Classification Of Elements NEET MCQs
NEET Chemistry For Classification Of Elements And Periodicity In Properties Multiple Choice Questions
Question 1. The IUPAC name of an element with atomic number 119 is
- Ununennium
- Unnilennium
- Unununnium
- Ununoctium.
Answer: 1. Ununennium
The IUPAC name of an element with atomic number 119 is ununennium.
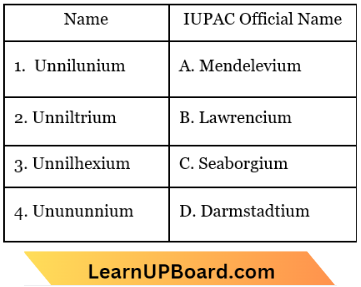
Question 2. Identify the incorrect match.
- 1-A
- 2-B
- 3-C
- 4-D
Answer: 4. 4-D
Unnilunium – Mendelevium ⇒ (1)-(A)
Unniltrium – Lawrencium ⇒ (2)-(B)
Unnilhexium – Seaborgium ⇒ (3)-(C)
Unununnium – Roentgenium ⇒ (4)-(D)
Question 3. The element Z = 114 has been discovered recently. It will belong to which of the following family/group and electronic configuration?
- Carbon family, \([\mathrm{Rn}] 5 f^{14} 6 d^{10} 7 s^2 7 p^2\)
- Oxygen family, \([\mathrm{Rn}] 5 f^{14} 6 d^{10} 7 s^2 7 p^4\)
- Nitrogen family, \([\mathrm{Rn}] 5 f^{14} 6 d^{10} 7 s^2 7 p^6\)
- Halogen family, \([\mathrm{Rn}] 5 f^{14} 6 d^{10} 7 s^2 7 p^5\)
Answer: 1. Carbon family, \([\mathrm{Rn}] 5 f^{14} 6 d^{10} 7 s^2 7 p^2\)
The electronic configuration of the element with Z = 114 (Flerovium) is \([\mathrm{Rn}] 5 f^4 6 d^{10} 7 s^2 7 p^2\)
Hence, it belongs to the carbon family which has the same outer electronic configuration.
Read and Learn More NEET MCQs with Answers
Classification of Elements NEET MCQs
Question 4. An atom has electronic configuration 1s2 2s2 2p6 3s2 3p6 3d3 4s2 you will place it in
- Fifth group
- Fifteenth group
- Second group
- Third group.
Answer: 1. Fifth Group
The electronic configuration of an atom: 1s2 2s2 2p6 3s2 3p6 3d3 4s2
In the configuration, the last electron of the atom is filled in d-subshell as 3d3. Thus, this element belongs to the d-block of the periodic table with group number VB or 5.
Question 5. The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p3 What is the atomic number of the element, which is just below the above element in the periodic table?
- 36
- 49
- 33
- 34
Answer: 3. 33
The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p3 What is the atomic number of the element
The atomic number of the given element is 15 and it belongs to group 15. Therefore the atomic number of the element below the above element = 15 + 18 = 33
Question 6. If the atomic number of an element is 33, it will be placed in the periodic table in the
- First group
- Third group
- Fifth group
- Seventh group.
Answer: 3. Fifth Group
The electronic configuration of an element with Z = 33 is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3
Hence, it lies in the VA or the 15th group
Periodicity in Properties NEET questions
Question 7. The electronic configuration of four elements is given below. Which elements do not belong to the same family as others?
- \([\mathrm{Xe}] 4 f^{14} 5 d^{10} 4 s^2\)
- \([\mathrm{Kr}] 4 d^{10} 5 s^2\)
- \([\mathrm{Ne}] 3 s^2 3 p^5\)
- \([\mathrm{Ar}] 3 d^{10} 4 s^2\)
Answer: 3. \([\mathrm{Ne}] 3 s^2 3 p^5\)
Elements (1), (2) and (4) belong to the same group since each one of them has two electrons in the valence shell. In contrast, element (3) has seven electrons in the valence shell, and hence it lies in another group.
Question 8. The element expected to form the largest ion to achieve the nearest noble gas configuration is
- N
- Na
- O
- F
Answer: 1. N
N3-, O2-, F– and Nat have 10 electrons each, hence these are isoelectronic. For isoelectronic species, the size of the species decreases as the nuclear charge increases. Hence, the size decreases as N3- > O2- > F– > Na+
Hence, among the given elements, nitrogen is expected to form the largest ion to achieve the nearest noble gas configuration.
Question 9. For the second-period elements, the correct increasing order of first ionization enthalpy is
- Li < Be < B < C < O < N < F < Ne
- Li < Be < B < C < N < O < F < Ne
- Li < B < Be < C < O < N < F < Ne
- Li < B < Be < C < N < O < F < Ne
Answer: 3. Li < B < Be < C < O < N < F < Ne
As we move across a period, ionisation enthalpy increases, because of increased nuclear charge and decrease in atomic radii. However, abnormal values are observed for Be, N and Ne due to the extra stability of half-filled and fully-filled orbitals.
Thus, the actual order is, Li<B<Be<C<O<N<F<Ne.
Question 10. Match the oxide given in column A with its property given in column B
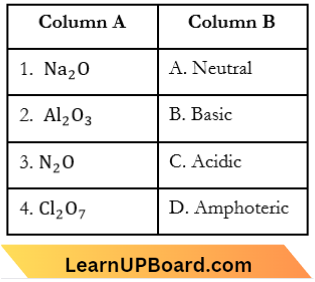
Which of the following options has all the correct pairs?
- (1)-B, (2)-A, (3)-D, (4)-C
- (1)-C, (2)-B, (3)-A, (4)-D
- (1)-A, (2)-D, (3)-B, (4)-C
- (1)-B, (2)-D, (3)-A, (4)-C
Answer: 4. (1)-B, (2)-D, (3)-A, (4)-C
Na2O – Basic oxide, AI2O3 – Amphoteric oxide, N2O – Neutrai oxide, Cl2O7 – Acidic oxide.
Periodicity in Properties NEET questions
Question 11. Which of the following oxides is most acidic in nature?
- MgO
- BeO
- BaO
- CaO
Answer: 2. BeO
In metals, on moving down the group, metallic character increases, so basic nature increases hence most acidic will be BeO
Question 12. In which of the following options the order of arrangement does not agree with the variation of the property indicated against it?
- I < Br < Cl < F (increasing electron gain enthalpy)
- Li < Na < K < Rb (increasing metallic radius)
- Al3+ < Mg2+ < Na+ < F– (increasing ionic size)
- B < C < N < O (increasing first ionisation enthalpy)
Answer: 1. I < Br < Cl < F (increasing electron gain enthalpy) and 4. B < C < N < O (increasing first ionisation enthalpy)
The correct order of increasing negative electron gain enthalpy is: I < Br < F < CI due to electron-electron repulsion in small-sized F atom and the correct order of increasing first ionisation enthalpy is B < C < O < N due to extra stability of half-filled orbitals in N-atom.
Question 13. The formation of the oxide ion, O2-(g) from the oxygen atom requires first an exothermic and then an endothermic step as shown below:
- \(\mathrm{O}_{(g)}+e^{-} \rightarrow \mathrm{O}_{(g)}^{-} ; \Delta_f H^{\circ}=-141 \mathrm{~kJ} \mathrm{~mol}^{-1} \)
- \(\mathrm{O}_{(g)}^{-}+e^{-} \rightarrow \mathrm{O}_{(g)}^{2-} ; \Delta_f H^{\circ}=+780 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
Thus, the process of formation of O2- in the gas phase is unfavourable even though O2- is isoelectronic with neon. It is due to the fact that,
- O– ion has a comparatively smaller size than the oxygen atom
- Oxygen is more electronegative
- The addition of an electron in oxygen results in a larger size of ion
- Electron repulsion outweighs the stability gained by achieving noble gas configuration.
Answer: 4. Electron repulsion outweighs the stability gained by achieving noble gas configuration.
NEET MCQs on Classification and Periodicity
Question 14. Which of the following orders of ionic radii is correctly represented?
- \(\mathrm{H}^{-}>\mathrm{H}^{+}>\mathrm{H}\)
- \(\mathrm{Na}^{+}>\mathrm{F}^{-}>\mathrm{O}^{2-}\)
- \(\mathrm{F}^{-}>\mathrm{O}^{2-}>\mathrm{Na}^{+}\)
- \(\mathrm{Al}^{3+}>\mathrm{Mg}^{2+}>\mathrm{N}^{3-}\)
Answer: None
Cations lose electrons and are smaller in size than the parent atom, whereas anions add electrons and are larger in size than the parent atom. Hence, the order is H–>H>H+.
For isoelectronic species, the ionic radii decreases with increase in atomic number i.e., orclear charge. Hence, the correct orders are \(\mathrm{O}^{2-}>\mathrm{F}^{-}>\mathrm{Na}^{+} \text {and } \mathrm{N}^{3-}>\mathrm{Mg}^{2+}>\mathrm{Al}^{3+}\)
Question 15. Which one of the following arrangements represents the correct order of least negative to most negative electron gain enthalpy for C, Ca, Al, F and O?
- Al < Ca < O < C <F
- Al < O < C < Ca < F
- C < F < O < Al < Ca
- Ca < Al < C < O < F
Answer: 4. Ca < Al < C < O < F
Electron gain enthalpy becomes less negative from top to bottom in a group while it becomes more negative from left to right within a period.
Question 16. In which of the following arrangements the given sequence is not strictly according to the property indicated against it?
- HF < HCl < HBr < HI: increasing acidic strength
- H2O < H2S < H2Se < H2Te: increasing pKa values
- NH3 < PH3 < AsH3 < SbH3: increasing acidic character
- CO2 < SiO2 < SnO2 < PbO2: increasing oxidising power
Answer: 2. H2O < H2S < H2Se < H2Te: increasing pKa values
The acidic strength of hydrides increases with an increase in molecular mass.
Thus, the order of acidic strength is
HF<HCl<HBr<HI
H2O < H2S < H2Se < H2Te
NH3 < PH3 < AsH3 < SbH3
and as acidic strength increases, pKa decreases. Thus order of \(\mathrm{p} K_p, \mathrm{H}_2 \mathrm{O}>\mathrm{H}_2 \mathrm{~S}>\mathrm{H}_2 \mathrm{Se}>\mathrm{H}_2 \mathrm{Te}\)
NEET MCQs on Classification and Periodicity
Question 17. Identify the wrong statement in the following.
- Amongst isoelectronic species, smaller the positive charge on the cation, smaller is the ionic radius.
- Amongst isoelectronic species, the greater the negative charge on the anion, the larger the ionic radius.
- The atomic radius of the elements increases as one moves down the first group of the periodic table.
- Atomic radius of the elements decreases as one moves across from left to right in the 2nd period of the periodic table.
Answer: 1. Amongst isoelectronic species, smaller the positive charge on the cation, smaller is the ionic radius.
As positive charge on the cation increases, effective nuclear charge increases. Thus, atomic size decreases.
Question 18. What is the value of electron gain enthalpy of Na+ if IE1 of Na = 5.1 eV?
- -5.1 eV
- -10.2 eV
- +2.55 eV
- +10.2 eV
Answer: 1. -5.1 eV
⇒ \(\mathrm{Na} \rightarrow \mathrm{Na}^{+}+e^{-} ; \Delta H=5.1 \mathrm{eV}\)
⇒ \(\mathrm{Na}^{+}+e^{-} \rightarrow \mathrm{Na} ; \Delta H=-5.1 \mathrm{eV}\)
Question 19. Which of the following oxides is amphoteric?
- SnO2
- CaO
- SiO2
- CO2
Answer: 1. SnO2
SnO2 reacts with acid as well as base. So, SnO2 is an amphoteric oxide.
⇒ \(\mathrm{SnO}_2+4 \mathrm{HCl} \longrightarrow \mathrm{SnCl}_2+2 \mathrm{H}_2 \mathrm{O}\)
⇒ \(\mathrm{SnO}_2+2 \mathrm{NaOH} \longrightarrow \mathrm{Na}_2 \mathrm{SnO}_3+\mathrm{H}_2 \mathrm{O}\)
Question 20. The correct order of the decreasing ionic radii among the following isoelectronic species is
- \(\mathrm{Ca}^{2+}>\mathrm{K}^{+}>\mathrm{S}^{2-}>\mathrm{Cl}^{-}\)
- \(\mathrm{Cl}^{-}>\mathrm{S}^{2-}>\mathrm{Ca}^{2+}>\mathrm{K}^{+}\)
- \(\mathrm{S}^{2-}>\mathrm{Cl}^{-}>\mathrm{K}^{+}>\mathrm{Ca}^{2+}\)
- \(\mathrm{K}^{+}>\mathrm{Ca}^{2+}>\mathrm{Cl}^{-}>\mathrm{S}^{2-}\)
Answer: 3 \(\mathrm{S}^{2-}>\mathrm{Cl}^{-}>\mathrm{K}^{+}>\mathrm{Ca}^{2+}\)
S2- > Cl– > K+ > Ca2+
Among isoelectronic species, ionic radii increase with the increase in negative charge. This happens because the effective nuclear charge (Zeff) decreases.
Similarly, ionic radii decrease with an increase in positive charge as Zeff increases.
NEET practice questions on Periodic Table
Question 21. Which of the following represents the correct order of increasing electron gain enthalpy with a negative sign for the elements O, S, F and Cl?
- Cl < F < O < S
- O < S < F < Cl
- F < S < O < Cl
- S < O < Cl < F
Answer: 2. O < S < F < Cl
Cl atom has the highest electron affinity in the periodic table. F being a member of group 17 has higher electron gain enthalpy than S which belongs to group 16. This in turn is higher than the electron affinity of O atom. Thus, Cl >F>S>O
It is worth noting that the electron gain enthalpy of oxygen and fluorine, the members of the second period, have less negative values of electron gain enthalpy than the corresponding elements sulphur and chlorine of the third period.
This is due to small size of the atoms of oxygen and fluorine. As a result, there is a strong inter-electronic repulsion when an extra electron is added to these atoms, i.e., the electron density is high and the addition of an extra electron is not easy.
Question 22. Among the elements Ca, Mg, P and Cl, the order of increasing atomic radii is
- Mg < Ca < Cl < P
- Cl < P < Mg < Ca
- P < Cl < Ca < Mg
- Ca < Mg < P < Cl
Answer: 2. Cl < P < Mg < Ca
The atomic radii decrease on moving from left to right in a period, the order of sizes for Cl, P and Mg is Cl < P < Mg. Down the group size increases. thus, overall order is Cl<P<Mg<Ca.
Question 23. Among the following which one has the highest cation to anion size ratio?
- CsI
- CsF
- LiF
- NaF
Answer: 2. CsF
The cation-to-anion size ratio will be maximum when the cation is of the largest size and the anion is of the smallest size.
Among the given species, Cs+ has the maximum size among given cations and F– has the smallest size among given anions, thus CsF has the highest rc/ra ratio.
Chemistry MCQs on Periodic Classification for NEET
Question 24. Amongst the elements with the following electronic configurations, which one of them may have the highest ionisation energy?
- \(\mathrm{Ne}\left[3 s^2 3 p^2\right]\)
- \({Ar}\left[3 d^{10} 4 s^2 4 p^3\right]\)
- \(\mathrm{Ne}\left[3 s^2 3 p^1\right]\)
- \(\mathrm{Ne}\left[3 s^2 3 p^3\right]\)
Answer: 4. \(\mathrm{Ne}\left[3 s^2 3 p^3\right]\)
Among options (1), (3) and (4), option (4) has the highest ionisation energy because of extra stability associated with, a half-filled 3p-orbital.
In option (2), the presence of 3d10 electrons offers a shielding effect, as a result, the 4p3 electrons do not experience much nuclear charge and hence, the electrons can be removed easily.
Question 25. Identify the correct order of the size of the following.
- \(\mathrm{Ca}^{2+}<\mathrm{K}^{+}<\mathrm{Ar}<\mathrm{Cl}^{-}<\mathrm{S}^{2-}\)
- \(\mathrm{Ar}<\mathrm{Ca}^{2+}<\mathrm{K}^{+}<\mathrm{Cl}^{-}<\mathrm{S}^{2-}\)
- \(\mathrm{Ca}^{2+}<\mathrm{Ar}<\mathrm{K}^{+}<\mathrm{Cl}^{-}<\mathrm{S}^{2-}\)
- \(\mathrm{Ca}^{2+}<\mathrm{K}^{+}<\mathrm{Ar}<\mathrm{S}^{2-}<\mathrm{Cl}^{-}\)
Answer: 1. \(\mathrm{Ca}^{2+}<\mathrm{K}^{+}<\mathrm{Ar}<\mathrm{Cl}^{-}<\mathrm{S}^{2-}\)
Among isoelectronic ions, ionic radii of anions is more than those of cations. Further size of the anion increases with an increase in negative charge and size of the cation decreases with an increase in positive charge.
Chemistry MCQs on Periodic Classification for NEET
Question 26. With which of the following electronic configuration an atom has the lowest ionisation enthalpy?
- \(1 s^2 2 s^2 2 p^3\)
- \(1 s^2 2 s^2 2 p^5 3 s^1\)
- \(1 s^2 2 s^2 2 p^6\)
- \(1 s^2 2 s^2 2 p^5\)
Answer: 2. \(1 s^2 2 s^2 2 p^5 3 s^1\)
The larger the atomic size, the smaller the value of the ionisation enthalpy. Again higher the screening effect, the lesser the value of ionisation potential. Hence, option (2) has the lowest ionisation enthalpy.
Question 27. Which one of the following ionic species has the greatest proton affinity to form a stable compound?
- \(\mathrm{NH}_2^{-}\)
- \(\mathrm{F}^{-}\)
- \(\mathrm{I}^{-}\)
- \(\mathrm{HS}^{-}\)
Answer: 1. \(\mathrm{NH}_2^{-}\)
In going from Ieft to right across a period in the periodic table, the basicity (l.e., proton affinity) decreases as the electronegativity of the atom possessing the lone pair of electrons increases.
Hence, the basicity of NH2– is higher than F–. On moving down a group, as the atomic size increases, basicity decreases.
Hence, F is more basic than I– and HO– is more basic than HS–. Hence, among the given ionic species, NH2– has maximum proton affinity.
Question 28. Which of the following is the most basic oxide?
- SeO2
- Al2O3
- Sb2O3
- Bi2O3
Answer: 4. Bi2O3
⇒ \(\mathrm{SeO}_2 \longrightarrow\) acidic oxide,
⇒ \(\mathrm{Al}_2 \mathrm{O}_3, \mathrm{Sb}_2 \mathrm{O}_3 \longrightarrow\) amphoteric, \(\mathrm{Bi}_2 \mathrm{O}_3 \longrightarrow\) basic oxide.
Periodic Table quiz for NEET
Question 29. What is the correct relationship between the pH of isomolar solutions of sodium oxide, Na2O (pH1), sodium sulphide, Na2S (pH2), sodium selenide, Na2Se (pH3) and sodium telluride Na2Te (pH4)?
- \(\mathrm{pH}_1>\mathrm{pH}_2>\mathrm{pH}_3>\mathrm{pH}_4\)
- \(\mathrm{pH}_1>\mathrm{pH}_2=\mathrm{pH}_3>\mathrm{pH}_4\)
- \(\mathrm{pH}_1<\mathrm{pH}_2<\mathrm{pH}_3<\mathrm{pH}_4\)
- \(\mathrm{pH}_1<\mathrm{pH}_2<\mathrm{pH}_3=\mathrm{pH}_4\)
Answer: 1. \(\mathrm{pH}_1>\mathrm{pH}_2>\mathrm{pH}_3>\mathrm{pH}_4\)
⇒ \(\begin{array}{l|l}
\mathrm{Na}_2 \mathrm{O} & \text { Basic character } \\
\mathrm{Na}_2 \mathrm{~S} & \text { decreases down the group } \\
\mathrm{Na}_2 \mathrm{Se} & \\
\mathrm{Na}_2 \mathrm{Te} &
\end{array}\)
pH ∝ basic character
Hence, pH1 > pH2 > PH3 > pH4
Question 30. Ionic radii are
- Inversely proportional to the effective nuclear charge
- Inversely proportional to the square of the effective nuclear charge
- Directly proportional to the effective nuclear charge
- Directly proportional to the square of effective nuclear charge.
Answer: 2. Inversely proportional to the square of effective nuclear charge
Question 31. The ions \(\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}\) and \(\mathrm{Al}^{3+}\) are isoelectronic. Their ionic radii show
- A significant increase from O2- to Al3+
- A significant decrease from O2- to Al3+
- An increase from O2- to F– and then decrease from Na+ to Al3+
- A decrease from O2- to F– and then increase from Na+ to Al3+
Answer: 2. A significant decrease from O2- to Al3+
The ions \(\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Na}^{+}, \mathrm{Mg}^{2+}\) and \(\mathrm{Al}^{3+}\) are isoelectronic.
Amongst isoelectronic ions, the ionic radii of anions are more than that of cations. Further size of the anion increases with an increase in the +ve charge and the size of the cation decreases with an increase in the +ve charge.
Hence, correct order is \(\mathrm{O}^{2-}>\mathrm{F}^{-}>\mathrm{Na}^{+}>\mathrm{Mg}^{2+}>\mathrm{Al}^{3+} .\)
Question 32. Which of the following orders is wrong?
- \(\mathrm{NH}_3<\mathrm{PH}_3<\mathrm{AsH}_3\)-acidic
- \(\mathrm{Li}<\mathrm{Be}<\mathrm{B}<\mathrm{C}-1^{\text {st }}\) IP
- \(\mathrm{Al}_2 \mathrm{O}_3<\mathrm{MgO}<\mathrm{Na}_2 \mathrm{O}<\mathrm{K}_2 \mathrm{O}-\) basic
- \(\mathrm{Li}^{+}<\mathrm{Na}^{+}<\mathrm{K}^{+}<\mathrm{Cs}^{+}-\) ionic radius.
Answer: 2. \(\mathrm{Li}<\mathrm{Be}<\mathrm{B}<\mathrm{C}-1^{\text {st }}\) IP
Li, Be, B, C – these elements belong to the same period. Generaliy the value of 1’t ionisation potential increases on moving from left to right in a period, since the nuclear charge of the elements also increase in the same direction.
But the ionisation potential of boron (B → 2s² 2p¹) is lower than that of beryllium (Be → 2s²) since in the case of boron, 2p¹ electron has to be removed to get B+ while in the case of Be (2s²), s-electron has to be removed to get Be+ (2s¹). p-electron can be removed more easily than s-electron so the energy required to remove electron will be less in case of boron.
The order will be Li < B < Be < C.
NEET question bank on Element Classification
Question 33. The correct order of 1st ionisation potential following elements Be, B, C, N, O is
- B<Be<C<O<N
- B<Be<C<N<O
- Be<B<C<N<O
- Be<B<C<O<N
Answer: 1. B<Be<C<O<N
The energy required to remove the most loosely bound electron from an isolated gaseous atom is called the ionisation energy. The ionisation potential increases as the size of the atom decreases. Atoms with fully or partly filled orbitals have high ionisation potential.
Question 34. Which of the following elements has the maximum electron affinity?
- I
- Br
- Cl
- F
Answer: 3. Cl
Among the halogens the electron affinity value of ‘F’ should be maximum. But due to small size, there is interelectronic repulsion thus, there is difficulty in the entry of new electrons. Thus, the E.A. value is slightly lower than chlorine andthe order is I < Br < F < Cl.
Question 35. The first ionization potentials (eV) of Be and B respectively are
- 8.29,8.29
- 9.32,9.32
- 8.29,9.32
- 9.32,8.29
Answer: 4. 9.32,8.29
⇒ \({ }_4 \mathrm{Be} \rightarrow 1 s^2 2 s^2,{ }_5 \mathrm{~B} \rightarrow 1 s^2 2 s^2 2 p^1\)
Due to the stable fully-fiIled ‘s’-orbital arrangement of electrons in the ‘Be’ atom, more energy is required to remove an electron from the valence shell than the ‘ B’-atom. Therefore ‘Be’ has a higher ionisation potential than ‘B’.
Question 36. Which one of the following is the correct order of the size of iodine species?
- \(I^{+}>I^{-}>\mathrm{I}\)
- \(I^{-}>I^{\text {I }^{+}}\)
- \(I>I^{-}>I^{+}\)
- \(I^{\text {I }^{+}}>I^{-}\)
Answer: 2. \(I^{-}>I^{\text {I }^{+}}\)
Positive ion is always smaller and negative ion is always larger than the parent atom.
Question 37. Which of the following ions is the largest in size?
- K+
- Ca2+
- Cl–
- S2-
Answer: 4. S2-
Since all of these ions contain 18 electrons each, these are isoelectronic. For isoelectronic ions, the anion having a large negative charge is the largest in size i.e., S2-.
NEET question bank on Element Classification
Question 38. Which of the following has the smallest size?
- \(\mathrm{Al}^{3+}\)
- \(\mathrm{F}\)
- \(\mathrm{Na}^{+}\)
- \(\mathrm{Mg}^{2+}\)
Answer: 1. \(\mathrm{Al}^{3+}\)
These are isoelectronic ions (ions with the same number of electrons) and for isoelectronic ions, the greater the positive charge, the greater the force of attraction on the electrons by the nucleus and the smaller the size of the ion. Thus, Al3+ has the smallest size.
Question 39. Among the following oxides, the one which is most basic is
- ZnO
- MgO
- Al2O3
- N2O5
Answer: 2. MgO
Al2O3 and ZnO are amphoteric. N2O5 is strongly acidic. MgO is the rnost basic.
Question 40. Which of the following has the largest size?
- Na
- Na+
- Na–
- Can’t be predicted.
Answer: 3. Na–
The cations are always smaller than the neutral atom and anions are always larger in size, Na– > Na > Na+
Question 41. \(\mathrm{Na}^{+}, \mathrm{Mg}^{2+}, \mathrm{Al}^{3+} \text { and } \mathrm{Si}^{4+}\) are isoelectronic. The order of their ionic size is
- \(\mathrm{Na}^{+}>\mathrm{Mg}^{2+}<\mathrm{Al}^{3+}<\mathrm{Si}^{4+}\)
- \(\mathrm{Na}^{+}<\mathrm{Mg}^{2+}>\mathrm{Al}^{3+}>\mathrm{Si}^{4+}\)
- \(\mathrm{Na}^{+}>\mathrm{Mg}^{2+}>\mathrm{Al}^{3+}>\mathrm{Si}^{4+}\)
- \(\mathrm{Na}^{+}<\mathrm{Mg}^{2+}>\mathrm{Al}^{3+}<\mathrm{Si}^{4+}\)
Answer: 3. \(\mathrm{Na}^{+}>\mathrm{Mg}^{2+}>\mathrm{Al}^{3+}>\mathrm{Si}^{4+}\)
In isoelectronic ions, the size of the cation decreases as the magnitude of the positive charge increases
Multiple choice questions on Periodicity in Properties for NEET
Question 42. In the periodic table from left to right in a period, the atomic volume
- Decreases
- Increases
- Remains same
- First decreases then increases.
Answer: 4. First decreases then increases.
Within a period from left to right, atomic volume first decreases and then increases.
Question 43. Which electronic configuration of an element has an abnormally high difference between second and third ionization energy?
- \(1 s^2, 2 s^2, 2 p^6, 3 s^1\)
- \(1 s^2, 2 s^2, 2 p^6, 3 s^1, 3 p^1\)
- \(1 s^2, 2 s^2, 2 p^6, 3 s^2, 3 p^2\)
- \(1 s^2, 2 s^2, 2 p^6, 3 s^2\)
Answer: 4. \(1 s^2, 2 s^2, 2 p^6, 3 s^2\)
An abnormally high difference between 2nd and 3rd ionisation energy means that the element has two valence electrons, which is the case in configuration (4)
Question 44. One of the characteristic properties of non-metals is that they
- Are reducing agents
- Form basic oxides
- Form cations by electron gain
- Are electronegative.
Answer: 4. Are electronegative.
Question 45. Which one of the following has the minimum value of the cation/anion ratio?
- NaCl
- KCl
- MgCl2
- CaF2
Answer: 3. MgCl2
The order of ionic size for given ions will be K2+ > Ca2+ > Mg2+ and that of CI– > F–. Therefore, MgCl2 has a minimum value of cation/anion (Mg2+/Cl–) ratio.
Question 46. Which of the following sets has the strongest tendency to form anions?
- Ga, Ni, Tl
- Na, Mg, Al
- N, O, F
- V, Cr, Mn
Answer: 3. N, O, F
N, O and F are highly electronegative non-metals and will have the strongest tendency to form anions by gaining electrons from metal atoms.
Multiple choice questions on Periodicity in Properties for NEET
Question 47. Elements of which of the following groups will form anions most readily?
- Oxygen family
- Nitrogen family
- Halogens
- Alkali metals
Answer: 3. Halogens
As halogens have seven electrons (ns²np5) in the valence shell, they have a strong tendency to acquire the nearest inert gas configuration by gaining an electron from the metallic atom and forming halide ions easily.
Question 48. In the periodic table, with the increase in atomic number, the metallic character of an element
- Decreases in a period and increases in a group
- Increases in a period and decreases in a group
- Increases both in a period and the group
- Decreases in a period and the group.
Answer: 1. Decreases in a period and increases in a group
Metallic character decreases in a period and increases in a group
Multiple choice questions on Periodicity in Properties for NEET
Question 49. Which of the following atoms will have the smallest size?
- Mg
- Na
- Be
- Li
Answer: 3. Be
The atomic size decreases within a period from left to right, therefore Li > Be and Na > Mg. The size increases in a group from top to bottom. Hence, the size of Na is greater than Li. Overall order Na > Mg > Li > Be. Thus, Be has the smallest size.
