. van der Waals’ real gas, acts as an ideal gas, at which conditions?
- High temperature, low pressure
- Low temperature, high pressure
- High temperature, high pressure
- Low temperature, low pressure
Answer: 1. High temperature, low pressure
At low-pressure and high-temperature van der Waals, real gas acts as an ideal gas and is observed to obey PV = nRT relation.
At very low pressure when the gas volume is quite large the space occupied by the molecules themselves becomes negligible comparatively and because the molecules are then far apart, the force of mutual attraction becomes too feeble, the real gas would satisfy the postulates of kinetic theory.
As the temperature is raised, the volume of the gas increases and
we can consider \(\left(P+\frac{n^2 a}{V^2}\right)\) term as P and at low pressure (V – nb) term as V. \(\left(P+\frac{n^2 a}{V^2}\right)(V-n b)=n R T\) (van der Waals equation)
This equation becomes PV = nRT
This is an ideal gas equation
Question 49. When is deviation more in the behaviour of a gas from the ideal gas equation PV = nRT.
- At high temperatures and low pressure
- At low temperatures and high pressure
- At high temperatures and high pressure
- At low temperatures and low pressure
Answer: 2. At low temperatures and high pressure
At low temperatures and high pressure, there is a deviation from the ideal behaviour in gases.
Question 50. A gas is said to behave like an ideal gas when the relation PV/T = constant. When do you expect a real gas to behave like an ideal gas?
- When the temperature is low.
- When both the temperature and pressure are low.
- When both the temperature and pressure are high
- When the temperature is high and pressure is low.
Answer: 4. When the temperature is high and pressure is low.
A gas is said to behave like an ideal gas when the relation PV/T = constant.
At high temperatures and low pressure, the effect of a/V² and b is negligible.
As we know, PV = nRT (Ideal gas equation)
PV = \(R T \text { or } \frac{P V}{R T}=1\)
∴ Z=1 [Z is compressibility factor]
Hence gas shows ideal behaviour.
States of Matter NEET question bank
Question 51. In van der Waals’ equation of state for a non-ideal gas, the term that accounts for intermolecular forces is
- \((V-b)\)
- \((R T)^{-1}\)
- \(\left(P+\frac{a}{V^2}\right)\)
- R T
Answer: 3. \(\left(P+\frac{a}{V^2}\right)\)
Vander Waals’ equation for 1 mole is \(\left(P+\frac{a}{V^2}\right)\)(V-b)=RT
Here, \(\left(P+\frac{a}{V^2}\right)\) represents the intermolecular forces and (V-b) is the correct volume.
Question 52. Given van der Waals’ constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquified?
- NH3
- H2
- O2
- HO2
Answer: 1. NH3
Given van der Waals’ constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59
van der Waals constant signifies the intermolecular forces of attraction between the particles of gas.
So, the higher the value of ‘a’, the easier will be the liquefaction of gas.
Question 53. An ideal gas, obeying the kinetic theory of gases cannot be liquefied, because
- It solidifies before becoming a liquid
- Forces acting between its molecules are negligible
- Its critical temperature is above 0°c
- Its molecules are relatively small in size.
Answer: 2. Forces acting between its molecules are negligible
A gas can only be liquefied, if some forces of attraction are acting in its molecules. According to kinetic theory, an ideal gas is devoid of the force of attraction in its molecules, therefore it cannot be liquefied.
States of Matter NEET question bank
Question 54. The beans are cooked earlier in a pressure cooker because
- The boiling point increases with increasing pressure
- The boiling point decreases with increasing pressure
- The extra pressure of the cooker softens the beans
- Internal energy is not lost while cooking in a pressure cooker
Answer: 1. Boiling point increases with increasing pressure
The more the pressure, the greater the boiling point.
