Thermodynamics NEET MCQs
NEET Chemistry For Thermodynamics Multiple Choice Questions
Question 1. Which of the following are not state functions?
- q+w
- q
- w
- H-TS
- (1), (2) and (3)
- (2) and (3)
- (1) and (4)
- (2),(3) and (4)
Answer: 2. (2) and (3)
State functions or state variables are those which depend only on the state of the system and not on how the state was reached.
q + w = ΔE (internal energy)
H – Ts = G (free energy)
Path function depends on the path followed during a process. Work and heat are the path functions.
Question 2. In a closed insulated container a liquid is stirred with a paddle to increase the temperature, which of the following is true?
- ΔE= W≠0, q=0
- ΔE= W=q≠0
- ΔE =0, W = q≠0
- W=0, ΔE=q≠0
Answer: 1. ΔE= W≠0, q=0
In a closed insulated container a liquid is stirred with a paddle to increase the temperature
The mathematical form of the first law of thermodynamics q = ΔE + w
Since the system is closed and insulated, q = 0
Paddlework is done on the system.
∴ w ≠ 0.
Temperature and hence internal energy of the system increases.
∴ ΔE ≠ 0.
Read and Learn More NEET MCQs with Answers
Thermodynamics NEET MCQs
Question 3. Which of the following is the correct equation?
- ΔU= ΔW+ ΔQ
- ΔU= ΔQ – W
- ΔW = ΔU+ ΔQ
- None of these
Answer: 2. ΔU= ΔQ – W
This is the mathematical relation of the first law of thermodynamics. Here ΔU = change in internal energy; ΔQ = heat absorbed by the system and W = work done by the system.
Question 4. Which of the following options is the correct relation between change in enthalpy and change in internal energy?
- ΔH – ΔU= -ΔnRT
- ΔH + ΔU = ΔnR
- ΔH = ΔU – ΔngRT
- ΔH= ΔU+ ΔngRT
Answer: 4. ΔH= ΔU+ ΔngRT
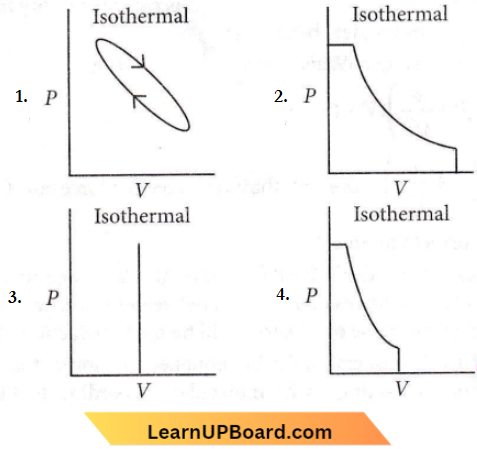
Question 5. Which of the following P-V curve represents maximum work done?
Answer: 2
Question 6. Which one among the following is the correct option for the right relationship between CP and CV for one mole of an ideal gas?
- CV=RCP
- CP+CV=R
- CP– CV=R
- CP = RCV
Answer: 3. CP – Cv=R
CP = CV+ nR
For one mole of an ideal gas, CP= CV + R or CP – CV = R
NEET questions on Thermodynamics
Question 7. The correct option for free expansion of ideal gas under adiabatic conditions is
- q = 0, ΔT = 0 and w = 0
- q = 0, ΔT < 0 and w > 0
- q < 0, ΔT = 0 and w = 0
- q > 0, ΔT > 0 and w > 0
Answer: 1. q = 0, ΔT = 0 and w = 0
For the free expansion of an ideal gas, Pex = 0,
w=-Pex ΔV=0
For adiabatic process, q = 0
According to the first law of thermodynamics, ΔU=q+w=0
As the internal energy of an ideal gas is a function of temperature, ΔU=0,
∴ T =0
Question 8. Under isothermal conditions, a gas at 300 K expands from 0.1 L to 0.25 L against a constant external pressure of 2 bar. The work done by the gas is [Given that 1 L bar = 100 J]
- 30 J
- -30 J
- 5 kJ
- 25J
Answer: 2. -30 J
Under isothermal conditions, a gas at 300 K expands from 0.1 L to 0.25 L against a constant external pressure of 2 bar.
Expansion of a gas against a constant external pressure is an irreversible process. The work done in an irreversible process
= \(-P_{\text {ext }} \Delta \mathrm{V}=-P_{\text {ext }}\left(V_2-V_1\right)=-2(0.25-0.1)\)
= \(-2 \times 0.15 \mathrm{~L} \text { bar }=-0.30 \times 100 \mathrm{~J}=-30 \mathrm{~J}\)
NEET questions on Thermodynamics
Question 9. Reversible expansion of an ideal gas under isothermal and adiabatic conditions as shown in the
AB → Isothermal expansion
AC → Adiabatic expansion
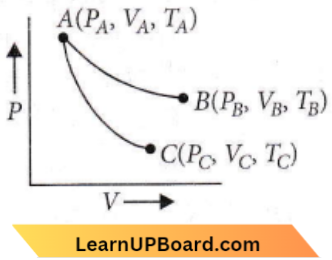
Which of the following options is not correct?
- \(\Delta S_{\text {isothermal }}>\Delta S_{\text {adiabatic }}\)
- \(T_A=T_B\)
- \(W_{\text {isothermal }}>W_{\text {adiabatic }}\)
- \(T_C>T_A\)
Answer: 4. \(T_C>T_A\)
For an ideal gas, internal energy is a function of temperature. The final temperature i.e., TC for adiabatic process is less than its initial temperature i.e., TA
∴ TC < TA
Question 10. An ideal gas expands isothermally from 10-3 m³ to 10-2 m³ at 300 K against a constant pressure of 105 N m-2. The work done on the gas is
- +270 kJ
- -900 J
- +900 kJ
- -900 kJ
Answer: 2. -900 J
An ideal gas expands isothermally from 10-3 m³ to 10-2 m³ at 300 K against a constant pressure of 105 N m-2.
w = \(-P d V=-P\left(V_2-V_1\right)\)
= \(-10^5 \mathrm{~N} \mathrm{~m}^{-2}\left(10^{-2}-10^{-3}\right) \mathrm{m}^3=-10^5 \mathrm{~N} \mathrm{~m}^{-2}\left(9 \times 10^{-3}\right) \mathrm{m}^3\)
= \(-9 \times 10^2 \mathrm{~N} \mathrm{~m}=-900 \mathrm{~J}\)
Thermodynamics multiple choice NEET
Question 11. A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy ΔU of the gas in joules will be
- -500 J
- -505 J
- +505 J
- 1136.25 J
Answer: 2. -505 J
A gas is allowed to expand in a well-insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L.
w = \(-P_{{ext}} \Delta V=-2.5(4.50-2.50)\)
= \(-5 \mathrm{~L} \text { atm }=-5 \times 101.325 \mathrm{~J}=-506.625 \mathrm{~J}\)
ΔU=q+w
As, the container is insulated, thus q=0
Hence, \(\Delta U=w=-506.625 \mathrm{~J}\)
Question 12. Equal volumes of two monatomic gases, A and B at the same temperature and pressure are mixed. The ratio of specific heat (CP/CV) of the mixture will be
- 0.83
- 1.50
- 3.3
- 1.67
Answer: 4. 1.67
Equal volumes of two monatomic gases, A and B at the same temperature and pressure are mixed.
CP for a monoatomic gas mixture of the same volume
= \(\frac{5}{2} R, C_V=\frac{3}{2} R\)
∴ \(\frac{C_P}{C_V}=\frac{\frac{5}{2} R}{\frac{3}{2} R}=\frac{5}{3}=1.67\)
Thermodynamics multiple choice NEET
Question 13. Which of the following is the correct option for free expansion of an ideal gas under adiabatic conditions?
- q=0, ΔT≠0, w = 0
- q≠0, ΔT= 0, w= 0
- q=0, ΔT=0, w=0
- q=0, ΔT<0, w≠0
Answer: 3. q=0, ΔT=0, w=0
For free expansion of an ideal gas under adiabatic condition q = 0, ΔT = 0, w = 0.
For free expansion, w = 0, adiabatic process, q = 0
ΔU = q + w = 0
Internal energy remains constant means ΔT = 0.
Question 14. Three moles of an ideal gas expanded spontaneously into the vacuum. The work done will be
- Infinite
- 3 Joules
- 9 Joules
- Zero.
Answer: 4. Zero.
Three moles of an ideal gas expanded spontaneously into the vacuum.
Since the ideal gas expands spontaneously into the vacuum, Pext = 0, hence work done is also zero.
Question 15. Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔE?
- \(2 \mathrm{CO}_{(\mathrm{g})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{CO}_{2(\mathrm{~g})}\)
- \(\mathrm{H}_{2(g)}+\mathrm{Br}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{HBr}_{(\mathrm{g})}\)
- \(\mathrm{C}_{(s)}+2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})} \rightarrow 2 \mathrm{H}_{2(\mathrm{~g})}+\mathrm{CO}_{2(\mathrm{~g})}\)
- \(\mathrm{PCl}_{5(g)} \rightarrow \mathrm{PCl}_{3(g)}+\mathrm{Cl}_{2(\mathrm{~g})}\)
Answer: 2. \(\mathrm{H}_{2(g)}+\mathrm{Br}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{HBr}_{(\mathrm{g})}\)
∴ \(\Delta H=\Delta E+\Delta n_g R T\)
For \(\mathrm{H}_{2(\mathrm{~g})}+\mathrm{Br}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{HBr}_{(\mathrm{g})}\)
∴ \(\Delta n_g=2-(1+1)=0 \text {. i.e. } \Delta H=\Delta E\)
NEET practice questions Thermodynamics
Question 16. The work done during the expansion of a gas from a volume of 4 dm³ to 6 dm³ against a constant external pressure of 3 atm is (1 L atm = 101.32 J)
- -6J
- -608J
- +304J
- -304J
Answer: 2. -608J
Work = -Pext x volume change = -3 x (6 – 4) x 101.32 = 6 x 101.32 = – 607.92 J = – 608 J
Question 17. For the reaction, \(\mathrm{C}_3 \mathrm{H}_{8(\mathrm{~g})}+5 \mathrm{O}_{2(g)} \rightarrow 3 \mathrm{CO}_{2(\mathrm{~g})}+4 \mathrm{H}_2 \mathrm{O}_{(l)}\) at constant temperature, ΔH – ΔE is
- +RT
- -3RT
- +3RT
- -RT
Answer: 2. -3RT
⇒ \(\mathrm{C}_3 \mathrm{H}_{g(g)}+5 \mathrm{O}_{2(g)} \rightarrow 3 \mathrm{CO}_{2(g)}+4 \mathrm{H}_2 \mathrm{O}_{(l)}\)
⇒ \(\Delta n_g=3-6=-3\)
∴ \(\Delta H=\Delta E+P \Delta V\) or \(\Delta H-\Delta E=P \Delta V\)
∴ \(\Delta H-\Delta E=\Delta n_g R T=-3 R T\)
Question 18. The molar heat capacity of water at constant pressure, C, is 75 J K-1 mol-1. When 1.0 kJ of heat is supplied to 100 g of water which is free to expand, the increase in temperature of water is
- 1.2 K
- 2.4 K
- 4.8 K
- 6.6 K
Answer: 2. 2.4 K
The molar heat capacity of water at constant pressure, C, is 75 J K-1 mol-1. When 1.0 kJ of heat is supplied to 100 g of water which is free to expand
Molar heat capacity = 75J K-1 mol-1
18 g of water = 1 mole = 75J K-1 mol-1
1 g of water = \(\frac{75}{18}\) J K-1
100 g ofwater= \(\frac{75}{18}\) x 100 J K-1
Q = \(m \cdot C \cdot \Delta T \text { or } 1000=100 \times \frac{75}{18} \times \Delta T\)
⇒ \(\Delta T=\frac{10 \times 18}{75}=2.4 \mathrm{~K}\)
NEET practice questions Thermodynamics
Question 19. When 1 mol of gas is heated at constant volume temperature is raised from 298 to 308 K. Heat supplied to the gas is 500 J. Then which statement is correct?
- q = w = 500 J, ΔE = 0
- q = ΔE = 500 J, w = 0
- q = w = 500 J, ΔE = 0
- ΔE = 0, q = w = -500 J
Answer: 2. q = ΔE = 500 J, w = 0
When 1 mol of gas is heated at constant volume temperature is raised from 298 to 308 K. Heat supplied to the gas is 500 J.
ΔH = ΔE + PΔV
When ΔV = 0; w = 0.
ΔH = ΔE + 0 or ΔH = ΔE
As ΔE = q + w, ΔE = q
In the present problem, ΔH = 500 J
ΔH= ΔE = 500 J, q = 500 J, w = 0
Question 20. For the reaction, \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}_{(l)}+3 \mathrm{O}_{2(g)} \rightarrow 2 \mathrm{CO}_{2(g)}+3 \mathrm{H}_2 \mathrm{O}_{(l)}\) which one is true?
- ΔH = ΔE – RT
- ΔH = ΔE + RT
- ΔH = ΔE + 2RT
- ΔH = ΔE – 2RT
Answer: 1. ΔH = ΔE – RT
ΔH = ΔE + PΔV also PV = nRT (ideal gas equation)
or PΔV = ΔngRT
Δng = Change in the number of gaseous moles
∴ ΔH= ΔE+ ΔngRT
⇒ Δng = 2 – 3 = -1
⇒ ΔH = ΔE – RT
Question 21. In an endothermic reaction, the value of ΔH is
- Negative
- Positive
- Zero
- Constant.
Answer: 2. Positive
In endothermic reactions, the energy of reactants is less than the energy of products. Thus, ER < EP.
ΔH = EP – ER = +ve
Chemistry MCQs Thermodynamics NEET
Question 22. One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres. The ΔE for this process is (R = 2cal mol-1 K-1)
- 1381.1 cal
- Zero
- 163.7 cal
- 9 L atm
Answer: 2. Zero
One mole of an ideal gas at 300 K is expanded isothermally from an initial volume of 1 litre to 10 litres.
Change in internal energy depends upon temperature. At constant temperature, the internal energy of the gas remains constant, so ΔE = 0.
Question 23. During isothermal expansion of an ideal gas, its
- Internal energy increases
- Enthalpy decreases
- Enthalpy remains unaffected
- Enthalpy reduces to zero.
Answer: 3. Enthalpy remains unaffected
During isothermal expansion of an ideal gas, ΔT = 0, ΔE = 0
∴ ΔH = 0
H = E + PV
ΔH = ΔE + Δ(PV) = ΔE + Δ(nRT)
∴ ΔH= ΔE + nRΔT = 0 + 0 = 0
Change in enthalpy is zero, which means its enthalpy remains the same or unaffected.
Question 24. For the reaction, \(\mathrm{N}_2+3 \mathrm{H}_2 \rightleftharpoons 2 \mathrm{NH}_3, \Delta H\)=?
- ΔE + 2RT
- ΔE – 2RT
- ΔH = RT
- ΔE – RT
Answer: 2. ΔE – 2RT
Δng =2 – 4= -2, ΔH = ΔE -2RT
Question 25. If ΔH is the change in enthalpy and ΔT, the change in internal energy accompanying a gaseous reaction, then
- ΔH is always greater than ΔT
- ΔH < ΔE only if the number of moles of the products is greater than the number of moles of the reactants
- ΔH is always less than ΔE
- ΔH < ΔE only if the number of moles of products is less than the number of moles of the reactant
Answer: 4. ΔH < ΔE only if the number of moles of products is less than the number of moles of the reactant
lf np < nr; Δng= np – nr= -ve.
Hence, ΔH < ΔE.
Chemistry MCQs Thermodynamics NEET
Question 26. Three thermochemical equations are given below:
- \(\mathrm{C}_{\text {(graphite) }}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{CO}_{2(g)} ; \Delta_{,} H^{\circ}=x \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(\mathrm{C}_{\text {(graphite) }}+\frac{1}{2} \mathrm{O}_{2(g)} \rightarrow \mathrm{CO}_{(g)} ; \Delta_r H^{\circ}=y \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(\mathrm{CO}_{(\mathrm{g})}+\frac{1}{2} \mathrm{O}_{2(g)} \rightarrow \mathrm{CO}_{2(g)} ; \Delta_{\mathrm{r}} H^{\circ}=z \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Based on the above equations, find out which of the relationships given below is correct.
- z = x + y
- x = y + z
- y = 2z – x
- x = y – z
Answer: 2. x = y + z
According to Hesst’s law, equation (1) is equal to equations (2) + (3) i.e., x = y + z
Question 27. The standard enthalpy of vaporisation ΔvapH° for water at 100°C is 40.66 kJ mol-1. The internal energy of the vaporisation of water at 100°C (in kJ mol-1) is
- +37.56
- -43.76
- +43.76
- +40.66
(Assume water vapour to behave like an ideal gas)
Answer: 1. +37.56
The standard enthalpy of vaporisation ΔvapH° for water at 100°C is 40.66 kJ mol-1.
∴ \(\Delta_{\text {vap }} H^{\circ}=40.66 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
T = \(100+273=373 \mathrm{~K}, \Delta E=?\)
∴ \(\Delta H=\Delta E+\Delta n_g R T \Rightarrow \Delta E=\Delta H-\Delta n_g R T\)
⇒ \(\Delta n_g\) = number of gaseous moles of products – number of gaseous moles of reactants
⇒ \(\mathrm{H}_2 \mathrm{O}_{(l)} \rightleftharpoons \mathrm{H}_2 \mathrm{O}_{(g)}\)
⇒ \(\Delta n_g=1-0=1\)
∴ \(\Delta E=\Delta H-R T\)
ΔE = (40.66 x 10³) – (8314 x 373)
= 37559 J/mol or 37.56 kJ/mol
Question 28. Consider the following processes:
⇒ \(\begin{array}{lc}
& \Delta \boldsymbol{H}(\mathbf{k J} / \mathbf{m o l}) \\
1 / 2 A \rightarrow B & +150 \\
3 B \rightarrow 2 C+D & -125 \\
E+A \rightarrow 2 D & +350
\end{array}\)
For \(B+D \rightarrow E+2 C, \Delta H\) will be
- \(525 \mathrm{~kJ} / \mathrm{mol}\)
- \(-175 \mathrm{~kJ} / \mathrm{mol}\)
- \(-325 \mathrm{~kJ} / \mathrm{mol}\)
- \(325 \mathrm{~kJ} / \mathrm{mol}\)
Answer: 2. \(-175 \mathrm{~kJ} / \mathrm{mol}\)
Adding all the equations, we get
⇒ \(\begin{array}{lc}
& \Delta H \\
A \rightarrow 2 B & 300 \mathrm{~kJ} / \mathrm{mol} \\
3 B \rightarrow 2 C+D & -125 \mathrm{~kJ} / \mathrm{mol} \\
2 D \rightarrow A+E & -350 \mathrm{~kJ} / \mathrm{mol} \\
\hline B+D \rightarrow E+2 C ; \Delta H=(300-125-350)=-175 \mathrm{~kJ} / \mathrm{mol}
\end{array}\)
Thermodynamics quiz for NEET
Question 29. The following two reactions are known
- \(\mathrm{Fe}_2 \mathrm{O}_{3(s)}+3 \mathrm{CO}_{(g)} \rightarrow 2 \mathrm{Fe}_{(s)}+3 \mathrm{CO}_{2(g)} ; \Delta H=-26.8 \mathrm{~kJ}\)
- \(\mathrm{FeO}_{(s)}+\mathrm{CO}_{(g)} \rightarrow \mathrm{Fe}_{(s)}+\mathrm{CO}_{2(g)} ; \Delta H=-16.5 \mathrm{~kJ}\)
The value of \(\Delta H\) for the following reaction \(\mathrm{Fe}_2 \mathrm{O}_{3(s)}+\mathrm{CO}_{(g)} \rightarrow 2 \mathrm{FeO}_{(s)}+\mathrm{CO}_{2(g)}\) is
- +10.3 kJ
- -43.3 kJ
- -10.3 kJ
- +6.2kJ
Answer: 4. +6.2kJ
⇒ \(\mathrm{Fe}_2 \mathrm{O}_{3(s)}+3 \mathrm{CO}_{(g)} \rightarrow 2 \mathrm{Fe}_{(s)}+3 \mathrm{CO}_{2(g)}\); \(\Delta H=-26.8 \mathrm{~kJ}\)…..(1)
⇒ \(\mathrm{FeO}_{(s)}+\mathrm{CO}_{(g)} \rightarrow \mathrm{Fe}_{(s)}+\mathrm{CO}_{2(g)} ; \Delta H=-16.5 \mathrm{~kJ}\)….(2)
⇒ \(\mathrm{Fe}_2 \mathrm{O}_{3(s)}+\mathrm{CO}_{(g)} \rightarrow 2 \mathrm{FeO}_{(s)}+\mathrm{CO}_{2(g)} ; \Delta H=?
\)…(3)
Equation (3) can be obtained as: (1) -2(2) = \(-26.8-2(-16.5)=-26.8+33.0=+6.2 \mathrm{~kJ}\)
Question 30. For which one of the following equations is \(\Delta H^{\circ} \text { reaction }\) equal to \(\Delta H^{\circ}{ }_f\) Affreaction equal to ïH°f, for the product?
- \(\mathrm{N}_{2(g)}+\mathrm{O}_{3(g)} \rightarrow \mathrm{N}_2 \mathrm{O}_{3(g)}\)
- \(\mathrm{CH}_{4(g)}+2 \mathrm{Cl}_{2(g)} \rightarrow \mathrm{CH}_2 \mathrm{Cl}_{2(b)}+2 \mathrm{HCl}_{(g)}\)
- \(\mathrm{Xe}_{(g)}+2 \mathrm{~F}_{2(g)} \rightarrow \mathrm{XeF}_{4(g)}\)
- \(2 \mathrm{CO}_{(\mathrm{g})}+\mathrm{O}_{2(g)} \rightarrow 2 \mathrm{CO}_{2(g)}\)
Answer: 3. \(\mathrm{Xe}_{(g)}+2 \mathrm{~F}_{2(g)} \rightarrow \mathrm{XeF}_{4(g)}\)
For (3), \(\Delta H_{\text {reaction }}^{\circ}\)
= \(\Delta H^{\circ}{ }_f\left(\mathrm{XeF}_4\right)-\left[\Delta H_f^{\circ}(\mathrm{Xe})+2 \Delta H^{\circ}{ }_f\left(\mathrm{~F}_2\right)\right]\)
Enthalpies of formation of elementary substances \(\mathrm{Xe}\) and \(\mathrm{F}_2\) are taken as zero.
Thus, \(\Delta H_{\text {reaction }}^{\circ}=\Delta H_f^{\circ}\left(\mathrm{XeF}_4\right)\)
Thermodynamics quiz for NEET
Question 31. Heat of combustion \(\Delta H\) for \(\mathrm{C}_{(s)}, \mathrm{H}_{2(g)}\) and \(\mathrm{CH}_{4(\mathrm{~g})}\) are -94,-68 and -213 \(\mathrm{kcal} / \mathrm{mol}\), then \(\Delta H\) for \(\mathrm{C}_{(s)}+2 \mathrm{H}_{2(g)} \rightarrow \mathrm{CH}_{4(g)}\) is
- -17 k cal
- -111 k cal
- -170 k cal
- -85 k cal
Answer: 1. -17 k cal
- \(\mathrm{C}_{(s)}+\mathrm{O}_{2(g)} \rightarrow \mathrm{CO}_{2(g)} ; \Delta H_{\mathrm{i}}=-94 \mathrm{kcal} / \mathrm{mole}\)
- \(2 \mathrm{H}_{2(\mathrm{gg})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{f})} ; \Delta H_{\mathrm{ii}}=-68 \times 2 \mathrm{kcal} / \mathrm{mole}\)
- \(\mathrm{CH}_{4(g)}+2 \mathrm{O}_{2(g)} \rightarrow \mathrm{CO}_{2(\mathrm{~g})}+2 \mathrm{H}_2 \mathrm{O}_{(l)} ; \Delta H_{\mathrm{iii}}=-213 \mathrm{kcal} / \mathrm{mole}\)
- \(\mathrm{C}_{(s)}+2 \mathrm{H}_{2(\mathrm{~g})} \rightarrow \mathrm{CH}_{4(\mathrm{~g}} ; \Delta H_{\mathrm{iv}}=\)?
By applying Hess’s law, we can compute \(\Delta H_{\mathrm{iv}}\).
∴ \(\Delta H_{\mathrm{iv}}=\Delta H_{\mathrm{1}}+\Delta H_{\mathrm{1}}-\Delta H_{\mathrm{3}}\)
= \((-94-68 \times 2+213) \mathrm{kcal}=-17 \mathrm{kcal}\)
Question 32. Change in enthalpy for reaction, \(2 \mathrm{H}_2 \mathrm{O}_{2(l)} \rightarrow 2 \mathrm{H}_2 \mathrm{O}_{(l)}+\mathrm{O}_{2(\mathrm{~g})}\) if the heat of formation of H2O2(l) and H2O(l) are -188 and -286 kJ/mol respectively, is
- -196 kJ/mol
- +196 kJ/mol
- +948 kJ/mol
- -948 kJ/mol
Answer: 1. -196 kJ/mol
∴ \(\Delta H_f^{\circ}=\Sigma H_{f \text { (products) }}^{\circ}-\Sigma H_{f \text { (reactants) }}^{\circ}\)
For the given reaction, \(2 \mathrm{H}_2 \mathrm{O}_{2(\mathrm{~J})} \rightarrow 2 \mathrm{H}_2 \mathrm{O}_{(\mathrm{J})}+\mathrm{O}_{2(\mathrm{~g})}\)
∴ \(\Delta H_f^{\circ}=2 \times \Delta H_{f\left(\mathrm{H}_2 \mathrm{O}\right)}^{\mathrm{o})}-2 \times \Delta H_{f\left(\mathrm{H}_2 \mathrm{O}_2\right)}^{\mathrm{o}}\)
= \(2 \times-286 \mathrm{~kJ} \mathrm{~mol}^{-1}-2 \times(-188) \mathrm{kJ} \mathrm{mol}^{-1}\)
= \(-196 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
NEET MCQs on Thermodynamics
Question 33. Enthalpy of \(\mathrm{CH}_4+1 / 2 \mathrm{O}_2 \rightarrow \mathrm{CH}_3 \mathrm{OH}\) is negative. If the enthalpy of combustion of CH4 and CH3OH are x and y respectively, then which relation is correct?
- x>y
- x<y
- x=y
- x≥y
Answer: 1. x>y
Enthalpy of \(\mathrm{CH}_4+1 / 2 \mathrm{O}_2 \rightarrow \mathrm{CH}_3 \mathrm{OH}\) is negative. If the enthalpy of combustion of CH4 and CH3OH are x and y respectively
⇒ \(\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}, \Delta H_1=-x\)…..(1)
⇒ \(\mathrm{CH}_3 \mathrm{OH}+\frac{3}{2} \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}, \Delta H_2=-y\)….(2)
Subtracting (2) from (1), we get \(\mathrm{CH}_4+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{CH}_3 \mathrm{OH}, \Delta H_3=- \text { ve }\)
i.e., \(-x-(-y)=-\mathrm{ve}\)
y – x = -ve. Hence, x>y.
Question 34. In the reaction \(\mathrm{S}+3 / 2 \mathrm{O}_2 \rightarrow \mathrm{SO}_3+2 x\) and \(\mathrm{SO}_2+1 / 2 \mathrm{O}_2 \rightarrow \mathrm{SO}_3+y\) kcal the heat of formation of SO2 is
- (2x + y)
- (x – y)
- (x + y)
- (2x – y)
Answer: 4. (2x – y)
⇒ \(\mathrm{S}+\frac{3}{2} \mathrm{O}_2 \rightarrow \mathrm{SO}_3+2 x \) kcal….(1)
⇒ \(\mathrm{SO}_2+\frac{1}{2} \mathrm{O}_2 \rightarrow \mathrm{SO}_3+y \) kcal….(2)
By subtracting equation (2) from (1) we get, \(\mathrm{S}+\mathrm{O}_2 \rightarrow \mathrm{SO}_2+(2 x-y)\) kcal
The heat of formation of \(\mathrm{SO}_2\) is \((2 x-y)\) kcal/mole.
NEET MCQs on Thermodynamics
Question 35. Given that \(\mathrm{C}+\mathrm{O}_2 \rightarrow \mathrm{CO}_2, \Delta H^{\circ}=-x \mathrm{~kJ}\); \(2 \mathrm{CO}+\mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2, \Delta H^{\circ}=-y \mathrm{~kJ}\) The enthalpy of formation of carbon monoxide will be
- \(\frac{y-2 x}{2}\)
- \(2 x-y\)
- \(y-2 x\)
- \(\frac{2 x-y}{2}\)
Answer: 1. \(\frac{y-2 x}{2}\)
⇒ \(\mathrm{C}_{(s)}+\mathrm{O}_{2(g)} \rightarrow \mathrm{CO}_{2(g)} ; \Delta H^{\circ}=-x \mathrm{~kJ}\)….(1)
⇒ \(\mathrm{CO}_{(g)}+\frac{1}{2} \mathrm{O}_{2(g)} \rightarrow \mathrm{CO}_{2(g)} ; \Delta H^{\circ}=\frac{-y}{2} \mathrm{~kJ}\)….(2)
By subtracting equation (2) from (1) we get, \(\mathrm{C}_{(s)}+\frac{1}{2} \mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{CO}_{(g)} ;\)
∴ \(\Delta H^{\circ}=-x-\left(-\frac{y}{2}\right)=\frac{y-2 x}{2} \mathrm{~kJ}\)
Question 36. If enthalpies of formation for C2H4(g), CO2(g) and H2O(l) at 25°C and 1 atm pressure are 52, -394 and -286 kJ/mol respectively, then enthalpy of combustion of C2H4(g) will be
- +141.2 kJ/mol
- +1412 kJ/mol
- -141.2 kJ/mol
- -1412 kJ/mol
Answer: 4. -1412 kJ/mol
⇒ \(\mathrm{C}_2 \mathrm{H}_4+3 \mathrm{O}_2 \rightarrow 2 \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}\)
∴ \(\Delta H^{\circ}=\Delta H_{\text {products }}^o-\Delta H_{\text {reactants }}^{\circ}\)
= \(2 \times(-394)+2 \times(-286)-(52+0)=-1412 \mathrm{~kJ} / \mathrm{mol}\)
Question 37. The bond dissociation energies of X2, Y2 and XY are in the ratio of 1: 0.5: 1. ΔH for the formation of XY is -200 kJ mol-1. The bond dissociation energy of X2 will be
- 200 kJ mol-1
- 100 kJ mol-1
- 800 kJ mol-1
- 400 kJ mol-1
Answer: 3. 800 kJ mol-1
The bond dissociation energies of X2, Y2 and XY are in the ratio of 1: 0.5: 1. ΔH for the formation of XY is -200 kJ mol-1.
Let B.E. of \(X_2, Y_2\) and \(X Y\) are \(x \mathrm{~kJ} \mathrm{~mol}^{-1}, 0.5 x \mathrm{~kJ} \mathrm{~mol}^{-1}\) and \(x \mathrm{~kJ} \mathrm{~mol}^{-1}\) respectively.
⇒ \(\frac{1}{2} X_2+\frac{1}{2} Y_2 \rightarrow X Y ; \Delta H=-200 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
⇒ \(\Delta H=\Sigma(B . E .)_{\text {Reactants }}-\Sigma(B . E .)_{\text {Products }}\)
∴ \(-200=\left[\frac{1}{2} \times(x)+\frac{1}{2} \times(0.5 x)\right]-[1 \times(x)]\)
B.E. of \(X_2=x=800 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
NEET MCQs on Thermodynamics
Question 38. The heat of combustion of carbon to CO2 is -393.5 kJ/mol. The heat released upon the formation of 35.2 g of CO2 from carbon and oxygen gas is
- +315 kJ
- -630 kJ
- -3.15 kJ
- 315 kJ
Answer: None
Given: \(\mathrm{C}_{(s)}+\mathrm{O}_{2(g)} \longrightarrow \mathrm{CO}_{2(g)}, \Delta H=-393.5 \mathrm{~kJ} / \mathrm{mol}\)
Amount of heat released on formation of 44 g CO2 = 393.5kJ
∴ Amount of heat released on formation of 35.2 g of CO2
= \(\frac{393.5}{44} \times 35.2=314.8 \approx 315 \mathrm{~kJ}\)
-ve or +ve sign considering the reaction is exothermic or endothermic.
Question 39. When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0ºC and 1 atmosphere, 16 litres of oxygen at the same temperature and pressure is consumed. The amount of heat released from this combustion in \(\mathrm{kJ}\left(\Delta H_{\text {comb }}\left(\mathrm{CH}_4\right)=890 \mathrm{~kJ} \mathrm{~mol}^{-1}\right.\), \(\left.\Delta H_{\text {comb }}\left(\mathrm{C}_3 \mathrm{H}_8\right)=2220 \mathrm{~kJ} \mathrm{~mol}^{-1}\right) is\)
- 38
- 317
- 477
- 32
Answer: 2. 317
When 5 litres of a gas mixture of methane and propane is perfectly combusted at 0ºC and 1 atmosphere, 16 litres of oxygen at the same temperature and pressure is consumed.
⇒ \(\mathrm{CH}_4+2 \mathrm{O}_2 \rightarrow \mathrm{CO}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{C}_3 \mathrm{H}_8+5 \mathrm{O}_2 \rightarrow 3 \mathrm{CO}_2+4 \mathrm{H}_2 \mathrm{O}\)
Number of moles in gaseous mixture \(\mathrm{CH}_4+\mathrm{C}_3 \mathrm{H}_8=\frac{5}{22.4}=0.22 \text { moles }\)
Number of moles of \(\mathrm{O}_2=\frac{16}{22.4}=0.71 \mathrm{moles}\)
Let x moles of CH4 be there in a gaseous mixture so, a number of moles of C3H8 would be 0.22 – x.
Then moles of O2 consumed, 2x + (0.22 – x)5 = 0.71 or x = 0.13
The total amount of heat liberated = 0.13 x 890 + 0.09 x 22210 = 315.5 J
Thermodynamics NEET question bank
Question 40. Enthalpy change for the reaction, 4 \(\mathrm{H}_{(g)} \rightarrow 2 \mathrm{H}_{2(g)} \text { is }-869.6 \mathrm{~kJ}\) The-dissociation energy of H – H bond is
- -434.8 kJ
- -869.6 kJ
- +434.8 kJ
- +217.4 kJ
Answer: 3. +434.8 kJ
The dissociation energy of H-H bond is \(\frac{869.6}{2}=434.8 \mathrm{~kJ}\)
Question 41. From the following bond energies:
- H – H bond energy: 431.37 k) mol-1
- C: C bond energy: 606.10 kJ mol-1
- C – C bond energy: 336.49 kJ mol-1
- C – H bond energy: 410.50 kJ mol-1
Enthalpy for the reaction, will be
- -243.6 kJ mol-1
- -120.0 kJ mol-1
- 553.0 kJ mol-1
- 1523.6 kJ mol-1
Answer: 2. -120.0 kJ mol-1
For the given reaction, the enthalpy of the reaction can be calculated as
= \(\Sigma B \cdot E_{.}(\text {reactants })-\Sigma B \cdot E .(\text { products) }\)
= \(\left[B \cdot E_{(\mathrm{C}=\mathrm{C})}+B \cdot E_{(\mathrm{H}-\mathrm{H})}+4 \times B_{\cdot} E_{(\mathrm{C}-\mathrm{H})}\right]\)
∴ \(-\left[B \cdot E_{\cdot(\mathrm{C}-\mathrm{C})}+6 \times B \cdot E_{\cdot(\mathrm{C}-\mathrm{H})}\right]\)
= \([606.10+431.37+4 \times 410.50]-[336.49+6 \times 410.50]\)
= \(2679.47-2799.49=-120.02 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Question 42. Bond dissociation enthalpy of H2, Cl2, and HCI are 434,242 and 431 kJ mol-1 respectively. Enthalpy of the formation of HCl is
- -93 kJ mol-1
- 245 kJ mol-1
- 93 kJ mol-1
- -245 kJ mol-1
Answer: 1. -93 kJ mol-1
⇒ \(\mathrm{H}_2+\mathrm{Cl}_2 \rightarrow 2 \mathrm{HCl}\)
⇒ \(\Delta H_{\text {reaction }}=\Sigma(B \cdot E)_{\text {reactants }}-\Sigma(B \cdot E)_{\text {products }}\)
= \(\left[(B \cdot E)_{\mathrm{H}-\mathrm{H}}+(B \cdot E)_{\mathrm{Cl}-\mathrm{Cl}}\right]-\left[2 B \cdot E_{(\mathrm{H}-\mathrm{Cl})}\right]\)
= \(434+242-(431) \times 2\)
∴ \(\Delta H_{\text {reaction }}=-186 \mathrm{~kJ}\)
Thermodynamics NEET question bank
Question 43. Consider the following reactions:
- \(\mathrm{H}_{(a q)}^{+}+\mathrm{OH}_{(a q)}^{-}=\mathrm{H}_2 \mathrm{O}_{(b)}, \Delta H=-X_1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(\mathrm{H}_{2(g)}+1 / 2 \mathrm{O}_{2(g)}=\mathrm{H}_2 \mathrm{O}_{(l)}, \Delta H=-X_2 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(\mathrm{CO}_{2(g)}+\mathrm{H}_{2(\mathrm{~g})}=\mathrm{CO}_{(g)}+\mathrm{H}_2 \mathrm{O}_{(l)}, \Delta H=-X_3 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(\mathrm{C}_2 \mathrm{H}_{2(g)}+5 / 2 \mathrm{O}_{2(g)}=2 \mathrm{CO}_{2(g)}+\mathrm{H}_2 \mathrm{O}_{(j)},\Delta H=+X_4 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Enthalpy of the formation of H2O(l) is
- \(+X_3 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(-X_4 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(+X_1 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
- \(-X_2 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Answer: 4. \(-X_2 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
The amount of heat absorbed or released when 1 mole of a substance is directly obtained from its constituent elements is called the heat of formation or enthalpy of formation.
Equation (1) represents the neutralisation reaction, (3) represents the hydrogenation reaction and (4) represents the combustion reaction.
Thus, enthalpy of formation of \(\mathrm{H}_2 \mathrm{O}_{(J)} \text {is }-X_2 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Question 44. Given that the bond energy of H – H and Cl – Cl are 430 kJ mol-1 and 240 kJ mol-1 respectively and ΔHf for HCl is -90 kJ mol-1, the bond enthalpy of HCl is
- 380 kJ mol-1
- 425 kJ mol-1
- 245 kJ mol-1
- 290 kJ mol-1
Answer: 2. 425 kJ mol-1
Given that the bond energy of H – H and Cl – Cl are 430 kJ mol-1 and 240 kJ mol-1 respectively and ΔHf for HCl is -90 kJ mol-1
∴ \(\frac{1}{2} \mathrm{H}_2+\frac{1}{2} \mathrm{Cl}_2 \rightarrow \mathrm{HCl}\)
ΔH = \(=\Sigma B \cdot E_{\text {(reactants) }}-\Sigma B \cdot E_{\text {(products) }}\)
= \(\frac{1}{2}\left[B \cdot E_{\left(\mathrm{H}_2\right)}+B \cdot E_{\cdot\left(\mathrm{Cl}_2\right)}\right]-B \cdot E_{(\mathrm{HCl})}=-90\)
∴ \(\frac{1}{2}(430+240)-B \cdot E_{\cdot(\mathrm{HCl})}=-90\)
∴ \(B \cdot E_{(\mathrm{HCl})}=\frac{1}{2}(430+240)+90=425 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Thermodynamics NEET question bank
Question 45. The absolute enthalpy of neutralisation of the reaction: \(]\mathrm{MgO}_{(s)}+2 \mathrm{HCl}_{(a q)} \rightarrow \mathrm{MgCl}_{2(a q)}+\mathrm{H}_2 \mathrm{O}_{(f)}\) will be
- -57.33 kJ mol-1
- Greater than -57.33 kJ mol-1
- Less than -57.33 kJ mol-1
- 57.33 kJ mol-1
Answer: 3. Less than -57.33 kJ mol-1
MgO is the oxide of a weak base and we know that the heat of neutralisation of 1 eq. of a strong acid with a strong base is -57.33 kJ/mol.
⇒ With a weak base, some heat is absorbed in the dissociation of the weak base.
⇒ The heat of neutralisation of weak base with strong acid will be less than -57.33kJ/mol
