NEET Biology Notes For Biomolecules Introduction
Biomolecules Introduction: Protoplasm is a complex mixture of both organic and inorganic compounds.
- Molecules found in the protoplasm of cells are called biomolecules.
- The collection of various types of molecules in a cell is called the cellular pool.
- The cellular pool consists of various types of biomolecules such as water, inorganic materials, and organic compounds.
- Small molecules of low molecular weight, simple molecular conformations, and higher solubilities are called macromolecules. These include minerals, water, amino acids, simple sugars, and nucleotides.
A List Of Representative Inorganic Constituents Of Living Tissues
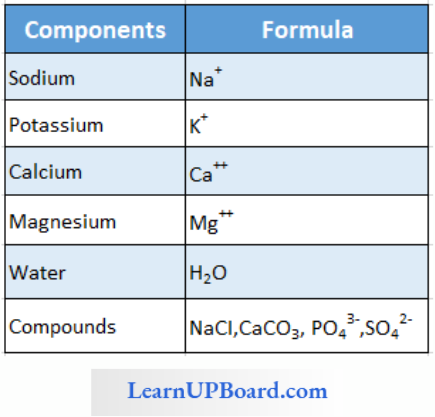
- Various minerals found in cells have many uses.
- Mitochondria are rich in manganese.
- Molybdenum is necessary for the fixation of nitrogen catalyzed by the enzyme nitrogenase.
- Copper occurs in cytochrome oxidase.
- Magnesium is essential for a large number of enzymes, particularly those utilizing ATP.
- Calcium and magnesium decrease the excitability of nerves and muscles.
- Sodium and potassium are responsible for the maintenance of extracellular and intracellular fluids through the osmotic effects of their concentrations. These two ions are also responsible for the maintenance of membrane potential and transmission of electrical impulses in the nerve cells. Both in cells and in extracellular fluids, diabasic phosphate (HPO42-) and monobasic phosphate (H2PO42-) act as acid-base buffers to maintain the W ion concentration.
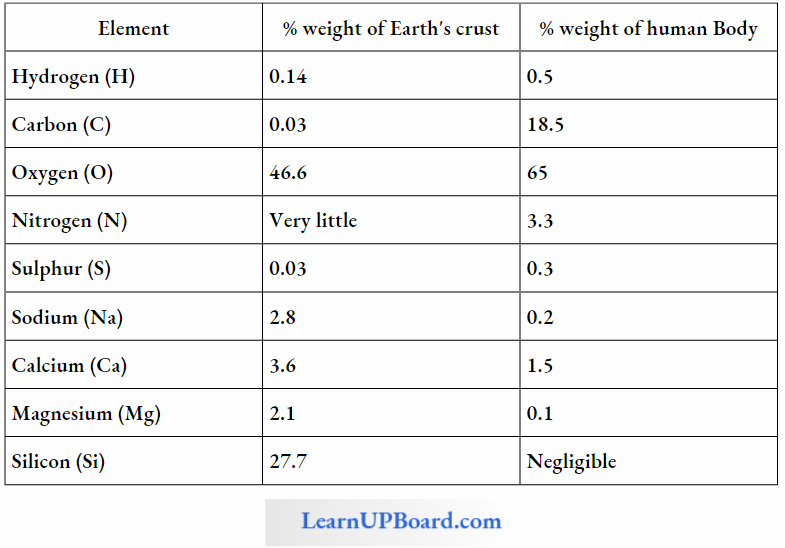
- The most abundant element in cell/living matter is oxygen (O > C > N > H ).
- Fe++ and Cu++ are found in cytochromes.
- The concentration of cations inside the cell is K > Na > Ca.
Read and Learn More NEET Biology Notes
A Comparison Of Elements Present In Non-Living And Living Matter
NEET Biology Notes For Biomolecules Methods To Analyze Chemical Composition
In order to study the various biomolecules found in living tissues (a vegetable, a piece of liver, etc.), the tissue is ground in trichloroacetic acid (Cl3CCOOH) using a pestle and mortar.
- The resultant slurry is strained through cheese cloth or cotton and we obtain two fractions.
- The filtrate is called an acid-soluble pool while the retentate is called an acid-insoluble fraction.
- The acid-soluble pool represents roughly the cytoplasmic composition.
- The macromolecules from the cytoplasm and organelles become the acid-insoluble fraction.
- Chemicals present in both fractions are further separated by various analytical techniques and identified.
Average Composition Of Cells
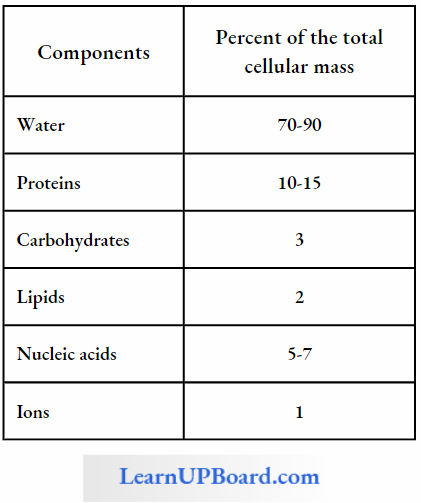
The acid-soluble pool contains chemicals called biomicromolecules as they have a small molecular mass of 18-800 daltons approximately.
biomolecules
- Biomacromolecules are large in size, high molecular weight, and complex molecules that are formed by the condensation of biomacromolecules.
- Their molecular mass is in the range of 10,000 daltons and above.
- Biomacromolecules are of three types—proteins, nucleic acids, and polysaccharides.
- Polymers occur in the form of threads. They are folded variously to form three-dimensional shapes required for their functioning.
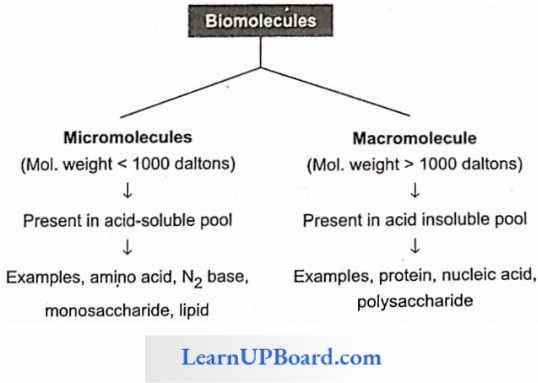
- Depending upon the molecular weight and solubility, biomolecules are divided into two categories.
- Micromolecules: These are small-sized, have low molecular weight, simple molecular structure, and high solubility in the intracellular fluid matrix. These include water, minerals, gases, carbohydrates, lipids, amino acids, and nucleotides.
- Macromolecules: These are large-sized, have larger molecular weight, complex conformation, and low solubility in the intracellular fluid matrix. They are generally formed by the polymerization of macromolecules. These include polysaccharides, proteins, and nucleic acids.
Analytical techniques, when applied to the compound, give us an idea of the molecular formula and the probable structure of the compound.
- All the carbon compounds that we get from living tissue can be called biomolecules. However, living organisms also have inorganic elements and compounds.
- When the tissue is fully burnt, all carbon compounds are oxidized to gaseous form (CO2, water vapor) and are removed.
- The residue is called ash. This ash contains inorganic elements such as calcium, magnesium, etc.
- Inorganic compounds such as sulfate, phosphate, etc., are also seen in the acid-soluble fraction. Therefore, elemental analysis gives the elemental composition of living tissues in the form of H, O, Cl, C, etc., while the analysis of compounds gives an idea of the kind of organic and inorganic constituents present in living tissues.
- From a chemistry point of view, one can identify functional groups such as aldehydes, ketones, aromatic compounds, etc. But from a biological point of view, we shall classify them into amino acids, nucleotide bases, fatty acids, etc.
NEET Biology Notes For Biomolecules Primary And Secondary Metabolites
The most exciting aspect of chemistry deals with isolating thousands of compounds, small and big, from living organisms, determining their structure and if possible, synthesizing them.
- If one were to make a list of biomolecules, such a list would have thousands of organic compounds including amino acids, sugars, etc. We can call these biomolecules as metabolites.
- In animal tissues, one notices the presence of all such categories of compounds. For example, proteins, carbohydrates, fats, amino acids, and nucleic acids.
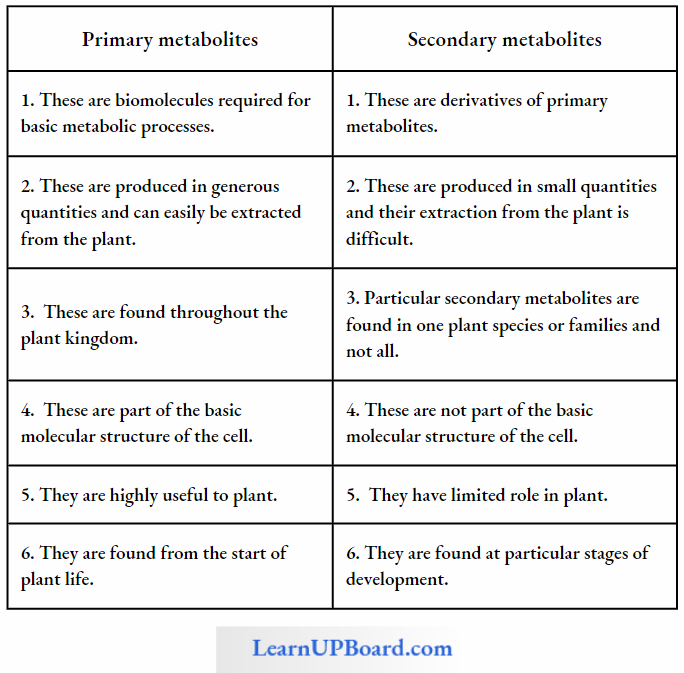
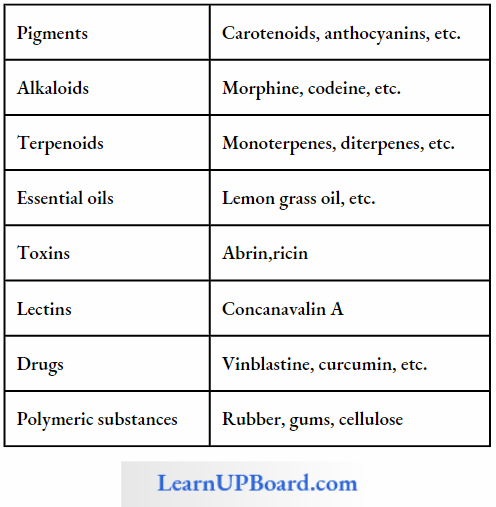
- These are called primary metabolites. However, when one analyzes plant, fungal, and microbial cells, one would see thousands of compounds other than these primary metabolites which are called secondary metabolites such as alkaloids, flavonoids, rubber, essential oils, antibiotics, colored pigments, scents, gums, and spices. Secondary metabolites are differentiated from primary metabolites in Table.
- Primary metabolites have identifiable functions and play known roles in normal physiological processes. Many of the secondary metabolites are useful to human welfare (for example, rubber, drugs, spices, scents, and pigments). Their physiological role is unknown.
- Some secondary metabolites have ecological importance too, which are mentioned in Table.
Difference Between Primary And Secondary Metabolites
Some Secondary Metabolites
NEET Biology Notes For Biomolecules Carbohydrates
Carbohydrates are mainly composed of carbon, hydrogen, and oxygen. Carbohydrates are so called because in most of them, the proportion of hydrogen and oxygen is the same as that in water (H2O), i.e., 2:1. These are also known as saccharides (compounds containing sugar).
- Carbohydrates are produced by green plants during photosynthesis. These constitute about 80% of the dry weight of plants.
- Carbohydrates Are Divided Into Three Main Classes: monosaccharides, oligosaccharides, and polysaccharides.
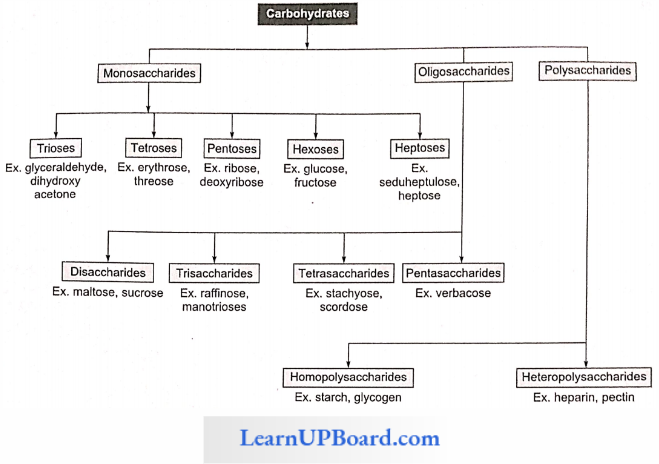
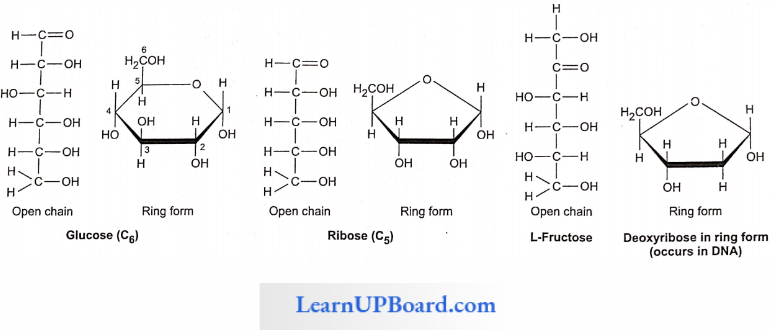
Monosaccharides: These are single saccharide units with CnH2nOn general formula which cannot be hydrolyzed further into smaller carbohydrates. These are composed of three to seven carbon atoms and are classified according to the number of C atoms as trioses (3C), tetroses (4C), pen-toses (5C), hexoses (6C), and heptoses (7C). Of these, pentoses and hexoses are the most common. Monosaccharides are important as energy sources and as building blocks for the synthesis of large molecules.
- All monosaccharides are either aldoses or ketoses. The simplest monosaccharides include trioses, for example, glyceraldehyde and dihydroxyacetone.
- Tetroses (for example, erythrose) are rare. Erythrose takes part in the synthesis of lignin and anthocyanin pigments.
- Ribose, ribulose, xylulose, and arabinose are pentoses. Xyluloses and arabinoses polymerize to form xylans and arabans which are cell wall materials.
- Glucose, fructose, mannose, and galactose are hexoses. These are white, sweet-tasting, crystalline, and extremely soluble in water.
- Glucose is called “universal sugar” and is also known as dextrose grape sugar or corn sugar.
Fructose is called fruit sugar and is also known as levulose. It is a naturally occurring sweetest sugar. Honey has two sugars: dextrose and levulose.
Heptoses have seven carbon atoms per molecule of sugar with the general formula C7H14O7, for example, sedoheptulose. It is an intermediate of respiratory and photosynthetic pathways.
- Pentoses and hexoses of monosaccharides occur in solid forms, i.e., open chain and ring form. There are two types of ring chains, i.e., pyranose ring, which has a hexagonal shape with five carbon atoms and one oxygen atom, and furanose ring, which has a pentagonal shape with four carbon atoms and one oxygen atom.
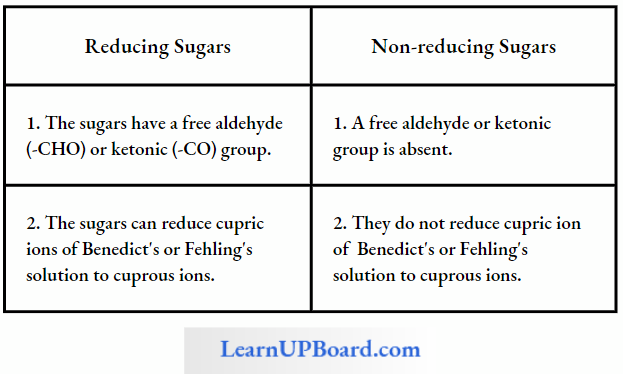
- Monosaccharides have a “tree” aldehyde or ketone group which can reduce Cu++ to Cu+ Hence, these are also called reducing sugars.
- Monosaccharides have two important chemical properties: first, sugars having a free aldehyde or ketone group can reduce Cu++ to Cu+ These are called reducing sugars. This property is the basis of Benedict’s test and Fehling’s test to detect the presence of glucose in urine.
- Second, the aldehyde or ketone group of monosaccharides can react and bind with an alcoholic group of another organic compound to join the two compounds together. This bond is called the glycosidic bond. This bond can be hydrolyzed to give the original reactants.
Differences Between Reducing And Non-Reducing Sugars
NEET Biology Notes For Biomolecules Carbohydrates Points To Remember
Derived Monosaccharides
- Deoxysugar: Loss of oxygen atom at the second carbon of ribose; yields deoxyribose, a constituent of DNA.
- Aminosugar: Monosaccharides having an amino group, for example, glucosamine, and galactosamine.
- Sugaracid: Examples, ascorbic acid, glucuronic acid, galacturonic acid.
- Sugaralcohol: Examples, glycerol, and mannitol (present in brown algae).
” biological molecules “
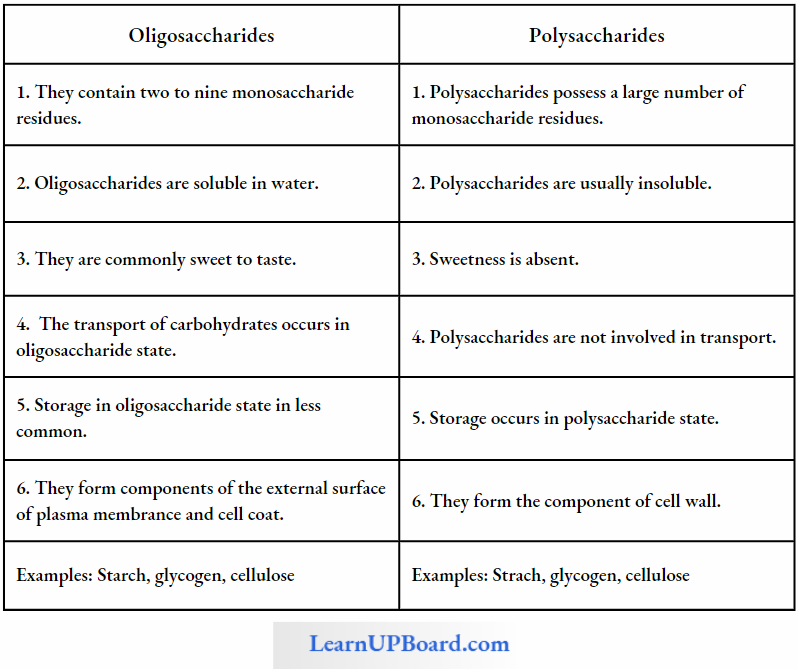
Oligosaccharides: They are the condensation product of two to nine monosaccharides. These include trisaccharides, tetrasaccharides, hexasaccharides, heptasaccharides, etc.
Disaccharides
- Disaccharides are formed by the condensation reactions between two monosaccharides (usually hexoses).
- The bond formed between two monosaccharides is called a glycosidic bond.
- It normally forms between C-atoms 1 and 4 of the neighboring units (1,4 bond).
- Once linked, the monosaccharide units are called residues.
- A molecule of sucrose is formed from a molecule each of glucose and fructose.
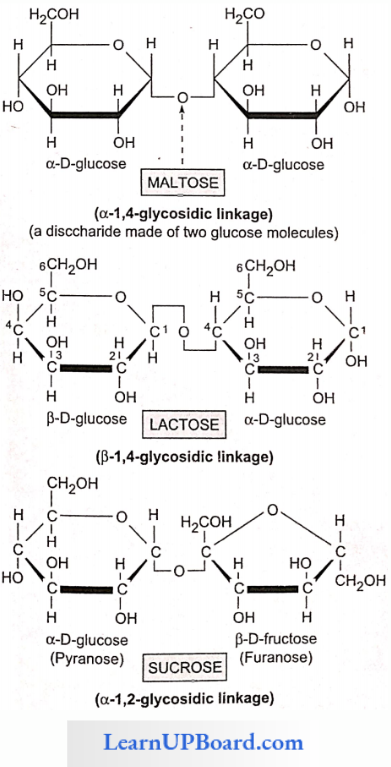
- Sucrose is the storage product of photosynthesis in sugarcane and sugarbeet.
- Lactose or milk sugar is found in human milk and cow’s milk.
- It is formed from one glucose molecule and one galactose molecule.
- Maltose or malt sugar is formed from two molecules of glucose during the germination of starchy seeds.
- Maltose and lactose are reducing disaccharides.
- Sucrose does not reduce Cu++ to Cu+. Hence, sucrose is a non-reducing sugar.
Trisaccharides
- Sugars composed of three monosaccharide units are called trisaccharides (for example, raffinose).
- Raffinose is a common trisaccharide found in plants.
- Upon hydrolysis, it yields one molecule each of glucose, fructose, and galactose.
- Larger oligosaccharides are attached to the cell membrane and enable cell-cell recognition due to their presence.
- Trisaccharides also take part in antigen specificity.
Polysaccharides
- These are polymers of monosaccharides and are branched or unbranched linear molecular chains.
- These are insoluble carbohydrates and are considered to be non-sugars.
- Starch, glycogen, cellulose, pectin, hemicellulose, and inulin are examples of polysaccharides.
- Body cells store carbohydrates as polysaccharides since these are easy to store and can be easily converted back into simple carbohydrates upon hydrolysis. These are in more condensed form and they have high molecular weight. These cannot pass through the plasma membrane.
Based on the types of structural components involved, polysaccharides are of two types:
- Hoinopolysaccharides: These consist of only one type of monosaccharide monomer for example starch, glycogen and cellulose, fructan, xylan, araban, and gnlactan.
- Heteropolysaccharides: These consist of more than one type of monosaccharide monomer for example chitin, agar, arabanogalactans, arabanoxylans etc.
- Based On Their Functions, Polysaccharides Are Of Three Main Types: storage (for example, starch and glycogen), structural (for example, chitin, cellulose), and mucopolysaccharides (for example, keratan sulfate, chondroitin sulfate, hyaluronic acid, agar, alginic acid, carrageenin. and heparin).
Storage Polysaccharides: Starch is found abundantly in rice, wheat, and other cereal grains and legumes, potato, tapioca, and bananas.
- It is formed during photosynthesis and serves as an energy-storing material.
- Glycogen found in the liver and muscles stores energy in mammals.
- Storing carbohydrates in the form of polysaccharides has two advantages: first, during their formation, many molecules of water are removed from monosaccharides. This helps in condensing the bulk to be stored. Second, unlike small carbohydrates, polysaccharides are relatively easy to store.
- When necessary, polysaccharides are broken down by enzymes for the release of energy.
Starch
- Starch is a polymer of a-D-glucose. It is the major reserve food in plants.
- Starch has two components: amylose (unbranched polymer) and amylopectin (branched polymer).
- Amylopectin: Consists of 2000-200,000 glucose molecules forming a straight chain and shows branching (after 25 glucose units). The branching point has a-1,6 glycosidic linkages.
- Amylose: Consists of a-1,4 glycosidic linkages between a-d glucose molecules. It is a straight chain of 200-1000 glucose units. Starch forms helical secondary structures; each turn consists of six glucose units.
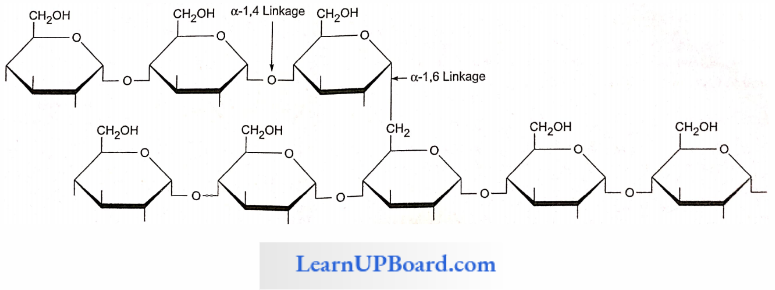
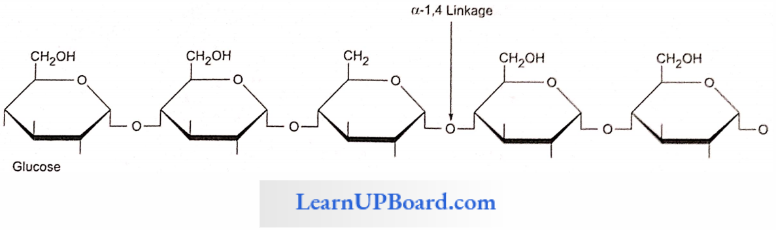
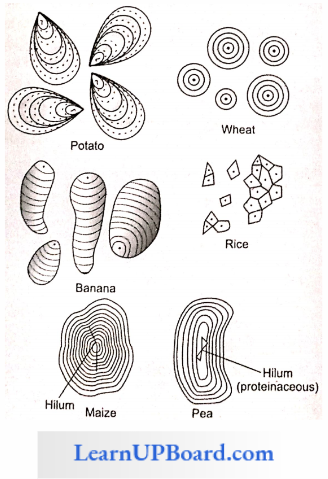
Starch Points To Remember: Starch molecules accumulate in the form of layers (stratifications) around a shifting organic center (hilum) to form starch grains.
- Hilum is made up of protein. In eccentric starch grains, hilum lies on one side. These are found in potatoes.
- In concentric starch grains, hilum is present in the center. These are found in wheat, maize, and pea.
- Dumb-bell-shaped starch grains are found in the latex of Euphorbia.
- Starch grains with a single hilum are called simple (for example, maize) but those with more than one hilum are called compound (for example, potato, rice).
- Starch turns blue with iodine as the helices in starch hold I2.
Glycogen: Glycogen is the animal equivalent of starch; many fungi also stoic it. Glycogen turns red-violet with iodine. It consists of 30,000 glucose units joined by α-1.4 bonds, much more branched than starch. Each branch point has α-1,6 linkages and branching occurs after 10-14 glucose units.
Inulin: It is an unusual polysaccharide and a polymer of fructose. It is stored particularly in roots and tubers of the family Compositae, for example, Dahlia tubers.
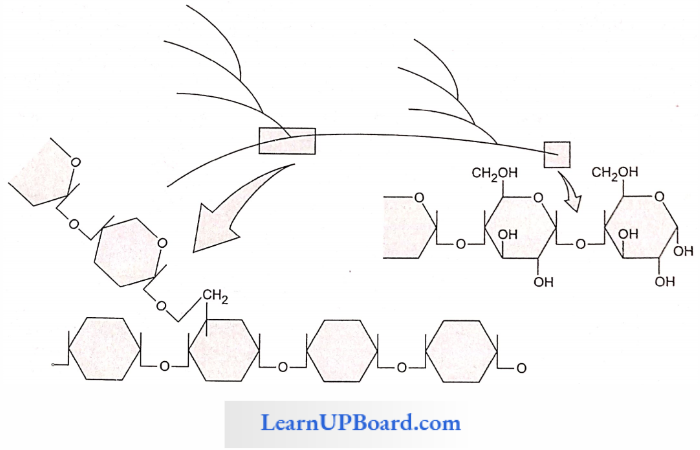
Structural Polysaccharides (Cellulose) (Hexosan Polysaccharide)
- Cellulose is the main structural unbranched homopolysaccharide of plants.
- One molecule of cellulose has about 6000 β-glucose residues.
- Cotton fibers contain the largest amount (90%) of cellulose among natural materials.
- Wood contains between 25 and 50% cellulose, the rest being hemicellulose and lignin.
- Fibers of cotton, linen, and jute are used for textiles and ropes.
- The artificial fiber rayon is manufactured by dissolving cellulosic materials in alkali and by extruding and coagulating the filaments.
- By treatment with other chemicals, cellulose is converted into cellulose acetate (used in fabrics, cellulosic plastics, and shatter-proof glass), cellulose nitrate (used in propellant explosives), and carboxymethyl cellulose (added to ice creams, cosmetics, and medicines to emulsify and give a smooth texture).
“intro to biomolecules “
Cellulose can be hydrolyzed to soluble sugars. Microbes can then convert these sugars to form ethanol, butanol, acetone, methane, and other useful chemicals.
- Cellulose is an unbranched homopolysaccharide of β-glucose.
- Cellulose is the most abundant carbohydrate in the biosphere.
- Cellulose is produced by plants and is used for building cell walls. It is also the most abundant organic compound in the biosphere.
- Wood and cotton contain large quantities of cellulose.
- Chitin is a polysaccharide found in the exoskeleton of insects, crabs, and prawns.
- Chitin is similar to cellulose in many ways except that its basic unit is not glucose, but a similar molecule that contains nitrogen (N-acetylglucosamine).
- Although chitin is soft and leathery, it becomes hard when impregnated with calcium carbonate or certain proteins.
- The insolubility of these polysaccharides in water helps to retain the form and strengthens the structure of organisms.
- Pectin and hemicelluloses are structural polysaccharides.
- Middle lamella which binds the cells together is composed of calcium pectate.
- Due to calcium pectinate, the water absorption capacity of the cell wall is increased.
- Fruit walls contain a high percentage of pectin.
- During ripening, pectin breaks down into simple sugars resulting in the sweetening and loosening of fruits.
- Hemicellulose is a mixture of n-xylose linked by β-1,4 glycosidic bond.
- Xylans, Arabians, and Galatians are hemicelluloses. Food such as dates have hemicellulose as the reserve food.
Mucopolysaccharides
- The slimy substances produced by plants are called mucilages.
- When you soak the seeds of isabgol (Phuuago ovate) or cut the fruit of okra (bhindi), you will notice the presence of a slimy substance.
- Mucilages are polysaccharides formed from galactose and mannose.
- Many seaweeds yield mucilages of commercial value such as agar, alginic acid, and carrageenin.
- Mucopolysaccharides are found in the cell walls of bacteria and in the connective tissues of animals as well as in body fluids.
- These bind proteins in cell walls and connective tissue and water in interstitial spaces thereby providing lubrication in ligaments and tendons.
- The vitreous humor of the eye and synovial fluid also contain mucopolysaccharides.
- Hyaluronic acid is found in connective tissue and in cell walls.
- Keratin sulfate and chondroitin sulfate occur in cartilage, cornea, and skin, and impart strength and flexibility to them.
- Keratin sulfate consists of acctylglucosaminc, galactose, and sulphuric acid. It provides strength and flexibility to the skin and cornea.
- Hyaluronic acid consists of d-glucuronic acid or iduronic acid and n-acetyl glucosamine, present in the vitreous humor of the eye, synovial fluid, cerebrospinal fluid, etc.
- Heparin is a polymer of α-1,4 glucosamine and glucuronic acid.
- It is an anticoagulant present in human blood.
- The husk of Plantago ovata and the mucilage of Aloe barbadensis are medicinally used.
- Agar, alginic acid, and carrageenin are obtained from marine algae.
- Artificial silk is a polysaccharide prepared from rayon.
“biomolecule definition “
Differences Between Oligosaccharides And Polysaccharides
NEET Biology Notes For Biomolecules Amino Acids
Amino acids are small molecules made of carbon, hydrogen, oxygen, nitrogen, and in some cases, sulfur.
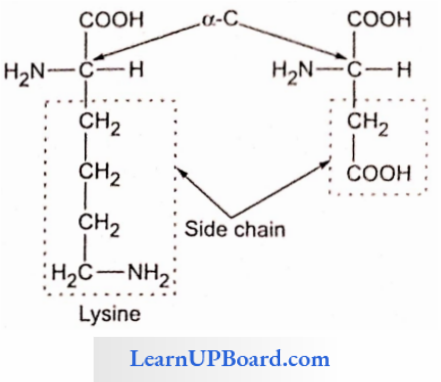
- Each amino acid has a free amino group, a free carboxyl group, and R as the side chain as the same substituents on the same carbon atom.
- The amino group lends basic character while the carboxylic group lends basic character to the molecule
- Lysine and arginine are basic amino acids because they carry two amino groups and one carboxylic group.
- Glutamic acid (glutamate) and aspartic acid (aspartate) contain one amino and two carboxyl groups each and are classified as acidic amino acids.
- Alanine, glycine, and valine are neutral amino acids as these contain one amino and one carboxyl group each.
- There are 20 different amino acids coded by our DMA that differ in the side chain.
- Most amino acids are laevorotatory while glycine is optically inactive.
- There are three important non-protein amino acids.
- They are ornithine, citrulline (both are involved in the ornithine cycle to synthesize urea), and diaminopimelic acid.
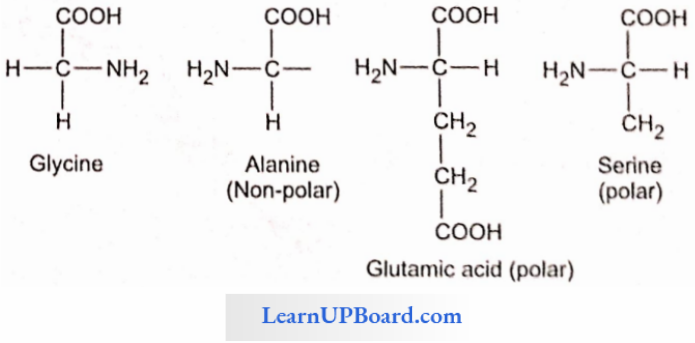
- A particular property of amino acids is the in/able nature of -NH2 and -COOH groups. Hence, in the solutions of different pH, the structure of amino acids changes.
- There are two types of amino acids, viz., essential and non-essential amino acids.
- There are seven essential amino acids in animals whereas eight essential amino acids in man.
- Threonine is an additional essential amino acid in human beings.
- Two amino acids, viz., arginine and histidine, are semi-indispensable amino acids as they can be synthesized by human beings but very slowly.
biomolecules functions
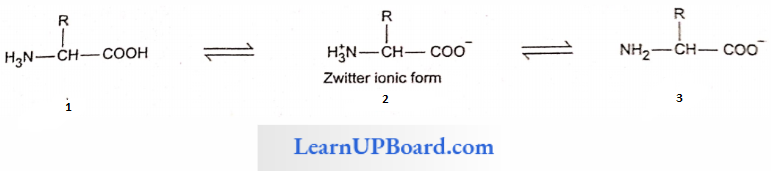
Differences Between Essential And Non-Essential Amino Acids
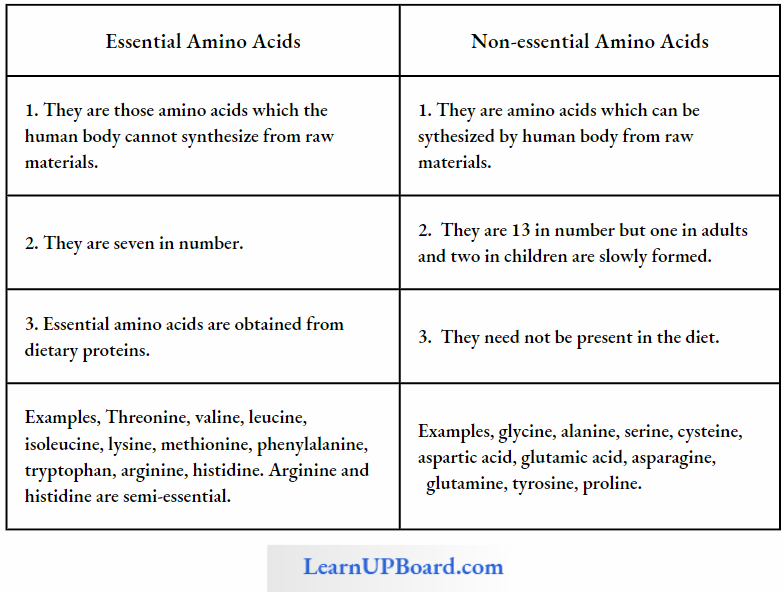
NEET Biology Notes For Biomolecules Amino Acids Points To Remembers
Amino acids are classified into the following groups:
- Neutral Amino Acid: With one -NH2 and one -COOH group, for example, glycine, and alanine (non-polar).
- Acidic Amino Acid: Have an extra -COOH group (mono-amino dicarboxylic), for example, glutamic and aspartic acid.
- Basic Amino Acid: Have additional NH2 group (diamino monocarboxylic), for example, arginine, and lysine.
- Sulfur-Containing Amino Acid: Have sulfur, for example, cysteine, cystine, and methionine.
- Alcoholic Amino Acid: Have —OH group, for example, serine, and threonine.
- Aromatic Amino Acid: Has cyclic structure having a side chain with -COOH and -NH2 groups, for example, phenylalanine, tryptophan, and tyrosine.
- Heterocyclic Amino Acid: N is present in the ring. for example. proline, histidine, hydroxyproline.
- Semi-Essential Amino Acid: Arginine and histidine are semi-essential amino acids required by children.
Protein amino acids are levorotatory and α-type except glycine. Glycine: simplest amino acid, involved in the formation of heme.
Functions Of Amino Acids
- Besides their principal function as building blocks lor proteins, specific amino acids are also converted into different types of biologically active compounds.
- For example, tyrosine is converted into the hormones thyroxin and adrenaline, as well as the skin pigment melanin. Glycine is involved in the formation of heme and tryptophan in the formation of the vitamin nicotinamide as well as the plant hormone indole-3-acetic acid.
- After the removal of the amino group, the carbon chain of many amino acids is converted into glucose.
- On losing the carboxyl groups as carbon dioxide, amino acids form biologically active amines such as histamine. Histamine is required for the functioning of muscles, blood capillaries, and gastric juices.
- Ornithine and citrulline are components of the urea cycle.
- Antibiotics contain non-protein amino acids.
- Amino acids form organic acids which form glucose by gluconeogenesis.
- Lysine is an essential amino acid because it is not formed in the body and has to be provided through diet.
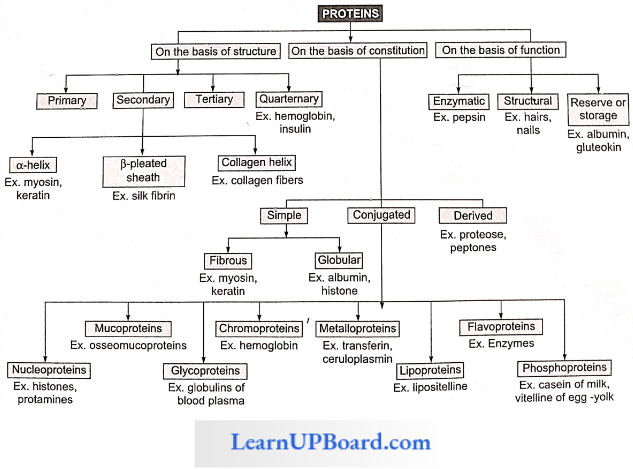
NEET Biology Notes For Biomolecules Proteins
Berzelius coined the term protein. Proteins are heteropolymers of amino acids. Two amino acids can join through the amino group of one and the carboxylic group of the other amino acid forming an anhydrous bond (CO-NH linkage) also known as a peptide bond by the loss of water molecule.
- A protein is a heteropolymer and not a homopolymer.
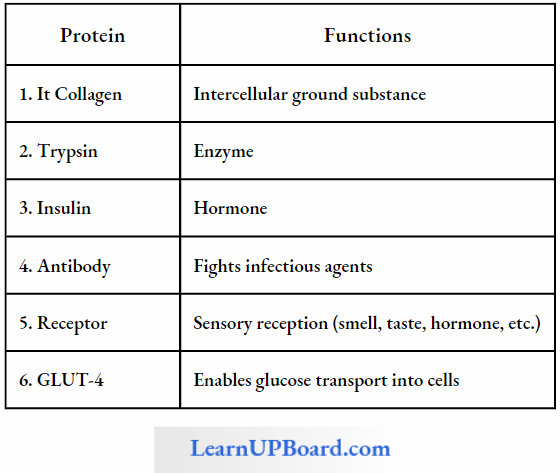
- Collagen is the most abundant protein in the animal world and RuBisCO (ribulose biphosphate. carboxylase oxygenase) is the most abundant protein in the whole biosphere.
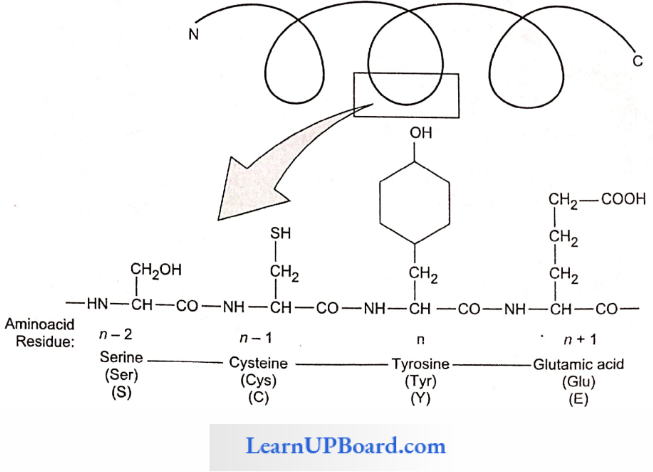
- N and C refer to the two termini of every protein. Single-letter codes and three-letter abbreviations of amino acids are also indicated.
Structure Of Proteins: The four levels of protein structure are
1. Primary Structure: The sequence of amino acids in the polypeptide chain gives the protein its primary structure.
- The primary structure is important as it determines the specificity of a protein but does not make a protein functional.
- To be functional, the protein must have a particular three-dimensional structure (conformation).
- A functional protein contains one or more polypeptide chains.
- The sequence of amino acids in the chain determines where the chain will bend or fold and where the various lengths will be attracted to each other.
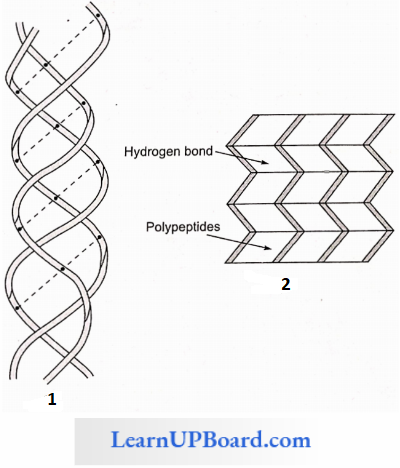
2. Secondary Structure: Through the formation of hydrogen bonds, peptide chains assume a secondary structure.
- When a chain is arranged like a coil, it is called an a-Helix.
- When two or more chains are joined together by intermolecular hydrogen bonds, the structure is called a pleated sheet.
- Helical structure is found in the keratin of hair and pleated structure is found in silk fibers.
- Each protein has a specific secondary structure also.
- It generally takes the form of an extended spiral spring, the a-helix, whose structure is maintained by many hydrogen bonds that are formed between adjacent -CO and -NH groups. The H-atom of the NH group of one amino acid is bonded to the O-atom of the CO group of three amino acids away. A protein which is entirely helical is keratin.
- The other type of secondary structure is called a β-pleated sheet. Here, two or more chains are joined together by intermolecular hydrogen bonds as in silk fibers.
- A special secondary structure is observed in collagen or tropocollagen helix which has three strands or polypeptides coiled around one another. The coil is strengthened by the establishment of a hydrogen bond between the -NH group of the glycine residue of each strand with the -CO group of the other two strands. The locking effect is due to proline and hydroxyproline.
“biomolecules carbohydrates “
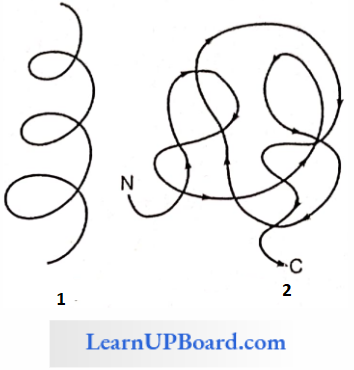
3. Tertiary Structure: Usually, the polypeptide chain bends and folds extensively and forms a compact “globular” shape to obtain functional conformation. This is termed as the tertiary structure.
- In a large protein such as hemoglobin, or in the case of an enzyme, the molecule undergoes further folding and coiling to attain functional conformation.
- The coils and folds of the protein molecule are so arranged as to hide non-polar amino acid side chains inside and expose the polar side chains.
- The three-dimensional conformation of a protein brings distant amino acid side chains closer.
- The active sites of proteins such as enzymes are thus formed.
- The conformation of proteins is easily changed by pH, temperature, and chemical substances, and hence the function of proteins is liable and subject to regulation.
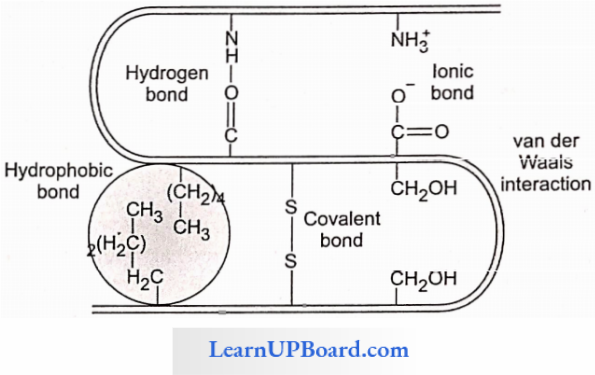
4. Quarternary Structure: Many complex proteins consist of an aggregation of polypeptide chains held together by hydrophobic interactions and hydrogen and ionic bonds.
- Their precise arrangement constitutes the quaternary structure.
- In aqueous media, proteins carry both cationic and anionic groups on the same molecule.
- The ionic state of the protein depends on the pH of the medium.
- A protein-rich in basic amino acids such as lysine and arginine exists as a cation and behaves as a base at the physiological pH of 7.4 (basic protein), for example, histones of nucleoproteins.
- Similarly, a protein with acidic amino acids exists as an anion and behaves as an acid, for example, most blood proteins (acidic proteins).
Types Of Proteins: On the basis of constitution, proteins are classified as simple, conjugated, and derived.
- Simple Proteins
- Simple proteins me composed of amino acids only.
- Some are small, globular molecules mostly soluble in water and not coagulated by heat (for example, histones).
- As the size of the protein molecule increases, it becomes less soluble and its heat coagulability increases.
- For example, larger globular proteins (such as egg albumin, scrum globulins, and glutelins of wheat or rice) are coagulated by heat.
- Fibrous proteins have long molecules and are insoluble in water (for example, keratin of skin and hair, and collagen of connective tissues).
- Conjugated Proteins
- Conjugated proteins are formed by binding of a simple protein with a non-protein called a prosthetic group, for example, nucleoproteins have nucleic acids as their prosthetic group.
- The conjugated proteins are of the following types
- Nucleoproteins: Prosthetic group is a nucleic acid, for example, protamines.
- Metalloproteins: The prosthetic group is a metal, for example, hemoglobin.
- Chromoproteins: Prosthetic group is a pigment, for example, cytochromes.
- Phosphoprotcins: Prosthetic group is a phosphoric acid, for example. casein of milk.
- Lipoproteins: Prosthetic group is lipids, for example, chylomicrons, HDL. LDL, etc.
- Glycoproteins: Prosthetic group is carbohydrates, for example, mucins.
- Glycoproteins (and glycolipids) play an important role in cell recognition.
The specificity of this recognition depends upon the particular sequence of sugars in carbohydrate portions. Ribulose bisphosphate carboxylase (an enzyme) present in large amounts in chloroplast stroma is the world’s most common protein.
- Storage proteins include albumin of egg and those that occur in seeds (glutelin of wheat). Prolamines are storage proteins.
- Protamines are basic proteins associated with the DNA of chromosomes, they are rich in lysine and arginine.
- Keratin and fibroin form protective structures.
- Antibodies arc defense proteins.
- Snake venom, ricin of castor, and bacterial toxins are proteinaceous in nature.
- Actin and myosin are essential for muscle contraction.
- Microtubules have tubulin protein.
- Hemoglobin and myoglobin are transport proteins.
Ovalbumin and glutelin are storage proteins present in cereals. Ferretin is an iron-storing protein of animal tissues. The types of prolamines and glutelins found in wheat are gliadin and glutenin.
- Insulin and parathormone are proteinaceous hormones.
- Fibrinogen and thrombin are blood-clotting proteins.
- Rhodopsin and iodopsin are photoreceptor pigments. These are present in rods and cones of the retina and are proteins.
- Proteins having all essential amino acids are called first-class proteins.
- Monellin, a protein, is the sweetest chemical obtained from an African berry.
- Cheese is a denatured protein.
- Resilin is a perfectly elastic protein found in the wings of some insects.
Some Proteins And Their Functions
NEET Biology Notes For Biomolecules Lipids
Lipids are made of carbon, hydrogen, and little oxygen. The number of oxygen atoms in a lipid molecule is always less as compared to the number of carbon atoms.
- Sometimes small amounts of phosphorus, nitrogen, and sulfur are also present.
- Lipids are insoluble in water, but soluble in non-polar solvents such as chloroform and benzene. Lipids contain fatty acids which may be saturated or unsaturated.
- Fatty acids are organic acids with a hydrocarbon chain ending in a carboxyl group (COOH).
Fatty acids are called saturated if they do not have any double bonds between the carbons of the molecular chain, for example, palmitic acid (16 C) and stearic acid (18 C).
CH3(CH2)14COOH Palmitic acid
CH3(CH2)16COOH Stearic acid
The general formula of saturated fatty acids is CnH2nO2.
- Their melting point is high.
- Unsaturated fatty acids have one or more double bonds between the carbons of the chain.
- The 18-C unsaturated fatty acids oleic, linoleic, and linolenic acids have 1, 2, and 3 double bonds, respectively.
CH3(CH2)7CH = CH(CH2)7COOH Oleic acid
The general formula of unsaturated fatty acids is CnH2n-2xO2 (where,v = number of double bonds). The arachidonic fatty acid has four double bonds.
Differences Between Saturated Fally Acids And Unsaturated Fatty Acids
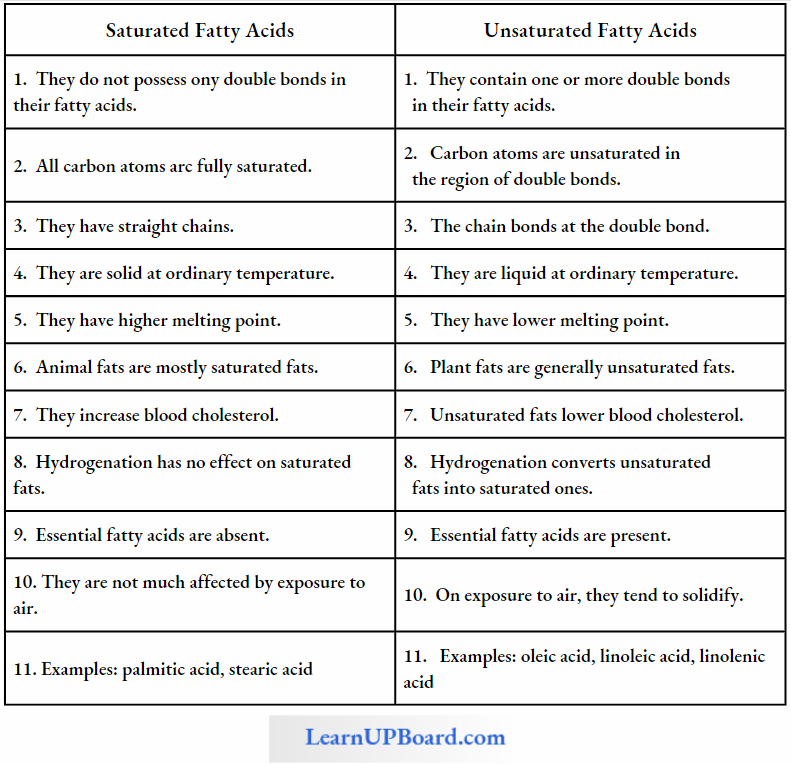
They have a bend at each double bond which keeps them in liquid form at ordinary temperature. They are called polyunsaturated fatty acids (PUFA) when they have more than one double bond in them.
- They are also called drying oils because they have a tendency to solidify on exposure.
- Oils of groundnut, mustard seed, sesame seed, and sunflower are rich in unsaturated fatty acids.
- The unsaturated fatty acids have lower melting points than those of saturated fatty acids.
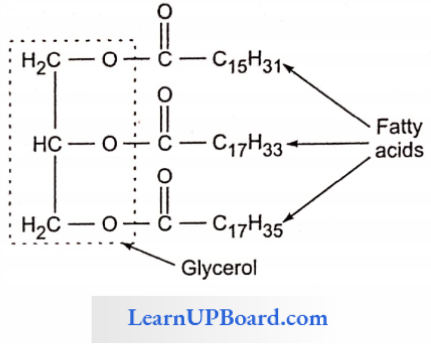
- In lipids, fatty acids are usually in the form of esters.
- Just as acids and bases react to form salts, similarly organic acids react with alcohol to form esters. Here alcohol is glycerol.
- Plants can synthesize all fatty acids.
- Animals cannot synthesize linoleic, linolenic, and arachidonic acid.
- These are called essential fatty acids. Their deficiency causes sterility, kidney failure, and stunted growth. The classification of lipids is given in detail
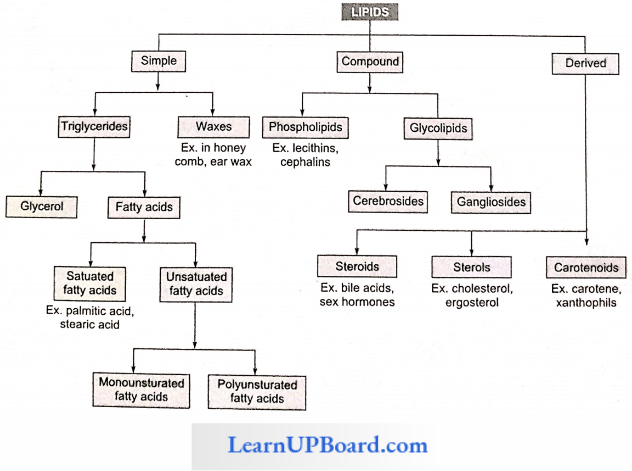
1. Simple Lipids: Simple lipids are esters of fatty acids with alcohol. For example, fats. oils, and waves.
- The simplest alcohol in fats is glycerol (trihydroxypropane).
- Triglycerides are common in nature.
- Fats are esters of fatty acids with glycerol (glycerine).
- Each molecule of glycerol can react with three molecules of fatty acids.
- Depending on the number of fatty acids that are attached to the glycerol molecule, the esters are called mono-, di-. or triglycerides.
- Fats that are generally liquid at room temperature are called oils.
- Oils are rich in unsaturated fatty acids and consequently have low melting points.
- On hydrogenation, the unsaturated fatty acids become saturated and the oil becomes a solid fat (vanaspati and margarine).
- Waxes are another class of simple lipids. They are formed by the combination of a long-chain fatty acid with a long-chain alcohol. Waxes play an important role in protection. They form water-insoluble coatings on the hair and skin of animals and stems, leaves, and fruits of plants.
Beeswax is formed from palmitic acid (C16H32O2) and medical alcohol (C30H61O2). Bee wax is also called as Hexacosyl palmitate. secreted by worker bees. Lanolin (wool fat), forms a waterproof coat around the animal fur.
- Bacteria that cause tuberculosis and leprosy produce a wax (wax-D) that contributes to their pathogenicity.
- Cutin is formed by the cross etherification and polymerization of hydroxy fatty acids and other fatty acids without esterification by alcohols other than glycerol. Cuticle has 50-90% cutin.
- Suberin is the condensation product of glycerol and phellonic acid. It makes the cell wall impermeable to water.
2. Compound Lipids: These lipids contain an additional group along with fatty acids and alcohols, for example, phospholipids, glycolipids, lipoproteins, and chromolipids.
Phospholipids
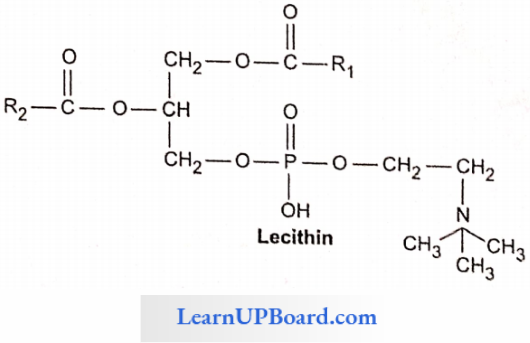
- These are straight-chain compounds of glycerol, fatty acids, and phosphoric acid.
- In these, only two fatty acids are attached to the glycerol molecule, and the third hydroxyl group of glycerol is esterified to phosphoric acid instead of fatty acid.
- Depending upon the type of phospholipid, this phosphate is also bound to a second alcohol molecule which can be choline, cthanolamine, inositol, or serine.
- Common phospholipids are lecithin and cephalin.
- Phospholipids are amphipathic molecules having hydrophilic (water-loving) polar regions and hydrophobic (water-repelling) non-polar regions.
- They are the basic constituents of biomembranes.
- Many phospholipids arrange themselves in a double-layered membrane in aqueous media (lipid bilayer).
- Cephalin is found in the brain and acts as an insulation material for nerves and also participates in blood coagulation.
- Lecithin takes part in cell permeability, osmotic tension, and surface conditioning of cells.
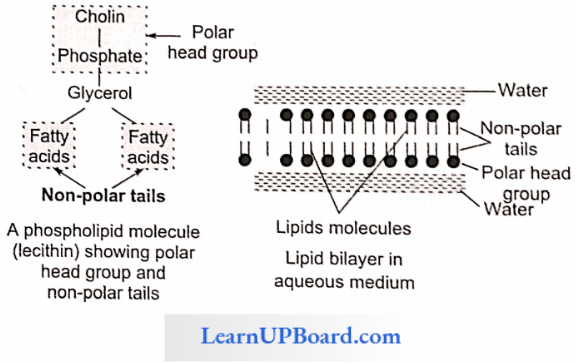
- The hydrocarbon chains of the fatty acids are the non-polar tails of the molecule.
- The phosphate and nitrogenous/non-nitrogenous groups form the polar head group of the molecule.
- Many phospholipid molecules may a nan go themselves in a double-layered membrane (lipid bilayer) in aqueous media. These have one or more simple sugars.
Glycolipids: They are lipids having sugar residues. Two common glycolipids are cerebrosides and gangliosides.
- Glycolipids Composition: Glycolipids contain fatty acids, alcohol sphingosine, and sugar such as galactose, glucose, etc.
- Glycolipids Function: The glycolipids are components of cell membranes, particularly in the myelin sheath of nerve fibers on outer surfaces of nerve cell, and in chloroplast membranes.
Lipoproteins: Lipoproteins contain lipids (mainly phospholipids) and proteins in their molecules.
Function: Membranes are composed of lipoproteins. Lipids are transported in the blood plasma and lymph as lipoproteins. Lipoproteins occur in milk and egg yolk.
Chromolipids: These contain pigments such as carot¬enoids, for example, carotene, and vitamin A.
3. Derived Lipids: These are isoprenoid structures, for example, steroids, terpenes, carotenoids, and prostaglandins.
- Sterols
- Sterols belong to a class of lipids that are not straight-chain compounds.
- These are composed of fused hydrocarbon rings and a long hydrocarbon side chain. One of the examples is cholesterol.
- Cholesterol is found in animals only.
- It exists either free or as a cholesterol ester with a fatty acid.
- Cholesterol is also the precursor of hormones such as progesterone, testosterone, estradiol, and cortisol.
- Another steroid compound, diosgenin, produced by the yam plant (Oioscorea) is used in the manufacturing of antifertility pills.
- Prostaglandins
- It is a group of hormone-like unsaturated fatty acids that function as messenger substances between cells.
- They are derived from arachidonic acid and related C20 fatty acids.
- Prostaglandins occur in human seminal fluid, menstrual fluid, amniotic fluid, and a number of tissues.
- They also circulate in blood.
- They produce a variety of effects in different organs.
- Prostaglandins regulate the production of acid in the stomach and stimulate the contraction of smooth muscles.
- They are used to induce labor because they cause uterine contractions.
- These can reduce the effects of asthma and gastric acidity. Analgesics such as aspirin inhibit prosta-glandin synthesis.
- Cholesterol helps in the absorption of fatty acids and the formation of sex hormones, vitamin D, and bile salts. Potato is rich in cholesterol.
- Terpenes are lipid-like carbohydrates formed of isoprene units (C5H8)n, for example, menthol, camphor, carotenoids, etc.
NEET Biology Notes For Biomolecules Nucleotides
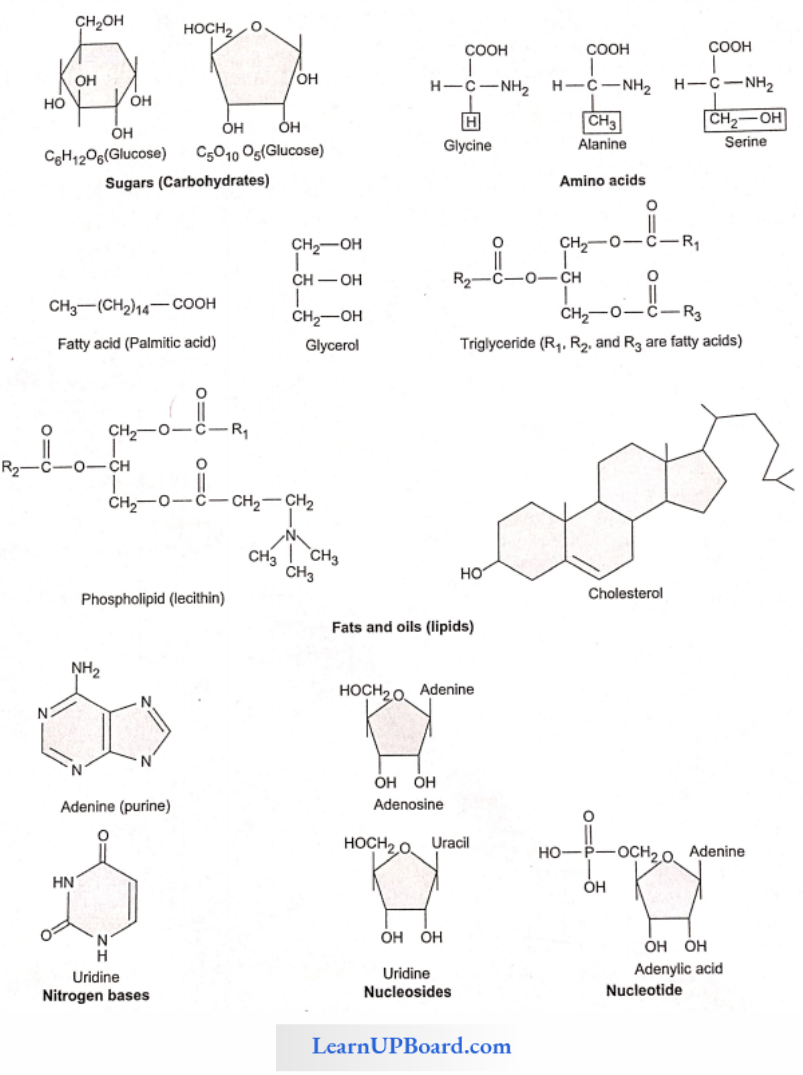
Nucleotide is a group of small complex molecules forming a part of the information transfer system in cells. They are the basic units of nucleic acids. They also participate in energy transfer systems.
- Nucleotides contain carbon, hydrogen, oxygen, nitrogen, and phosphorus.
- Each nucleotide is made up of a cyclic nitrogenous base, a pentose, and one to three phosphate groups.
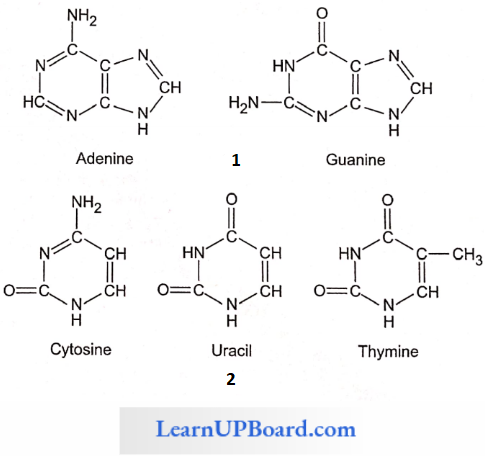
- The nitrogenous bases occurring in nucleotides are either a purine or a pyrimidine.
- Major purines are adenine and guanine. Thymine, uracil, and cytosine are pyrimidines.
- The sugar pentose is either ribose or deoxyribose.
- The nucleotides are, thus, called ribonucleotides or deoxyribonucleotides.
- Examples of ribonucleotides and deoxyribonucleotides are adenylic acid (AMP) and deoxyadenylic acid (dAMP), respectively.
- Ribonucleotides arce the basic units of ribonucleic acids (RNA) and deoxyribonucleotides arc the basic units of deoxyribonucleic acids (DNA).
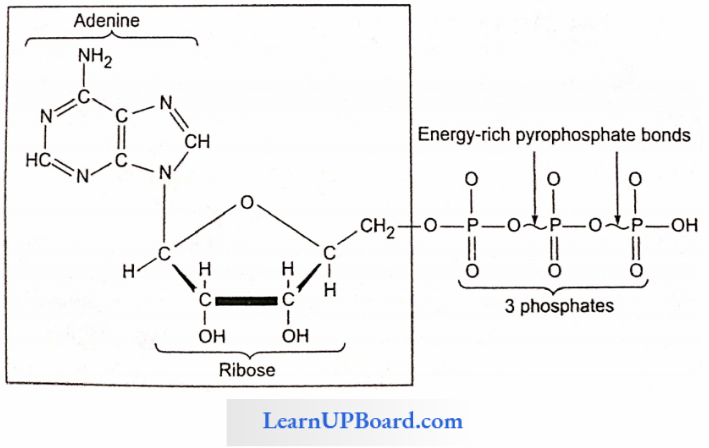
- Nucleotides are mono-, dk, or triphosphate of nucleosides. For example, adenylic acid or adenosine monophosphate (AMP).
- Adenosine diphosphate (ADP) and adenosine triphosphate (ATP) arc higher adenine nucleotides.
- Nucleotides with more than one phosphate group arc are called higher nucleotides, for example, ATP and ADP. Likewise. other purines and pyrimidines can also form higher nucleotides.
- Higher nucleotides of purines and pyrimidines occur in the free state, for example. ATP and ADP. Their third and second phosphate bonds can release about 8 kcal or more of tree energy per mole on hydrolysis. This far exceeds the energy released on the hydrolysis of most other covalent bonds. Therefore, these phosphate bonds of higher nucleotides are called high-energy bonds.
- Nucleotides of the vitamins nicotinamide and riboflavin in occur either freely or in combination with specific proteins, thus working as coenzymes. They do not participate in the formation of nucleic acids. Instead, they act along with oxidizing enzymes and participate in oxidation reactions occurring in the cell.
Nicotinamide And Riboflavin Nucleotides
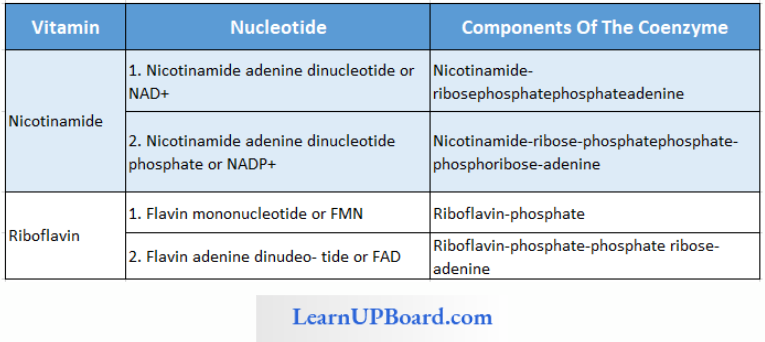
Functions Of Nucleotides
- Purine and pyrimidine nucleotides polymerize to form nucleic acids.
- Higher purine and pyrimidine nucleotides, particularly ATP, store energy in their high-energy phosphate bonds.
- They arc formed during photosynthesis and respiration.
- Hydrolysis of the phosphate bonds of ATP releases their bond energy for driving energy-dependent reactions and processes.
- Nicotinamide and riboflavin nucleotides act as coenzymes of oxidizing enzymes.
NEET Biology Notes For Biomolecules Nucleic Acids
First discovered by Meischer, nucleic acids are giant molecules having a variety of functions. There are two major types of nucleic acids deoxyribonucleic acid or DNA and ribonucleic acid or RNA.
- DNA is found mainly in the nucleus but also occurs in chloroplasts and mitochondria.
- It is the genetic material and contains all the information needed for the development and existence of an organism.
- RNA occurs as genetic material in some viruses.
- Nucleic acids are linear polymers of purine and pyrimidine nucleotides.
- The nucleotides are linked serially by phosphate groups, each linking the C’5(5′ – C) and C’3(3′ – C) of the pentoses of the successive nucleotides.
- A DNA molecule consists of a double chain of nucleo¬tides, whereas RNA consists of a single chain.
- The nucleotides of DNA contain the bases adenine (A), thymine (T), guanine (G), and cytosine (C), while RNA contains A, G, C, and uracil (U) instead of T.
- The backbone of the nucleic acid is uniformly made up of alternating pentose and phosphate groups.
- The pentose in DNA is deoxyribose (C5H10O4) and that in RNA is ribose (C5H10O3).
Double Helix Structure Of DNA
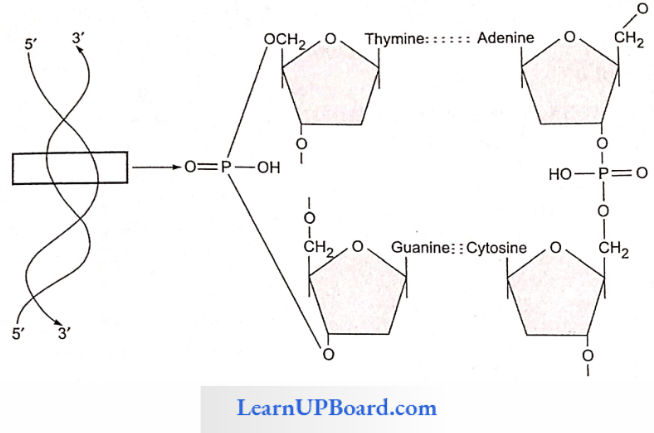
- In the double-stranded DNA, the bases of the opposite strands pair in a specific relationship by means of hydrogen bonds.
- A always pairs with T and G always pairs with C. This complementarity is known as the base pairing rule.
- Nucleic acids exhibit a wide variety of secondary structures. For example, one of the secondary structures exhibited by DNA is the famous Watson-Crick model.
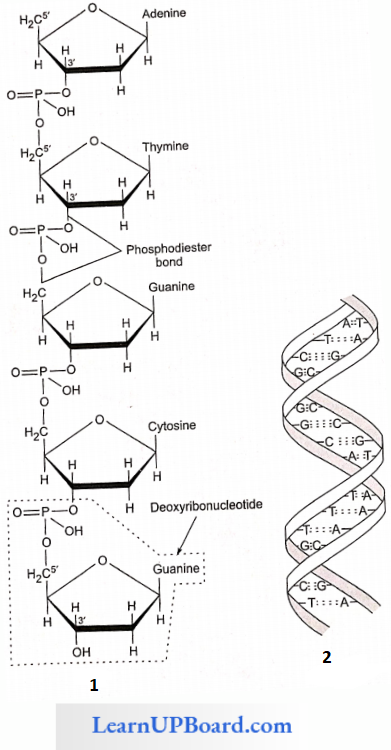
- The double helix model says that DNA exists as a double helix.
- The two strands of polynucleotides arc antiparallel i.e., run in the opposite direction.
- The backbone is formed by the sugar-phosphate-sugar chain.
- The nitrogen bases are projected more or less perpendicular to this backbone but face inside.
- A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand.
- There are two hydrogen bonds between A and T.
- There are three hydrogen bonds between G and C.
- Each strand appears like a helical staircase.
- Each step of the ascent is represented by a pair of bases.
- At each step of ascent, the strand turns 36°.
- One full turn of the helical strand would involve 10 steps or 10 base pairs.
- Attempt drawing a line diagram.
- The pitch would be 34 Å.
- The rise per base pair would be 3.4 Å.
- This form of DNA with the above-mentioned salient features is called B-DNA.
In 1950, Erwin Chargaff found that in any DNA molecule:
- The amount of purines and pyrimidines is equal, i.e., A+ G = T + C.
- The amount of adenine is always equal to that of thymine; and the amount of guanine is always equal to that of cytosine (i.e., A = T and G = C).
- The base ratio (A + T)/(G + C) may vary from one species to another but is constant for a given species.
- The deoxyribose sugar and phosphate components occur in equal proportions.
Structure Of RNA
- RNA is usually single-stranded, but sometimes (as in reovirus and rice dwarf virus) it is double-stranded.
- RNA does not follow Chargaff’s rules, i.e., 1: a 1 ratio does not exist between purines and pyrimidines bases due to its single-stranded nature and lack of complementarity.
Types Of RNA: There are three types of non-genetic RNA.
- Messenger RNA (mRNA): It is produced in the nucleus and carries the information for the synthesis of proteins; it was discovered by Jacob and Monod (1961).
- Ribosomal RNA (rRNA): It is the largest RNA and constitutes about 80% of the total cellular RNA. It is found in the ribosomes where protein synthesis takes place.
- Transfer RNA or soluble RNA or adaptive RNA (s-RNA, t-RNA): It is the smallest type of RNA and constitutes about 10-15% of the total cellular RNA. These are found in the cytoplasm and are of different types (as many types as those of amino, acids, usually 20). Their function is to collect amino acids from the cytoplasm for protein synthesis. t-RNA molecule is folded to form a clover leaf-like structure. This structure was given by Holley.
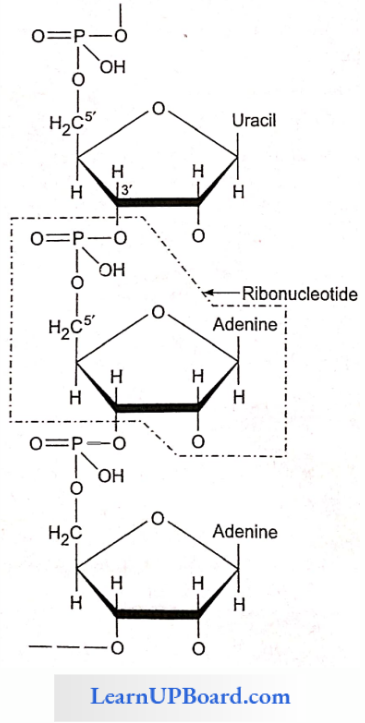
NEET Biology Notes For Biomolecules Nucleic Acids Points To Remember
The study of X-ray diffraction patterns of DNAs isolated from various organisms by Wilkins, Franklin, and Astbury revealed that DNA has a right-handed helical structure.
- Using all the available chemical and physical information, James Watson and F.C. Crick of Cambridge gave the double helix model of DNA for which they were awarded the Nobel Prize in 1962.
- The width between the two backbones is constant and equal to the width of a base pair (i.e., the width of a purine + a pyrimidine).
- Along the axis of the molecule, the base pairs are spaced at intervals of 0.34 nm. Therefore, one complete turn of the double helix comprises 3.4 nm (10 base pairs).
- There is no restriction on the sequence of bases in one chain. However, due to the rule of base pairing, the sequence of one chain determines the sequence in the other. The two chains are thus said to be complementary.
- As a result, the (purine) adenine in either chain is associated with (pyrimidine) thymine in the other. Similarly, the (purine) guanine in either chain is associated with the (pyrimidine) cytosine in the other.
- The two chains are held together by hydrogen bonding between the bases (joined together in pairs), a single base from one chain being hydrogen-bonded to the complementary base from the complementary chain.
- The adenine-thymine pair has two hydrogen bonds and the guanine-cytosine pair has three hydrogen bonds.
- The double helix has a diameter of 20 Å, i.e., the distance between two strands is 19.8 Å (or 20 Å).
- DNA with a higher percentage of G = C has more density than those with a higher percentage of A = T.
- Upon heating at temperatures above SO-90 C, the two strands of DNA uncoil and separate (denaturation). On cooling, the strands come closer and are rejoined together (denaturation/annealing). The low melting area of DNA is A = T base pairs.
- 1 μm of DNA contains about 3000 base pairs.
DNA is mostly right-handed. This type of DNA exists in four forms:
- B form: The usual DNA, having 10 base pairs per turn.
- A form: Having 11 base pairs (instead of 10 base pairs per turn), the base pairs are not perpendicular to the axis, but are tilted.
- C form: Like B form, but have nine base pairs per turn.
- D form: Like B form, but have eight base pairs per turn.
DNA with left-handed coiling is called Z-DNA. In this, the repeating unit is dinucleotide. In some cases, as in ∅ x 174 and S-13 viruses, the DNA is single-stranded.
Palindromic And Repetitive DNA: DNA duplex possessing areas of the same sequences of nucleotides is called palindromic DNA. Repetitive DNA has a sequence of nitrogen bases repeated several times in tandem.
NEET Biology Notes For Biomolecules Enzymes
Enzymes are proteinaceous, biocatalysts. The first enzyme was discovered by Buchner. The term enzyme was given by Kuhne.
- Zymase (from yeast) was the first discovered enzyme (Buchner)
- The first purified and crystalized enzyme was urease (by J.B. Sumner) from the Canavaliat/jack bean (labia plant).
- The proteinaceous nature of the enzyme was established by Northrop and Sumner.
- Enzymes are biocatalysts made up of proteins (except ribozyme), which increase the rate of biochemical reactions by lowering the activation energy.
- The first discovered ribozyme was L19 RNAase by T. Cech from rRNA of a protozoan Tetrahymena thermophila and RNAase P or Ribonuclease P by Altman in a prokaryotic cell (Nobel prize).
General Properties Of Enzymes
- Large-sized biomolecules, colloidal nature with high molecular weight.
- The large size (equal to colloid particles) provides more surface area and, hence possesses a large number of active sites. A large number of substrates get converted into products by one molecule of enzyme at a time.
- The highest molecular weight is of the enzyme pyruvate dehydrogenase complex (46 lakh), which participates in the link reaction of respiration.
Proteinous Nature Of Enzymes
- The monomer unit of an enzyme is an amino acid.
- Amino acids are linked together to form a polypeptide chain.
- Most of the enzymes are arranged in the tertiary structure of protein or globular proteins except isoenzyme (quaternary structure).
- The tertiary structure of proteins provides stability and water-soluble nature to enzymes.
- The synthesis of enzymes occurs on ribosomes under the control of genes.
Enzymes Specificity
- Enzymes are specific in the case of pH, temperature, and substrate.
- The common pH range for enzyme activity is 6-8.
- Every enzyme works on a specific pH, pepsin: 2.5 pH, hydrolase: 4-5.
- RuBisCO, per case: 8.5 pH, trypsin: 8.5 pH.
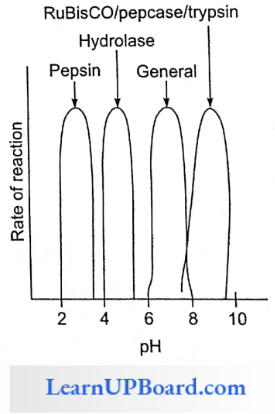
Enzymes Temperature
- The common range of temperature for enzyme activity is 20-40°C.
- Enzymes work on the body temperature of an organism not on environmental temperature.
- Plant enzymes are affected by environmental temperature change as plants do not show homeostasis.
- At low temperatures, enzymes become functionally inactive and at high temperatures, denatured.
Enzymes Substrate
- Every enzyme works on a specific substrate.
- The substrate binds at the active site of the enzyme which is made up of a specific sequence of amino acids and recognizes its substrate.
- An example is succinic dehydrogenase acts on succinic acid while pyruvate dehydrogenase acts on pyruvic acid.
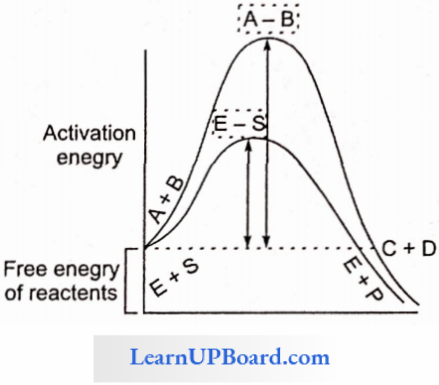
- Enzymes increase the rate of reaction by decreasing activation energy.
- Activation Energy: Minimum amount of energy required more than the free energy of reactants to reach the transition state of a chemical reaction or to undergo the chemical reaction.
Enzymes Turn Over Number (TON): The number of reactant molecules converted into products by one molecule of enzyme in a unit of time. The highest TON is of carbonic anhydrase (360 lakh/minute)

Enzymes KM Constant
- Enzymes follow the Michaelis-Menten reaction kinetics.
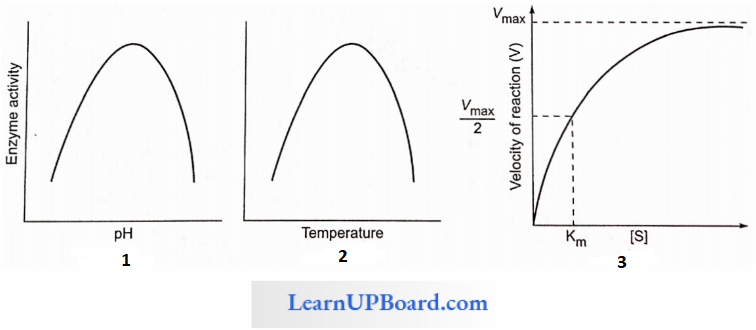
- It represents the substrate concentration at which the rate of enzymatic reaction becomes half of the maximum velocity or rate.
- If an enzyme passes a high KM constant then its affinity towards the substrate is low and the rate of reaction is also low.
- The energy required for a chemical reaction to proceed is called activation energy.
- Enzymes lower the activation energy. (Remember that enzymes cannot start the chemical reaction.)
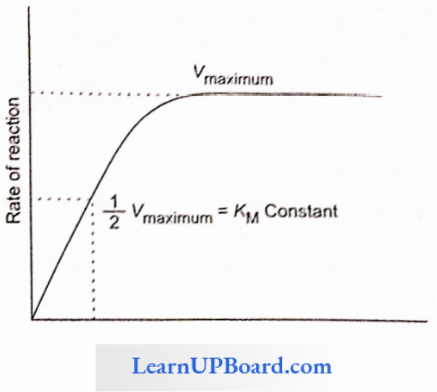
- With the increase in concentration of substrate, the enzymatic velocity also increases.
- At a certain value, all the active sites of enzyme molecules are saturated, and an increase in the substrate concentration does not increase the velocity of the enzymatic reaction. (The concentration of substrate at which the velocity of enzymatic action reaches half of its maximum value is called KM value or Michaelis constant).
The higher is the affinity of an enzyme for a substrate, the lower is its KM value, i.e., KM value ∝ 1/Affinity
Ki Constant (Enzyme Inhibitor Complex Dissociation Constant): The substrate concentration at which the enzyme inhibitor complex dissociates and the reaction becomes normal. It is applicable only for competitive reversible inhibitions.
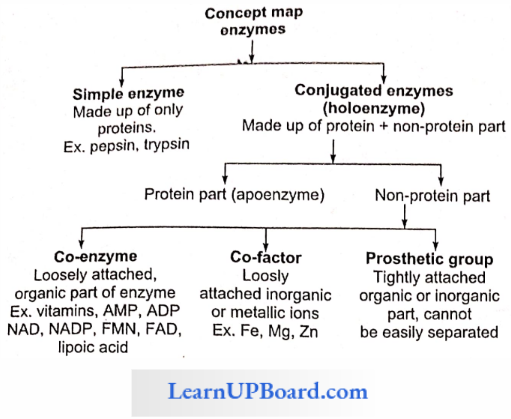
Structure Of Enzyme
- Simple Enzymes: They are made up of proteins only, for example, pepsin, trypsin, etc.
- Conjugated Enzymes: They are made up of protein as well as non-protein parts.
- Co-enzymes: Co-enzymes are non-protein, organic groups, which are loosely attached to apoenzymes. They are generally made up of vitamins.
- Prosthetic Group: When a non-protein part is tightly or firmly attached to apoenzymes, a prosthetic group is formed.
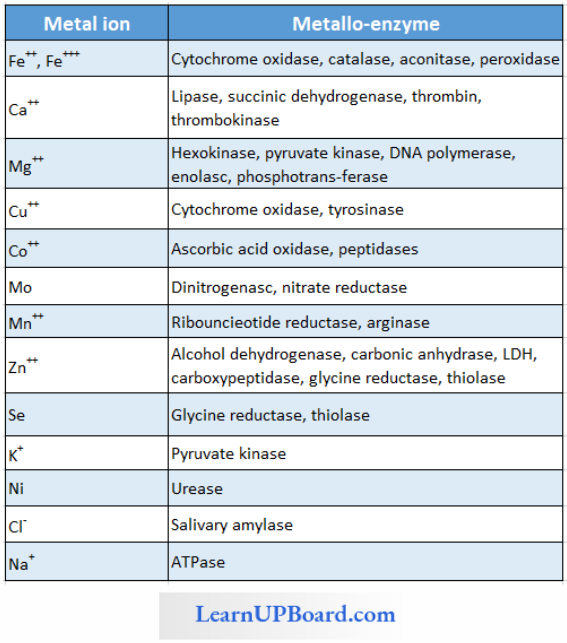
- Metal Activators/Co-Factors/Metallic Factor: Loosely attached inorganic co-factor, for example, Mn, Fe, Co, Zn, Ca, Mg, Cu, etc
Enzyme Active Site: The part of the polypeptide chain made up of a specific sequence of amino acids at which a specific substrate is to be bound and catalyzed is known as an active site. The specific sequence of amino acids at the active site is determined by genetic codes.
Enzyme Allosteric Site: Besides the active site, some enzymes possess additional sites, at which chemical other than the substrate (allosteric modulators) are bound. These sites are known as allosteric sites and enzymes with allosteric sites are called as allosteric enzymes, for example, hexokinase, and phosphofructokinase.
NEET Biology Notes For Biomolecules Enzymes Points To Remember
- Endoenzymes: Enzymes that are functional only inside the cells. For example, enzymes of metabolism.
- Exoenzymes: Enzymes catalyze the reactions outside the cell. For example, enzymes of digestion, some enzymes of insectivorous plants, zymase complex of fermentation, etc.
- Proenzyme/zymogen: These are precursors of enzymes or inactive forms of enzymes. For example, pepsinogen, trypsinogen, etc.
- Isoenzymes: Enzymes having Similar action, but little difference in their molecular configuration are called isoenzymes. Sixteen forms of α-amylase of wheat, five forms of LDH (lactate dehydrogenase), and three forms of per case are known. These isoenzyme forms are synthesized by different genes and are tissue- and organ-specific.
- Inducible Enzymes: When the formation of the enzyme is induced by substrate availability. For example, lactase, nitrogenase, β-galactosidase, etc.
- Extremozymes: Enzymes that may also function at extremely adverse conditions (very high temperature), for example, Taq polymerase.
- Abzynics: When monoclonal antibodies (Mab) are used as enzymes.
- Biodetergents: Enzymes used in washing powders are known as detergents, for example, amylase, lipase, and proteolytic enzymes.
House-keeping/constitutive Enzymes: These are always present in constant amounts and are also essential to cells. For example, enzymes of cell respiration.
Classification Of Enzymes
- Enzyme names such as ptyalin (salivary amylase), pepsin, and trypsin give no indication of their action.
- Other enzymes such as amylase, sucrase, protease, and lipase were named after the substrates on which they act: amylose (starch), sucrose, protein, and lipids, respectively.
- Still others were named according to the source from which they were obtained papain from papaya, bromelain from pineapple (belongs to the family Bromeliaceae).
- Some like DNA polymerase indicate its specific action, polymerization.
- Duclaux (1883) provided a system for naming enzymes by adding the suffixase at the end of the enzyme name.
- In this system, each enzyme ends with an -ase and consists of two parts, the first part indicates its substrate and the second the reaction catalyzed.
- For example, glutamate pyruvate transaminase transfers an amino group from the substrate glutamate to another substrate pyruvate. However, arbitrary names such as ptyalin and trypsin still continue to be used because of their familiarity.
Enzymes Are Grouped Into Six Major Classes:
- Class 1 Oxidoreductases: These catalyze oxidation or reduction of their substrates and act by removing or adding electrons (and/or H+) from or to substrates, for example, cytochrome oxidase oxidizes cytochrome.
- Class 2 Transferases: These transfer specific groups from one substrate to another. The chemical group transferred in the process is not in a free state, for example, glutamate pyruvate transaminase.
- Class 3 Hydrolases: These break down large molecules into smaller ones by the introduction of water (hydrolysis) and the breaking of specific covalent bonds. Most digestive enzymes belong to this category, for example, amylase which hydrolyzes starch, and lipases.
- Class 4 Lyases: These catalyze the cleavage of specific covalent bonds and removal of groups without hydrolysis, for example, histidine decarboxylase cleaves the C-C bond in histidine to form carbon dioxide and histamine.
- Class 5 Isomerases: These catalyze the rearrangement of molecular structure to form isomers, for example, phosphohexose isomerase changes glucose-6-phosphate to fructose-6-phosphate (both are hexose phosphates).
- Class 6 Ligases: These catalyze the covalent bonding of two substrates to form a large molecule. The energy for the reaction is derived from the hydrolysis of ATP. Pyruvate carboxylase combines pyruvate and carbon dioxide to form oxaloacetate at the expense of ATP.
Factors Affecting Enzyme Activity: The activity of an enzyme can be affected by a change in the conditions which can alter the tertiary structure of the protein. These include temperature, pH, change in substrate concentration, or binding of specific chemicals that regulate enzyme activity.
- Enzymes generally function in a narrow range of temperatures and pH.
- Each enzyme shows its highest activity at a particular temperature and pH called the optimum temperature and optimum pH.
- Activity declines both below and above the optimum value.
- Low temperature preserves the enzyme in a temporarily inactive state, whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Enzyme Optimum Temperature
- Enzymes generally work over a narrow range of temperatures.
- Usually, it corresponds to the body temperature of the organism. For instance, human enzymes work at the normal body temperature.
- Each enzyme shows its highest activity at a particular temperature called the optimum temperature
- Activity declines both above and below the optimum temperature.
- Every enzyme has a specific optimum temperature.
- According to the general rule of thumb, Q10 (temperature coefficient) for enzymes is 2-3, i.e., in between minimum and optimum temperature (5-40°C), the rate of reaction increases 2-3 times with rise in 10°C temperature.
If the temperature is reduced to near or below freezing point, the enzymes are inactivated (not denatured).
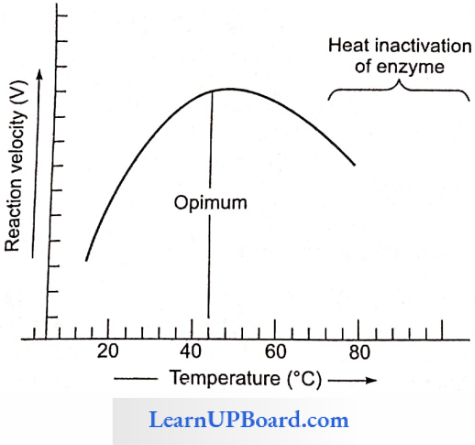
- Most enzymes show maximum activity in a temperature range of 25-40°C.
- Enzymes are thermolabile, i.e., they get denatured at high temperatures.
- The loss of catalytic properties begins at 35°C and is almost complete around 60°C. However, dried enzyme extracts can endure temperatures of 100-120°C or even higher. That is why dry seeds can endure higher temperatures than germinating seeds.
- Thermal stability is thus an important quality of some enzymes isolated from thermophilic organisms.
Enzyme Optimum pH
- Each enzyme shows its highest activity at a specific pH. This is called optimum pH.
- Activity declines both above and below the optimum pH.
- Most intracellular enzymes function best around neutral pH.
- Some digestive enzymes have their optimum pH in the acidic or alkaline range. For example, the protein-digesting enzyme pepsin found in the stomach has an optimum pH of 2.0.
- Another protein-digesting enzyme, trypsin, found in the duodenum functions best at an alkaline pH of 8.0.
Enzyme Concentration Of Substrate: With an increase in substrate concentration, the velocity of the enzymatic reaction rises at first.
- The reaction ultimately reaches a maximum velocity (Vmax) which is not exceeded by any further rise in the concentration of substrate.
- This is because the enzyme molecules are fewer than the substrate molecules and after the saturation of these molecules, there are no free enzyme molecules to bind with the additional substrate molecules.
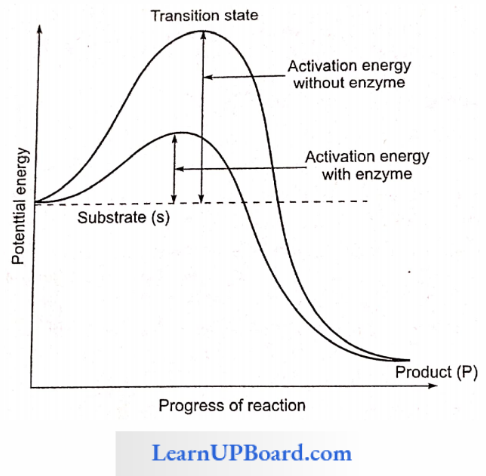
How Enzymes Speed Up Reactions: A certain amount of energy is necessary to initiate any chemical reaction. This is called activation energy or free energy of activation.
- In a population of molecules of each substrate, the majority have average kinetic energy, some have higher and some have lower than the average energy.
- Under normal temperature, only the molecules having relatively high energy are likely to react to form the product. Therefore, the reaction takes place very slowly.
- One way to make the reaction go faster is to raise the temperature of the mixture.
- Heat increases the kinetic energy of the molecules, causing their collisions and reactions.
- The other method of quickening the reaction is by adding an enzyme.
- The enzyme lowers the activation energy of the reaction and allows a large number of molecules to react at a time.
- Exactly how the enzymes lower the activation energy is not clear. However, it is known that the enzymes combine with the substrate molecules and bring them close together which favors their collisions in the most suitable directions and locations for the reaction to occur.
- The inorganic catalysts work in the same manner.
- It is now held that conformational changes in the active sites of the enzymes actually “push” the substrate molecules toward an interaction.
- The hydrolysis of starch into glucose is an organic chemical reaction.
- The rate of a physical or chemical process refers to the amount of product formed per unit time. The rate can also be called velocity if the direction is specified for a given reaction.
- Reactants absorb energy from surroundings to climb the hill of activation energy (EA) and reach the unstable, short-lived, transition state Enzyme speeds up the reaction by reducing the uphill climb to the transition state. In the transition state, the reactants are in an unstable condition and a reaction can occur.
Mechanism Of Enzyme Action: Two hypotheses have been put forward to explain the mode of enzyme action.
1. Lock And Key Hypothesis
- This hypothesis was given by Emil Fischer (1894).
- According to this hypothesis, both enzyme and substrate molecules have specific geometrical shapes.
- It is similar to the system of lock and key, which have special geometrical shapes in the region of their activity.
- The active sites contain special groups having -NH2, -COOH, -SH, etc., for establishing contact with the substrate molecules.
- Just as a lock can be opened by its specific key, a substrate molecule can be acted upon by a particular enzyme. This also explains the specificity of enzyme action.
- After coming in contact with the active site of the enzyme, the substrate molecules or reactants form a complex called enzyme-substrate complex.
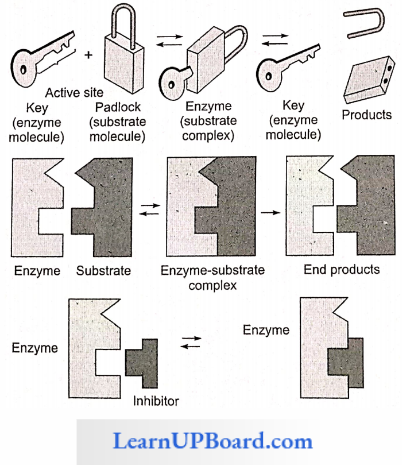
Enzyme + Substrate ⇔ Enzyme -Substrate Complex
Enzyme-Substrate Complex ⇔ Enzyme + End Products
- In the enzyme-substrate complex, the molecules of the substrate undergo chemical change and form products.
- The product no longer fits into the active site and escapes in the surrounding medium, leaving the active site free to receive more substrate molecules. This theory explains how a small concentration of an enzyme can act upon a large amount of the substrate. It also explains how the enzyme remains unaffected at the end of a chemical reaction.
- The lock and key theory explains how a substance having a structure similar to the substrate can work as a competitive inhibitor.
2. Induced Fit Hypothesis
- This hypothesis was proposed by Koshland (1960).
- According to this hypothesis, the active site of the enzyme does not initially exist in a shape that is complementary to the substrate but is induced to assume the complementary shape as the substrate becomes bound to the enzyme.
- According to Koshland, “the active site is induced to assume a complementary shape in much the same way as a hand induces a change in the shape of a glove.”
- An active site of an enzyme is a crevice or a pocket into which the substrate fits. Thus, enzymes through their active site catalyze reactions at a high rate. Hence, according to this model, the enzyme (or its active site) is flexible.
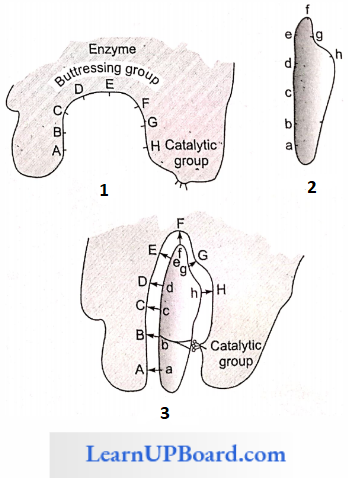
The active site of the enzyme contains two groups:
- The buttressing group is meant to support the substrate.
- The catalytic group is meant to catalyze the reaction.
When a substrate comes in contact with the buttressing group, the active site changes to bring the catalytic group opposite the substrate bonds to be broken.
Isoenzymes: Multiple molecular forms of an enzyme (synthesized by different genes) occurring in the same organism and having a similar substrate activity are called iso-enzymes. Over 100 enzymes are known to have iso-enzymes such as
- α-amylase of wheat endosperm has 16-iso-enzymes.
- Lactic acid dehydrogenase has five iso-enzymes.
- Alcohol dehydrogenase has four iso-enzymes.
Site of Enzyme Action: All enzymes are produced in the living cells. About 2000 enzymes have been recorded. These are of two types with regard to the site where they act: intracellular and extracellular.
- Intracellular Enzymes
- Most of the enzymes remain and function inside the cells. They are called intracellular enzymes or endoenzyines.
- Some are dissolved in the cytoplasmic matrix.
- The water extract of ground-up liver cells contains all the 11 enzymes necessary to change glucose to lactic acid.
- Certain enzymes are bound to particles such as ribosomes, mitochondria, and chloroplast.
- The respiratory enzymes needed to convert lactic acid to carbon dioxide and water are found in mitochondria.
- Extracellular Enzymes
- Certain enzymes leave the cells and function outside them.
- They are called extracellular enzymes or exoenzymes.
- They mainly include the digestive enzymes, for example, salivary amylase, gastric pepsin, and pancreatic lipase; secreted by the cells of the salivary glands, gastric glands, and pancreas, respectively.
- Lysozyme present in tears and nasal secretion is also an exo-enzyme.
- Rennet tablets, containing the enzyme rennin from the calf’s stomach, are used to coagulate milk protein carcinogen for cheese (casein) formation.
Inhibition of Enzyme Activity
- Any substance that can diminish the velocity of an enzyme-catalyzed reaction is called an inhibitor.
- Reversible inhibitors bind to enzymes through noncovalent bonds.
- Dilution of the enzyme-inhibitor complex results in the dissociation of the reversibly-bound inhibitor and recovery of enzyme activity.
- Irreversible inhibition occurs when an inhibited enzyme does not regain activity upon dilution of the en¬zyme-inhibitor complex.
- Some irreversible inhibitors act by forming covalent bonds with specific groups of enzymes; for example, the neurotoxic effects of certain insecticides are due to their irreversible binding at the catalytic site of the enzyme acetylcholinesterase.
- The two most commonly encountered types of inhibition are competitive and non-competitive.
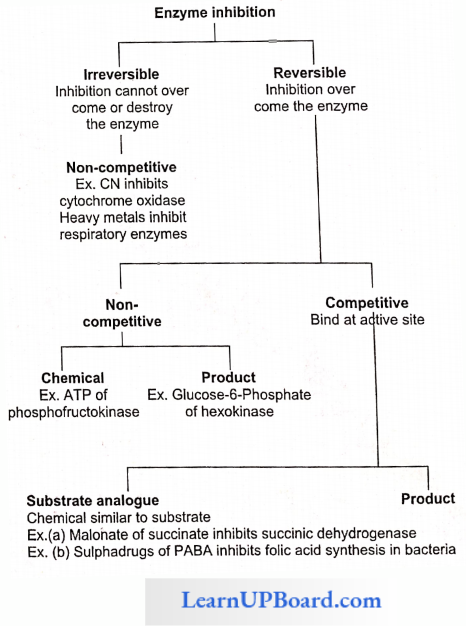
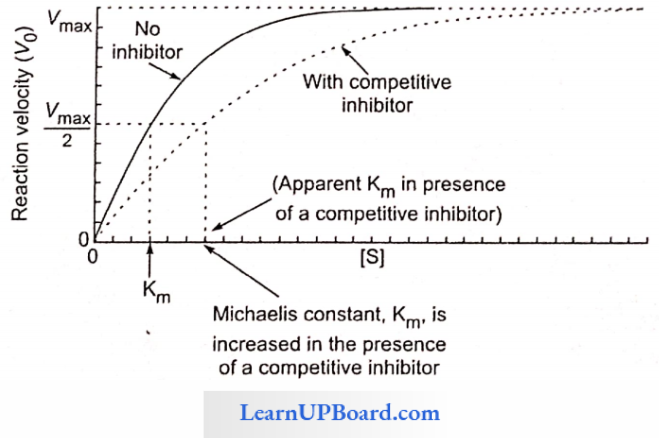
Competitive Inhibition: This type of inhibition occurs when the inhibitor binds reversibly to the same site that the substrate would normally occupy and, therefore, competes with the substrate for that site.
- Effect On Vmax: The effect of a competitive inhibitor is reversed by increasing [S]. At a sufficiently high substrate concentration, the reaction velocity reaches the Vmax observed in the absence of an inhibitor.
- Effect On Km: A competitive inhibitor increases the apparent for a given substrate. This means that in the presence of a competitive inhibitor, more substrate is needed to achieve (1/2) Vmax, for example, sulpha drugs for folic acid synthesis in bacteria and inhibition of succinic dehydrogenase by malonate.
Non-competitive inhibition: This type of inhibition is recognized by its characteristic effect on Vmax. Non-competitive inhibition occurs when the inhibitor and substrate bind at different sites on the enzyme. The non-competitive inhibitor can bind cither free enzyme or the ES complex, thereby preventing the reaction from occurring.
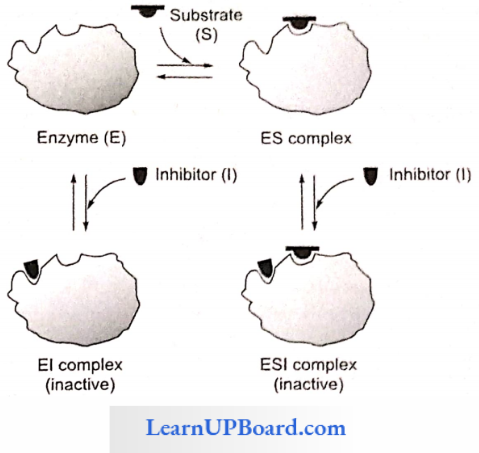
- Effect On Vmax: Noncompetitive inhibition cannot be overcome by increasing the concentration of substrate. Thus, non-competitive inhibitors decrease Vmax of the reaction.
- Effect On Km: Non-competitive inhibitors do not interfere with the binding of substrate to enzyme. Thus, the enzyme shows the same Km in the presence or absence of the non-competitive inhibitor. For example, cyanide kills an animal by inhibiting cytochrome oxidase.
NEET Biology Notes For Biomolecules Assertion Reasoning Questions And Answers
In the following questions, an Assertion (A) is followed by a corresponding Reason (R). Mark the correct answer.
- If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
- If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.
- If Assertion is true, but Reason is false.
- If both Assertion and Reason are false.
Question 1. Assertion: Heparin is a natural anticoagulant inside the blood vessels.
Reason: It is an example of homopolysaccharide.
Answer: 3. If Assertion is true, but Reason is false.
Question 2. Assertion: Hemoglobin is a monomeric protein.
Reason: It is made up of two polypeptide chains.
Answer: 4. If both Assertion and Reason are false.
Question 3. Assertion: Saturated fatty acids are non-essential fatty acids.
Reason: They can be synthesized in an animal body.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 4. Assertion: Lipids provide more energy as compared to carbohydrates on oxidation.
Reason: Lipid is the first respiratory substance.
Answer: 3. If Assertion is true, but Reason is false.
Question 5. Assertion: In protoplasm, protein functions as a buffer.
Reason: The protein molecule is amphoteric.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 6. Assertion: Phospholipids form a bimolecular layer in an aqueous medium.
Reason: Phospholipid molecules are amphipathic.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 7. Assertion: Starch is the storage polysaccharide in plants.
Reason: Starch is a polymer of p-glucose.
Answer: 3. If Assertion is true, but Reason is false.
Question 8. Assertion: Lecithin is important in membranes.
Reason: It has an amphipathic nature.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 9. Assertion: Glucose is dextrose.
Reason: The open chains of glucose have four asymmetrical carbons.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 10. Assertion: Histones are acidic proteins.
Reason: These join all nucleic acids.
Answer: 4. If both Assertion and Reason are false.
Question 11. Assertion: Disaccharides show optical activity.
Reason: Cellobiose is an example of a disaccharide.
Answer: 4. If both Assertion and Reason are false.
Question 12. Assertion: Isabgol is used as a medicine.
Reason: The husk of isabgol contains mucilage.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion
Question 13. Assertion: Monellin is the sweetest chemical.
Reason: Monellin is a protein.
Answer: 2. If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.
Question 14. Assertion: Natural silk is made up of protein.
Reason: Artificial silk is a polysaccharide.
Answer: 2. If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.
Question 15. Assertion: Specific substrate binds at the active site of the enzyme.
Reason: Enzymes increase the activation energy of the substrate.
Answer: 3. If Assertion is true, but Reason is false.
Question 16. Assertion: Enzymes become denatured at high temperatures.
Reason: The tertiary structure of proteins gets damaged at high temperatures.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
Question 17. Assertion: All enzymes are proteins.
Reason: Ribozymes are enzymes without proteins.
Answer: 2. If both Assertion and Reason are true, but the Reason is not the correct explanation of the Assertion.
Question 18. Assertion: Allosteric modulators accelerate or retard the rate of catalysis of an allosteric enzyme.
Reason: Allosteric modulators modulate the configura¬tion of the active site of enzyme.
Answer: 1. If both Assertion and Reason are true and the Reason is the correct explanation of the Assertion.
