Structure Of Atom NEET MCQs
NEET Chemistry For Structure Of Atom Multiple Choice Questions
Question 1. Select the correct statements from the following
- Atoms of all elements are composed of two fundamental particles.
- The mass of an electron is 9.10939 x 10-31 kg.
- All the isotopes of a given element show the same chemical properties.
- Protons and electrons are collectively known as nucleons.
- Dalton’s atomic theory regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
- 1 and 5 only
- 2, 3 and 5 only
- 1, 2 and 3 only
- 3, 4 and 5 only
Answer: 2. 2, 3 and 5 only
Atoms of all elements are composed of three fundamental particles: electrons, Protons, and neutrons.
Protons and neutrons are collectively known as nucleons
Hence, 2, 3, and 5 are correct statements
Question 2. From the following pairs of ions which one is not an iso-electronic pair?
- \(\mathrm{Fe}^{2-}, \mathrm{Mn}^{2-}\)
- \(\mathrm{O}^{2-}, \mathrm{F}\)
- \(\mathrm{Na}^{+}, \mathrm{Mg}^{2+}\)
- \(\mathrm{Mn}^{2+}, \mathrm{Fe}^{3-}\)
Answer: 1. \(\mathrm{Fe}^{2-}, \mathrm{Mn}^{2-}\)
Among the given pairs of ions, Fe2+ and Mn2+ is not an iso-electronic pair.
Question 3. The number of protons, neutrons and electrons in \({ }_{71}^{175} \mathrm{Lu}_3\) respectively, are:
- 71, 104 and 71
- 104, 71 and 71
- 71, 71 and 104
- 175,104 and 71
Answer: 1. 71, 104 and 71
⇒ \({ }_{71}^{175} \mathrm{Lu}_3\) Number of protons = Number of electrons =
Atomic number = 71
Number of neutrons = Mass number – Atomic number
= 175 – 71 = 104
Read and Learn More NEET MCQs with Answers
Question 4. Be2+ is isoelectronic with which of the following ions?
- \(\mathrm{H}^{+}\)
- \(\mathrm{Li}^{+}\)
- \(\mathrm{Na}^{+}\)
- \(\mathrm{Mg}^{2+}\)
Answer: 2. \(\mathrm{Li}^{+}\)
⇒ \(\begin{array}{cc}
\text { Species } & \text { No. of electrons } \\
\mathrm{Be}^{2+} & 2 \\
\mathrm{H}^{+} & 0 \\
\mathrm{Li}^{+} & 2 \\
\mathrm{Na}^{+} & 10 \\
\mathrm{Mg}^{2+} & 10
\end{array}\)
Structure of Atom NEET MCQs
Question 5. Isoelectronic species are
- \(\mathrm{CO}, \mathrm{CN}^{-}, \mathrm{NO}^{+}, \mathrm{C}_2^{2-}\)
- \(\mathrm{CO}^{-}, \mathrm{CN}^{-} \mathrm{NO}, \mathrm{C}_2^{-}\)
- \(\mathrm{CO}^{+}, \mathrm{CN}^{+}, \mathrm{NO}^{-}, \mathrm{C}_2\)
- \(\mathrm{CO}, \mathrm{CN}, \mathrm{NO}, \mathrm{C}_2\)
Answer: 1. \(\mathrm{CO}, \mathrm{CN}^{-}, \mathrm{NO}^{+}, \mathrm{C}_2^{2-}\)
Species having the same number of electrons are called isoelectronic species.
The no. of electrons in \(\mathrm{CO}=\mathrm{CN}^{-}=\mathrm{NO}^{+}=\mathrm{C}_2^{2-}=14\). So, these are isoelectronic species
Question 6. The ion that is isoelectronic with CO is
- \(\mathrm{CN}^{-}\)
- \(\mathrm{N}_2^{+}\)
- \(\mathrm{O}^{2-}\)
- \(\mathrm{N}_2^{-}\)
Answer: 1. \(\mathrm{CN}^{-}\)
Since both CO and CN– have 14 electrons, therefore these are isoelectronic species (i.e. having the same number of electrons)
Question 7. Which one of the following is not isoelectronic with O2-?
- \(\mathrm{Tl}^{+}\)
- \(\mathrm{Na}^{+}\)
- \(\mathrm{N}^{3-}\)
- \(\mathrm{F}^{-}\)
Answer: 1. \(\mathrm{Tl}^{+}\)
The number of electrons in O2- N+2, F-, and Nan is 10 each, but a number of electrons in Tl+ is 80.
Question 8. A particular station of All India Radio, New Delhi, broadcasts on a frequency of 1, 368 kHz (kilohertz). The wavelength of the electromagnetic radiation emitted by the transmitter is [speed of light. c = 3.0 x 108 ms-1]
- 21.92 cm
- 219.3 m
- 219.2 m
- 2192 m
Answer: 2. 219.3 m
A particular station of All India Radio, New Delhi, broadcasts on a frequency of 1, 368 kHz (kilohertz).
v = \(\frac{c}{\lambda} ; \lambda=\frac{c}{v}=\frac{3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}}{1368 \times 10^3 \mathrm{~s}^{-1}}=219.3 \mathrm{~m}\)
Question 9. Which of the following series of transitions in the spectrum of hydrogen atom tails in the visible region?
- Brackett series
- Lyman series
- Balmer series
- Paschen series
Answer: 3. Balmer series
Lyman series: UV region
Balmer series: Visible region
Paschen series: IR region
Brackett series: IR region
NEET questions on Structure of Atom
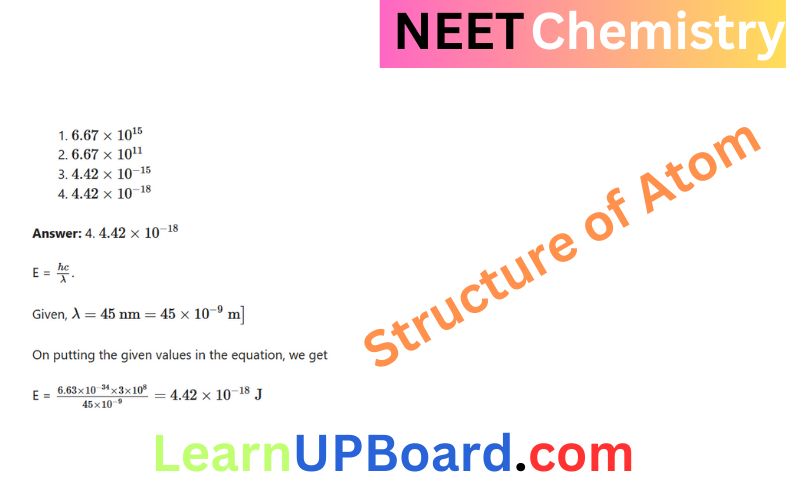
Question 10. Calculate the energy in joule corresponding to light of wavelength 45 nm. (Planck’s constant, h = 6.63 x 10-34J s, speed of light, c = 3 x 108 m s-1)
Question 11. The value of Planck’s constant is 6.63 x 10-34. The speed of light is 3 x 1017 nm s-1. Which value is closest to the wavelength in the nanometer of a quantum of light with a frequency of 6 x 1015 s-1?
- 50
- 75
- 10
- 25
Answer: 1. 50
The value of Planck’s constant is 6.63 x 10-34. The speed of light is 3 x 1017 nm s-1.
c = vλ
λ = \(\frac{c}{v}=\frac{3 \times 10^{17}}{6 \times 10^{15}}=50 \mathrm{~nm}\)
Question 12. According to the law of photochemical equivalence, the energy absorbed (in ergs/mole) is given as (h = 6.62 x 10-27 ergs, c = 3 x 1010 cm s-1 NA = 6.02 x 1023 mol-1)
- \(\frac{1.196 \times 10^8}{\lambda}\)
- \(\frac{2.859 \times 10^{16}}{\lambda}\)
- \(\frac{2.859 \times 10^5}{\lambda}\)
- \(\frac{1.196 \times 10^{16}}{\lambda}\)
Answer: 1. \(\frac{1.196 \times 10^8}{\lambda}\)
We know that, E = \(\frac{h c N_A}{\lambda}\)
= \(\frac{6.62 \times 10^{-27} \times 3 \times 10^{10} \times 6.02 \times 10^{23}}{\lambda}\)
= \(\frac{1.1955 \times 10^8}{\lambda}=\frac{1.196 \times 10^8}{\lambda} \mathrm{ergs} \mathrm{mol} \mathrm{m}^{-1}\)
Question 13. The energies E1 and E2 of the two radiations are 25 eY and 50 eV respectively. The relation between their wavelengths i.e., λ1 and λ2 will be
- \(\lambda_1=\lambda_2\)
- \(\lambda_1=2 \lambda_2\)
- \(\lambda_1=4 \lambda_2\)
- \(\lambda_1=\frac{1}{2} \lambda_2\)
Answer: 2. \(\lambda_1=2 \lambda_2\)
The energies E1 and E2 of the two radiations are 25 eY and 50 eV respectively.
∴ \(E_1=\frac{h c}{\lambda_1}\) and \(E_2=\frac{h c}{\lambda_2} ; \frac{E_1}{E_2}=\frac{h c}{\lambda_1} \times \frac{\lambda_2}{h c}=\frac{\lambda_2}{\lambda_1}\)
or \(\frac{25}{50}=\frac{\lambda_2}{\lambda_1}\) or \(\frac{1}{2}=\frac{\lambda_2}{\lambda_1} \Rightarrow \lambda_1=2 \lambda_2\)
NEET questions on Structure of Atom
Question 14. The value of Planck’s constant is 6.63 x 10-34 s. The velocity of light is 3.0 x 108 m s-1. Which value is closest to the wavelength in nanometers of a quantum of light with a frequency of 8 x 1015 s-1?
- \(2 \times 10^{-25}\)
- \(4 \times 10^1\)
- \(5 \times 10^{-18}\)
- \(3 \times 10^7\)
Answer: 3. \(5 \times 10^{-18}\)
The value of Planck’s constant is 6.63 x 10-34 s. The velocity of light is 3.0 x 108 m s-1.
Applying v = c/λ
λ = \(\frac{c}{v}=\frac{3 \times 10^8}{8 \times 10^{15}}=37.5 \times 10^{-9} \mathrm{~m}=37.5 \mathrm{~nm}=4 \times 10^1 \mathrm{~nm}\)
Question 15. For given energy, E = 3.03 x 10-19 joules corresponding wavelength is (h = 6.626 x 10-34 J sec, c = 3 x 108 m/sec)
- 65.6 nm
- 6.56 nm
- 3.4 nm
- 656 nm
Answer: 4. 656 nm
E = \(\frac{h c}{\lambda} \Rightarrow \lambda=\frac{6.626 \times 10^{-34} \times 3 \times 10^8}{3.03 \times 10^{-19}}=656 \mathrm{~nm}\)
Question 16. What will be the longest wavelength line in the Balmer series of spectra?
- 546 nm
- 656 nm
- 566 nm
- 556 nm
Answer: 2. 656 nm
The longest wavelength means the lowest energy.
We know that relation for wavelength \(\frac{1}{\lambda}=R_{\mathrm{H}}\left(\frac{1}{n_1^2}-\frac{1}{n_2^2}\right) \)
(\(R_{\mathrm{H}}\), Rydberg constant = \(109677 \mathrm{~cm}^{-1}\))
For \(n_1=2, n_2=3\)
∴ \(\frac{1}{\lambda}=109677\left(\frac{1}{(2)^2}-\frac{1}{(3)^2}\right)=15233\)
or, \(\lambda=\frac{1}{15233}=6.56 \times 10^{-5} \mathrm{~cm}\)
= \(6.56 \times 10^{-7} \mathrm{~m}\)
= \(656 \mathrm{~nm}\)
Atomic Structure multiple choice NEET
Question 17. If the radius of the second Bohr orbit of the He+ ion is 105.8 pm, what is the radius of the third Bohr orbit of the Li2+ ion?
- 158.7 pm
- 1587 pm
- 1.587 pm
- 158.7 Å
Answer: 1. 158.7 pm
The radius of the second Bohr orbit of the He+ ion is 105.8 pm,
Radius \(=r_0 \times \frac{n^2}{Z}\)
For \(\mathrm{He}^{+}, n=2 ; Z=2\)
∴ \(\mathrm{r}_{\mathrm{He}^{+}}=r_0 \times \frac{2 \times 2}{2} ; 105.8=r_0 \times 2\)
∴ \(r_0=\frac{105.8}{2} \mathrm{pm}\)
For \(\mathrm{Li}^{2+} ; n=3 ; Z=3\)
∴ \(r_{\mathrm{Li}^{2+}}=r_0 \times \frac{(3)^2}{3}=\frac{105.8}{2} \times 3=158.7 \mathrm{pm}\) or 1.587Å
Question 18. Based on equation E = \(-2.178 \times 10^{-18} \mathrm{~J}\left(\frac{Z^2}{n^2}\right),\) certain conclusions are written. Which of them is not correct?
- The equation can be used to calculate the change in energy when the electron changes orbit.
- For n = 1, the electron has a more negative energy than it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit.
- The negative sign in the equation simply means that the energy of the electron bound to the nucleus is lower than it would be if the electrons were at an infinite distance from the nucleus.
- The larger the value of n, the larger is the orbit radius.
Answer: 2. For n = 1, the electron has a more negative energy than it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit.
The electron is more tightly bound in the smallest allowed orbit.
Question 19. According to the Bohr theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon?
- n = 6 to n = 1
- n = 5 to n = 4
- n = 6 to n = 5
- n = 5 to n = 3
Answer: 3. n = 6 to n = 5
We know that \(\Delta E \propto\left[\frac{1}{n_1^2}-\frac{1}{n_2^2}\right]\) where \(n_2>n_1\)
∴ n = 6 to n = 5 will give the least energetic photon.
Question 20. The energy of the second Bohr orbit of the hydrogen atom is -328 kJ mol-1; hence the energy of the fourth Bohr orbit would be
- \(41 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(-82 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(-164 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(-1312 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Answer: 2. \(-82 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
⇒ \(E_n=-K\left(\frac{Z}{n}\right)^2\)
Z=1 for hydrogen; n=2
∴ \(E_2=\frac{-K \times 1}{4} \Rightarrow E_2=-328 \mathrm{~kJ} \mathrm{~mol}^{-1} ; K=4 \times 328\)
∴ \(E_4=\frac{-K \times 1}{16} \Rightarrow E_4=-4 \times 328 \times \frac{1}{16}=-82 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Question 21. The frequency of radiation emitted when the electron falls from n = 4ton=lina hydrogen atom will be (Given ionization energy of H = 2.18 x 10-18 J atom-1 and h = 6.626 x 10-34 J s)
- \(1.54 \times 10^{15} \mathrm{~s}^{-1}\)
- \(1.03 \times 10^{15} \mathrm{~s}^{-1}\)
- \(3.08 \times 10^{15} \mathrm{~s}^{-1}\)
- \(2.00 \times 10^{15} \mathrm{~s}^{-1}\)
Answer: 3. \(3.08 \times 10^{15} \mathrm{~s}^{-1}\)
E = hv or v = E/h
For \(\mathrm{H}\) atom, \(E=\frac{-21.8 \times 10^{-19}}{n^2} \mathrm{~J} \mathrm{atom}^{-1}\)
ΔE= \(-21.8 \times 10^{-19}\left(\frac{1}{4^2}-\frac{1}{1^2}\right)=20.44 \times 10^{-19} \mathrm{Jatom}^{-1}\)
v = \(\frac{20.44 \times 10^{-19}}{6.626 \times 10^{-34}}=3.08 \times 10^{15} \mathrm{~s}^{-1}\)
Atomic Structure multiple choice NEET
Question 22. In a hydrogen atom, the energy of the first excited state is -3.4 eV. Then find out K.E. of the same orbit of the hydrogen atom.
- +3.4 eV
- +6.8 eV
- -13.6 eV
- +13.6 eV
Answer: 1. +3.4 eV
Kinetic energy = \(\frac{1}{2} m v^2\) = \(\left(\frac{\pi e^2}{n h}\right)^2 \times 2 m\)
(because \(v=\frac{2 \pi e^2}{\pi h}\))
Total energy, \(E_n=-\frac{2 \pi^2 m e^4}{n^2 h^2}=-\left(\frac{\pi e^2}{n h}\right)^2 \times 2 m=-K . E\).
∴ Kinetic energy = -En
Energy’s first excited state is -3.4 eV.
∴ The kinetic energy of the same orbit (n = 2) will be +3.4 eV
Question 23. Who modified Bohr’s theory by introducing elliptical orbits for electron path?
- Rutherford
- Thomson
- Hund
- Sommerfeld
Answer: 4. Sommerfeld
Sommerfeld modified Bohr’s theory considering that in addition to circular orbits electrons also move in elliptical orbits.
Question 24. The Bohr orbit radius for the hydrogen atom (n = 1) is approximately 0.530 Å. The radius for the first excited state (n = 2) orbit is (in Å)
- 4.77
- 1.06
- 0.13
- 2.12
Answer: 4. 2.12
For nth orbit of ‘H’ atom, rn = n² x r1
⇒ the radius of 2nd Bohr’s orbit.
r2 = 4 x r1 = 4 x 0.53 = 2.12Å
NEET practice questions Structure of Atom
Question 25. In Bohr’s model of an atom, when an electron jumps from n = 1 to n = 3, how much energy will be emitted or absorbed?
- \(2.389 \times 10^{-12} \text { ergs }\)
- \(0.239 \times 10^{-10} \text { ergs }\)
- \(2.15 \times 10^{-11} \mathrm{ergs}\)
- \(0.1936 \times 10^{-10} \mathrm{ergs}\)
Answer: 4. \(0.1936 \times 10^{-10} \mathrm{ergs}\)
The energy of an atom when n = 1
∴ \(E_1=-\frac{1312}{(1)^2}=-1312 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Similarly energy when n = 3, \((E_3)=-\frac{1312}{(3)^2}=-145.7 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
The energy absorbed when an electron jumps from n = 1 to n = 3, \(E_3-E_1=-145.7-(-1312)=1166.3 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
= \(\frac{1166.3}{6.023 \times 10^{23}}=193.6 \times 10^{-23} \mathrm{~kJ}\)
= \(193.6 \times 10^{-20} \mathrm{~J} \quad\left[1 \text { joule }=10^7 \text { ergs }\right]\)
∴ \(193.6 \times 10^{-13} \text { ergs }=0.1936 \times 10^{-10} \text { ergs }\)
Question 26. The radius of a hydrogen atom in the ground state is 0.53 Å. The radius of Li2+ ion (atomic number = 3) in a similar state is
- 0.53 Å
- 1.06 Å
- 0.17 Å
- 0.265 Å
Answer: 3. 0.17 Å
Due to the ground state, the state of hydrogen atom (n) = 1;
The radius of hydrogen atom (r) = 0.53 Å
The atomic number of Li (Z) = 3
Now, radius of \(\mathrm{Li}^{2+} \text { ion }=r \times \frac{n^2}{Z}=0.53 \times \frac{(1)^2}{3}\) =0.17Å
Question 27. The energy of an electron in the nth Bohr orbit of a hydrogen atom is
- \(\frac{13.6}{n^4} \mathrm{eV}\)
- \(\frac{13.6}{n^3} \mathrm{eV}\)
- \(\frac{13.6}{n^2} \mathrm{eV}\)
- \(\frac{13.6}{n} \mathrm{eV}\)
Answer: 3. \(\frac{13.6}{n^2} \mathrm{eV}\)
Energy of an electron in nth Bohr orbit of hydrogen atom = \(\frac{-13.6}{n^2} \mathrm{eV}\)
Question 28. The spectrum of He is expected to be similar to that
- H
- Li+
- Na
- He+
Answer: 2. Li+
Both He+ and Li+ contain 2 electrons each.
NEET practice questions Structure of Atom
Question 29. If r is the radius of the first orbit, the radius of the nth orbit of H-atom is given by
- rn²
- rn
- r/n
- r2n2
Answer: 1. rn2
The radius of nth orbit of H-atom = r0n2
where r0 = radius of the first orbit.
Question 30. In a hydrogen atom, the de Broglie wavelength of an electron in the second Bohr orbit is [Given that Bohr radius, a0 = 52.9 pm]
- 211.6 pm
- 211.6 π pm
- 52.9 π pm
- 105.8 pm
Answer: 2. 211.6 π pm
Bohr radius, ao = 52.9 pm
n = 2, rn = n²a0= (2)2a0= 4x 52.9 pm = 211.6 pm
The angular momentum of an electron in a given stationary state can be expressed as in the equation,
mvr = \(n \cdot \frac{h}{2 \pi}=2 \times \frac{h}{2 \pi}=\frac{h}{\pi} \Rightarrow m v r \pi=h\)…..(1)
de-Broglie equation, \(\lambda=\frac{h}{m v} ; \lambda m v=h\)…..(2)
From equations (1) and (2), we get λ = πr
Putting the value of r, λ = 211.6 π pm
Chemistry MCQs Structure of Atom NEET
Question 31. A 0.66 kg ball is moving at a speed of 100 m/s. The associated wavelength will be (h = 6.6 x 10-34 J s)
- \(6.6 \times 10^{-32} \mathrm{~m}\)
- \(6.6 \times 10^{-34} \mathrm{~m}\)
- \(1.0 \times 10^{-35} \mathrm{~m}\)
- \(1.0 \times 10^{-32} \mathrm{~m}\)
Answer: 3. \(1.0 \times 10^{-35} \mathrm{~m}\)
According to de-Broglie equation, \(\lambda=\frac{h}{m v}\)
Given, \(h=6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s} ; m=0.66 \mathrm{~kg} ; v=100 \mathrm{~m} \mathrm{~s}^{-1}\)
∴ \(\lambda=\frac{6.6 \times 10^{-34}}{0.66 \times 100}=1 \times 10^{-35} \mathrm{~m}\)
Question 32. If uncertainty in position and momentum are equal, then uncertainty in velocity is
- \(\frac{1}{m} \sqrt{\frac{h}{\pi}}\)
- \(\sqrt{\frac{h}{\pi}}\)
- \(\frac{1}{2 m} \sqrt{\frac{h}{\pi}}\)
- \(\sqrt{\frac{h}{2 \pi}}\)
Answer: 3. \(\frac{1}{2 m} \sqrt{\frac{h}{\pi}}\)
From the Heisenberg uncertainty principle, \(\Delta p \cdot \Delta x \geq \frac{h}{4 \pi}\) or \(m \Delta v \times \Delta x \geq \frac{h}{4 \pi}\)
or \((m \Delta v)^2 \geq \frac{h}{4 \pi}\) (because \(\Delta x=\Delta p\)) or \(\Delta v \geq \frac{1}{2 m} \sqrt{\frac{h}{\pi}}\)
Question 33. The measurement of the electron position is associated with an uncertainty in momentum, which is equal to 1 x 10-18 g cm s-1. The uncertainty in electron velocity is (mass of an electron is 9 x 10-28 g)
- \(1 \times 10^5 \mathrm{~cm} \mathrm{~s}^{-1}\)
- \(1 \times 10^{11} \mathrm{~cm} \mathrm{~s}^{-1}\)
- \(1 \times 10^9 \mathrm{~cm} \mathrm{~s}^{-1}\)
- \(1 \times 10^6 \mathrm{~cm} \mathrm{~s}^{-1}\)
Answer: 3. \(1 \times 10^9 \mathrm{~cm} \mathrm{~s}^{-1}\)
The measurement of the electron position is associated with an uncertainty in momentum, which is equal to 1 x 10-18 g cm s-1.
Uncertainty in momentum (mΔv) = 1 x 10-18 g cm s-1
Uncertainty in velocity (Δv) = \(\frac{1 \times 10^{-18}}{9 \times 10^{-28}}=1.1 \times 10^9 \mathrm{~cm} \mathrm{~s}^{-1}\)
Chemistry MCQs Structure of Atom NEET
Question 34. Given: The mass of an electron is 9.11 x 10-31 kg, the Planck constant is 6.626 x 10-34 J s, and the uncertainty involved in the measurement of velocity within a distance of 0.1 Å is
- \(5.79 \times 10^5 \mathrm{~m} \mathrm{~s}^{-1}\)
- \(5.79 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\)
- \(5.79 \times 10^7 \mathrm{~m} \mathrm{~s}^{-1}\)
- \(5.79 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}\)
Answer: 2. \(5.79 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\)
∴ \(\Delta x \cdot m \Delta v=h / 4 \pi\)
0.1 x \(10^{-10} \times 9.11 \times 10^{-31} \times \Delta v=\frac{6.626 \times 10^{-34}}{4 \times 3.143}\)
∴ \(\Delta v=\frac{6.626 \times 10^{-34}}{0.1 \times 10^{-10} \times 9.11 \times 10^{-31} \times 4 \times 3.143}\)
= \(5.79 \times 10^6 \mathrm{~m} \mathrm{~s}^{-1}\)
Question 35. The uncertainty in the momentum of an electron is 1 x 10-5 kg m/s. The uncertainty in its position will be (h = 6.62 x 10-34 kg m²/s)
- \(8.0 \times 10^{-26} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
- \(80 \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
- \(50 \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
- \(5.0 \times 10^{-26} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
Answer: 1. \(8.0 \times 10^{-26} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
∴ \(\Delta x \times \Delta p=\frac{h}{4 \pi}\) (Heisenberg uncertainty principle)
⇒ \(\Delta x=\frac{6.62 \times 10^{-34}}{4 \times 3.14 \times 10^{-5}}=5.27 \times 10^{-30} \mathrm{~m}\)
Question 36. The de Broglie wavelength of a particle with a mass 1 g and velocity of 100 m/s is
- \(6.63 \times 10^{-35} \mathrm{~m}\)
- \(6.63 \times 10^{-34} \mathrm{~m}\)
- \(6.63 \times 10^{-33} \mathrm{~m}\)
- \(6.65 \times 10^{-35} \mathrm{~m}\)
Answer: 3. \(6.63 \times 10^{-33} \mathrm{~m}\)
λ = \(\frac{h}{m \nu}=\frac{6.63 \times 10^{-27} \mathrm{erg} \mathrm{sec}}{1 \mathrm{~g} \times 10^4 \mathrm{~cm} / \mathrm{s}}\)
= \(6.63 \times 10^{-31} \mathrm{~cm}=6.63 \times 10^{-33} \mathrm{~m}\)
Structure of Atom quiz for NEET
Question 37. The position of both, an electron and a helium atom is known within 1.0 nm. Further, the momentum of the electron is known within 5.0 x 10-26 kg m s-1. The minimum uncertainty in the measurement of the momentum of the helium atom is
- \(8.0 \times 10^{-26} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
- \(80 \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
- \(50 \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
- \(5.0 \times 10^{-26} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
Answer: 4. \(5.0 \times 10^{-26} \mathrm{~kg} \mathrm{~m} \mathrm{~s}^{-1}\)
The position of both, an electron and a helium atom is known within 1.0 nm. Further, the momentum of the electron is known within 5.0 x 10-26 kg m s-1.
According to the uncertainty principle, the product of uncertainty in position and uncertainty in momentum is constant for a particle.
i.e., \(\Delta x \times \Delta p=\frac{h}{4 \pi}\)
As, Δx =1.0 nm for both electron and helium atom, so Δp is also the same for both the particles.
Thus, the uncertainty in the momentum of the helium atom is also 5.0 x 10-26 kg m s-1.
Question 38. Uncertainty in position of an electron (Mass = 9.1 x 10-28 g) moving with a velocity of 3 x 104 cm/s accurate upto 0.001% will be (Use h/(4π) in uncertainty expression where h = 6.626 x 10-27 erg second)
- 5.76 cm
- 7.68 cm
- 1.93 cm
- 3.84 cm
Answer: 3. 1.93 cm
Mass of an electron (m) = 9.1 x 10-28 g
Velocity of electron (v) = 3 x 104 cm/s
Accuracy = 0.001% = \(\frac{0.001}{100}\) and
Planck constant (h)= 6.626 x 10-27 erg-second.
We know that actual velocity of the electron \((\Delta v)=3 \times 10^4 \times \frac{0.001}{100}=0.3 \mathrm{~cm} / \mathrm{s}\)
Therefore, uncertainty in the position of the electron, \((\Delta x)=\frac{h}{4 \pi m \Delta v}=\frac{6.626 \times 10^{-27}}{4 \pi \times\left(9.1 \times 10^{-28}\right) \times 0.3}=1.93 \mathrm{~cm}\)
Structure of Atom quiz for NEET
Question 39. Which of the following statements do not form a part of Bohr’s model of the hydrogen atom?
- The energy of the electrons in the orbits is quantized.
- The electron in the orbit nearest the nucleus has the lowest energy.
- Electrons revolve in different orbits around the nucleus.
- The position and velocity of the electrons in the orbit cannot be determined simultaneously.
Answer: 4. The position and velocity of the electrons in the orbit cannot be determined simultaneously.
It is Heisenberg’s uncertainty principle and not Bohr’s postulate.
Question 40. The relation between nm (nm = the number of permissible values of magnetic quantum number (m)) for a given value of azimuthal quantum number
- \(n_m=2 l^2+1\)
- \(n_m=l+2\)
- \(l=\frac{n_m-1}{2}\)
- \(l=2 n_m+1\)
Answer: 3. \(l=\frac{n_m-1}{2}\)
∴ \(n_m=2 l+1 \quad \text { or } \quad l=\frac{n_m-1}{2}\)
Question 41. Identify the incorrect statement from the following.
- All the five 5d orbitals are different in size when compared to the respective 4d orbitals.
- The five 4d orbitals have shapes similar to the respective 3d orbitals.
- In an atom, all five 3d orbitals are equal in energy in a free state.
- The shapes of dxy, dyz, and dzx orbitals are similar to each other; and \(d_{x^2-y^2} \text { and } d_{z^2}\) are similar to each other
Answer: 4. The shapes of dxy, dyz, and dzx orbitals are similar to each other; and \(d_{x^2-y^2} \text { and } d_{z^2}\) are similar to each other
The shapes of dxy, dxz,, dyz, and \(d_{x^2-y^2}\) are similar to each other, whereas that of d; is different from others. AIl five 3d orbitals are equivalent in energy. The d-orbitals for which n is greater than 3 (4d, 5d, …) also have shapes similar to 3d-orbital but differ in energy and size.
Question 42. 4d, 5p, 5/and 6p orbitals are arranged in the order of decreasing energy. The correct option is
- 5f> 6p > 4d > 5p
- 5f> 6p> 5p> 4d
- 6p > 5f> 5p > 4d
- 6p > 5f> 4d > 5p
Answer: 2. 5f> 6p> 5p> 4d
The higher the value of (n + 1) for an orbital, the higher its energy. However, if two different types of orbitals have the same value of (n + 1), the orbital with the lower value of n has lower energy. Therefore, the decreasing order of energy of the given orbitals is 5f > 6p > 5p > 4d.
NEET MCQs on Atomic Structure
Question 43. Orbital having 3 angular nodes and 3 total nodes is
- 5p
- 3d
- 4f
- 6d
Answer: 3. 4f
Number of spherical/radial nodes in any orbital =n-l-1
Number of planar/angular nodes in orbital = l = 3
.’. Totalnumberofnodesinanyorbital= n -1=3
∴ n = 4
Thus, the orbital is 4f
Question 44. Which one is a wrong statement?
- The total orbital angular momentum of an electron in the s-orbital is equal to zero.
- An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers.
- The electronic configuration of the N atom is
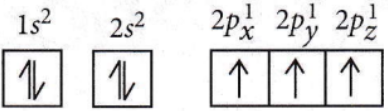
- The value of m for dz2 is zero.
Answer: 3. The electronic configuration of the N atom is
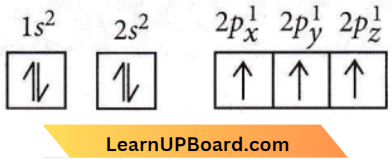
According to Hund’s rule of maximum multiplicity, the correct configuration of ‘N’ is
Structure of Atom NEET question bank
Question 45. Which one is the wrong statement?
- The uncertainty principle is \(\Delta E \times \Delta t \geq \frac{h}{4 \pi}\)
- Half-filled and fully-filled orbitals have greater stability due to greater exchange energy, greater symmetry, and a more balanced arrangement.
- The energy of 2s-orbital is less than the energy of 2p-orbital in the case of hydrogen-like atoms.
- de-Broglie’s wavelength is given by \(\lambda=\frac{h}{m v}\) where m = mass of the particle, v = group velocity of the particle.
Answer: 3. The energy of 2s-orbital is less than the energy of 2p-orbital in the case of hydrogen-like atoms
In the case of hydrogen-like atoms, energy depends on the principal quantum number only. Hence, 2s-orbital will have energy equal to 2p-orbital.
Question 46. Consider the following sets of quantum numbers:
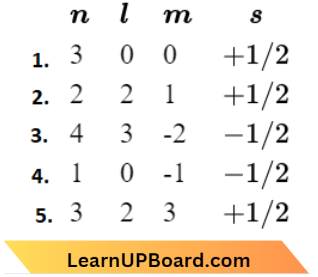
Which of the following sets of quantum numbers is not possible?
- (1), (2), (3) and (4)
- (2), (4), and (5)
- (1) and (3)
- (2), (3), and (4)
Answer: 2. (2), (4) and (5)
- Represents an electron in 3s orbital.
- This is not possible as the value varies from 0, 1, … (n-1).
- Represents an electron in 4/orbital.
- Is not possible as the value of varies from -l… +l.
- This is not possible as the value of z varies from -l …+l, It can never be greater than l.
Question 46. The following quantum numbers are possible for how many orbitals? n = 3, l = 2, m = +2
- 1
- 2
- 3
- 4
Answer: 1. 1
n = 3, l= 2, m = +2
It symbolizes one of the five d-orbitals (3d).

Question 47. For which of the following sets of four quantum numbers, an electron will have the highest energy?
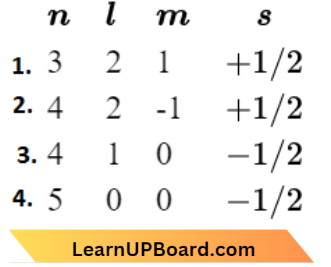
Answer: 2.
The energy of an electron depends on the value of (n + 1). The subshell are 3d, 4d, 4p and 5s, out of which 4d has highest energy
Structure of Atom NEET question bank
Question 48. The electronic configuration of calcium atoms can be written as
- [Ne]4p²
- [Ar]4s²
- [Ne]4s²
- [Kr]4p²
Answer: 2. [Ar]4s²
The atomic number of Ca = 20
∴ Electronic configuration of Ca = [Ar]4s²
Question 49. For azimuthal quantum number l = 3, the maximum number of electrons will be
- 2
- 6
- 0
- 14
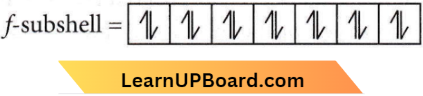
Answer: 4. 14
l = 3 means f subshell
Maximum no. of electrons in f subshell = 14