Solid State NEET MCQs
NEET Chemistry For The Solid State Multiple Choice Question
Question 1. The pure crystalline substance on being heated gradually first forms a turbid liquid at constant temperature and still at higher temperature turbidity completely disappears. The behaviour is a characteristic of substance-forming
- Allotropic crystals
- Liquid crystals
- Isomeric crystals
- Isomorphous crystals.
Answer: 2. Liquid crystals
The pure crystalline substance on being heated gradually first forms a turbid liquid at constant temperature and still at higher temperature turbidity completely disappears.
Liquid crystals on heating first become turbid and then on further heating turbidity completely disappears.
Question 2. Glass is a
- Liquid
- Solid
- Supercooled liquid
- Transparent organic polymer.
Answer: 3. Supercooled liquid
Glass is a supercooled liquid which forms a noncrystalline solid without a regular lattice.
Question 3. Most crystals show good cleavage because their atoms, ions or molecules are
- Weakly bonded together
- Strongly bonded together
- Spherically symmetrical
- Arranged in planes.
Answer: 4. Arranged in planes.
Crystals show good cleavage because their constituent particles are arranged in planes.
Question 4. The ability of a substance to assume two or more crystalline structures is called
- Isomerism
- Polymorphism
- Isomorphism
- Amorphism.
Answer: 2. Polymorphism
The phenomenon of the existence of a substance in two or more crystalline structures is called polymorphism.
Solid State NEET MCQs
Question 5. Cation and anion combine in a crystal to form the following type of compound
- Ionic
- Metallic
- Covalent
- Dipole-dipole.
Answer: 1. The electrostatic force of attraction which exists between oppositely charged ions is called an ionic bond.
Question 6. For two ionic solids CaO and KI, identify the wrong statement among the following.
- CaO has a high melting point.
- The lattice energy of CaO is much larger than that of KI.
- KI has a high melting point.
- KI is soluble in benzene.
Answer: 4. KI is soluble in benzene.
KI is an ionic compound while benzene is not.
Read and Learn More NEET MCQs with Answers
Question 7. The correct option for the number of body-centred unit cells in all 14 types of Bravais lattice unit cells is
- 3
- 7
- 5
- 2
Answer: 1. 3
Out of 14 types of Bravaisiattice, three body-centred units crisis are there which are: orthorhombic, tetragonal and cubic
Question 8. For the orthorhombic system, axial ratios are α = β = γ = 90° and the axial angles are
- α = β = γ ≠ 90°
- α = β = γ = 90°
- α = γ = 90°, β ≠ 90°
- α ≠ β ≠ γ ≠ 90°
Answer: 2. α = β = γ = 90°
For orthorhombic system, α = β = γ = 90°
NEET questions on Solid State
Question 9. The number of carbon atoms per unit cell of the diamond unit cell is
- 6
- 1
- 4
- 8
Answer: 4. 8
Diamond is like ZnS (Zincblende).
Carbon forms ccp (fcc) and also occupies half of the tetrahedral voids.
Total no. of carbon atoms per unit cell = 8 x 1/8 + 6 x 1/2 + 4 = 8
Question 10. In a face-centred cubic lattice, a unit cell is shared equally by how many unit cells?
- 2
- 4
- 6
- 8
Answer: 3. 6
Here given unit cell is shared equally by six faces in the fcc which is shared equally by six different unit cells.
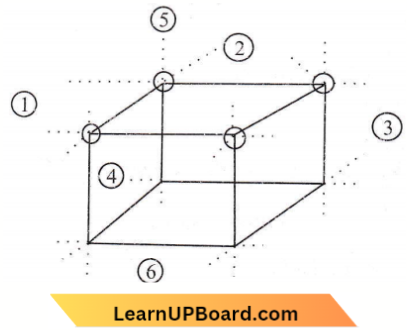
Question 11. When Zn converts from its melted state to its solid state, it has a hcp structure and then finds the number of nearest atoms.
- 6
- 8
- 12
- 4
Answer: 3. 12
hcp is a closed-packed arrangement in which the unit cell is hexagonal and the coordination number is 12
NEET questions on Solid State
Question 12. The fee crystal contains how many atoms in each unit cell?
- 6
- 8
- 4
- 5
Answer: 3. 4
The contribution of eight atoms of face-centred cubic unit cell = 8 x 1/8 = 1 atom. There is one atom at each of the six faces, which is shared by 2 unit cells each.
The contribution of 6 face-centred atoms = 6 x 1/2 = 3
Therefore n = L + 3 = 4
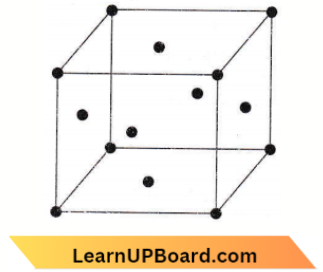
Question 13. The number of atoms contained in a fee unit cell of a monatomic substance is
- 1
- 2
- 4
- 6
Answer: 3. 4
fcc crystal contains = 8 x 1/8 + 6 x 1/2 = 4 atoms in a unit cell
Solid State multiple choice questions NEET
Question 14. A compound is formed by two elements A and B. The element B forms a cubic close-packed structure and atoms of A occupy 1/3 of tetrahedral voids. If the formula of the compound is Ax By, then the value of x + y is in the option
- 3
- 2
- 5
- 4
Answer: 3. 5
A compound is formed by two elements A and B. The element B forms a cubic close-packed structure and atoms of A occupy 1/3 of tetrahedral voids. If the formula of the compound is Ax By,
Let, the number of atoms in ccp unit cell = N
Thus, the number of B atoms = N
Number of tetrahedral voids = 2N
Number of A atoms = \(\frac{2 N}{3}\)
Molecular ratio A:B = \(\frac{2 N}{3}\)
The formula of the compound is A2B2
Hence, x+y=2+3=5
Question 15. What fraction of one edge-centred octahedral void lies in one unit cell of fcc?
- 1/4
- 1/12
- 1/2
- 1/3
Answer: 1. 1/4
There is an octahedral void at each edge of the unit cell such that 1/4th of the void is in one cell.
Question 16. The right option for the number of tetrahedral and octahedral voids in the hexagonal primitive unit cells are
- 12,6
- 8,4
- 6,12
- 2, 1
Answer: 1. 12,6
Number of atoms in hexagonal unit cell = N = 6
Number of octahedral void = N = 6
Number of tetrahedral void = 2N = 12
Solid State multiple choice questions NEET
Question 17. A compound is formed by cation C and anion A. Hie anions form hexagonal close-packed (hep) lattice and the cations occupy 75% of octahedral voids. The formula of the compound is
- \(C_4 A_3\)
- \(C_2 A_3\)
- \(C_3 A_2\)
- \(C_3 A_4\)
Answer: 4. \(C_3 A_4\)
A compound is formed by cation C and anion A. Hie anions form hexagonal close-packed (hep) lattice and the cations occupy 75% of octahedral voids.
Number of atoms per unit cell in hcp = 6
Number of octahedral voids in hcp = 6
Number of anions per unit cell = 6
Number of octahedral voids occupied by cations = 6 x \(\frac{75}{100}\) = \(\frac{9}{2}\)
∴ Formula of compound = C9/2A6 = C3A4
Question 18. In calcium fluoride, having the fluorite structure, the coordination numbers for calcium ion (Ca2+) and fluoride ion (F–) are
- 4 and 2
- 6 and 6
- 8 and 4
- 4 and 8
Answer: 3. 8 and 4
In fluorite structure, Ca2+ ions are in the face-centred cubic arrangement. Each Ca2+. is connected to 4 F– ions below it and to another set of 4 F– ions above it i.e. Ca2+ has a coordination number of 8 and each F1 ion has a coordination number 4.
Question 19. The ionic radii of A+ and B– ions are 0.98 x 10-10 m and 1.81 x 10-10 m. The coordination number of each ion in AB is
- 8
- 2
- 6
- 4
Answer: 3. 6
Radius ration, \(\frac{r_{+}}{r_{-}}=\frac{0.98 \times 10^{-10}}{1.81 \times 10^{-10}}=0.54\)
It lies in the range of 0.414 to 0.732 hence, the coordination number of each ion will be 6 as the compound will have NaCl type structure i.e., octahedral arrangement.
Question 20. The number of octahedral voids (s) per atom present in a cubic close-packed structure is
- 1
- 3
- 2
- 4
Answer: 1. 1
The number of octahedral voids is the same as the number of atoms
Solid State multiple choice questions NEET
Question 21. The structure of a mixed oxide is cubic close-packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One-fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B. The formula of the oxide is
- \(\mathrm{ABO}_2\)
- \(A_2 B_2\)
- \(A_2 B_3 \mathrm{O}_4\)
- \(A B_2 \mathrm{O}_2\)
Answer: 4. \(A B_2 \mathrm{O}_2\)
The structure of a mixed oxide is cubic close-packed (ccp). The cubic unit cell of mixed oxide is composed of oxide ions. One-fourth of the tetrahedral voids are occupied by divalent metal A and the octahedral voids are occupied by a monovalent metal B.
Number of atoms in ccp = 4= O2-
Number of tetrahedral voids = 2 x N = 2 x 4 = B
Number of A2+ ions = 8 x 1/4 = 2
Number of octahedral voids = Number of B+ ions = N = 4
Ratio, O2-: A2+ : Bn = 4 : 2 : 4 = 2 : 1 : 2
Formula of oxide = AB2A2
Question 22. A solid compound XY has a NaCl structure. If the radius of the cation is 100 pm, the radius of the anion (Y–) will be
- 275.1 pm
- 322.5 pm
- 241.5 pm
- 165.7 pm
Answer: 3. 241.5 pm
For NaCl, \(\frac{r_{+}}{r_{-}}=0.414\)
Given: radius of cation =100pm
⇒ \(\frac{100}{r_{-}}=0.414 \Rightarrow \frac{100}{0.414}=r_{-} \Rightarrow r_{-}=241.5 \mathrm{pm}\)
Question 23. A compound formed by elements X and Y crystallises in a cubic structure in which the X atoms are at the corners of a cube and the Y atoms are at the face- centres. The formula of the compound is
- XY3
- X3Y
- XY
- XY2
Answer: 1. XY3
A compound formed by elements X and Y crystallises in a cubic structure in which the X atoms are at the corners of a cube and the Y atoms are at the face- centres.
In a unit cell, X atoms at the corners = \(\frac{1}{8}\) = 1
Y atoms at the face centres = \(\frac{1}{2}\) x 6 = 3
The ratio of X and Y = 1 : 3. Hence formula is XY
NEET practice questions Solid State
Question 24. In a cube of any crystal, an A-atom is placed at every corner and a B-atom is placed at every centre of the face. The formula of a compound is
- AB
- AB3
- A2B2
- A2B3
Answer: 2. AB3
In a cube of any crystal, an A-atom is placed at every corner and a B-atom is placed at every centre of the face.
‘A’ atoms are at ‘8’ corners of the cube.
Thus, no. of ‘A’atoms per unit cell = 8 x \(\frac{1}{8}\) = 1
‘B’ atoms are at the face centre of six faces. Thus, no. of ‘B’ atoms per unit cell = 6 x \(\frac{1}{2}\) = 3
The formula is AB3
Question 25. In crystals of which one of the following ionic compounds would you expect the maximum distance between centres of cations and anions?
- Csl
- CsF
- LiF
- Lil
Answer: 1. Csl
As Cs+ ion has a larger size than Li+ and I– has a larger size than F–, so maximum distance between centres of cations and anions is in CsI.
Question 26. The second-order Bragg diffraction of X-rays with λ = 1.00 Å from a set of parallel planes in a metal occurs at an angle of 60°. Die distance between the scattering planes in the crystal is
- 2.00 Å
- 1.00 Å
- 0.575 Å
- 1.15 Å
Answer: 4. 1.15 Å
The second-order Bragg diffraction of X-rays with λ = 1.00 Å from a set of parallel planes in a metal occurs at an angle of 60°.
According to Bragg’s equation, nλ = 2d sin θ
As, n = 2λ = 1.00Å, θ = 60°, d = ?
2dsinθ = nλ
2dsin60°=2×1Å
2d x \(\frac{\sqrt{3}}{2}=2 \Rightarrow d=\frac{2}{\sqrt{3}}=1.15\) Å (because \(\sin 60^{\circ}=\frac{\sqrt{3}}{2}\))
NEET practice questions Solid State
Question 27. The intermetallic compound LiAg crystallizes in the cubic lattice in which both lithium and silver have a coordination number of eight. The crystal class is
- Face-centred cube
- Simple cube
- Body-centred, cube
- None of these.
Answer: 3. Body-centred, cube
The intermetallic compound LiAg crystallizes in the cubic lattice in which both lithium and silver have a coordination number of eight.
A body-centred cubic unit cell consists of 8 atoms at the corners and one atom at the centre.
Question 28. In the fluorite structure, the coordination number of Ca2+ ions is
- 4
- 6
- 8
- 3
Answer: 3. 8
In fluorite (CaF2) structure, C.N. of Ca2+ =8, C.N. of F– = 4
Question 29. An element has a body-centred cubic (bcc) structure with a cell edge of 288 pm. The atomic radius is
- \(\frac{\sqrt{3}}{4} \times 288 \mathrm{pm}\)
- \(\frac{\sqrt{2}}{4} \times 288 \mathrm{pm}\)
- \(\frac{4}{\sqrt{3}} \times 288 \mathrm{pm}\)
- \(\frac{4}{\sqrt{2}} \times 288 \mathrm{pm}\)
Answer: 1. \(\frac{\sqrt{3}}{4} \times 288 \mathrm{pm}\)
For bcc structure, \(r=\frac{\sqrt{3}}{4} a\), where a is the unit cell edge length and r is the radius of the sphere (atom).
r = \(\frac{\sqrt{3}}{4} \times 288 \mathrm{pm}\)
Chemistry MCQs Solid State NEET
Question 30. The Vacant space in bcc lattice unit cell is
- 48%
- 23%
- 32%
- 26%
Answer: 3. 32%
Packing efficiency of bcc lattice = 68%
Hence, empathy space = 32%
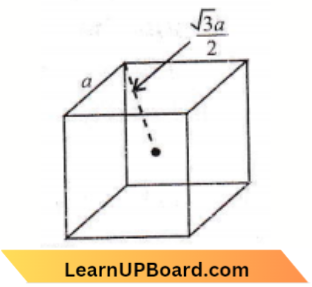
Question 31. If a is the length of the side of a cube, the distance between the body-centred atom and one corner atom in the cube will be
- \(\frac{2}{\sqrt{3}} a\)
- \(\frac{4}{\sqrt{3}} a\)
- \(\frac{\sqrt{3}}{4} a\)
- \(\frac{\sqrt{3}}{2} a\)
Answer: 4. \(\frac{\sqrt{3}}{2} a\)
The distance between the body-centred atom and one corner atom is \(\frac{\sqrt{3} a}{2}\) i.e. half of the body diagonal.
Question 32. A metal crystallises with a face-centred cubic lattice. The edge of the unit cell is 408 pm. The diameter of the metal atom is
- 288 pm
- 408 pm
- 144 pm
- 204 pm
Answer: 1. 288 pm
For a face-centred cubic (fcc) structure.
r = \(\frac{a}{2 \sqrt{2}}, a=408 \mathrm{pm}, r=\frac{408}{2 \sqrt{2}}=144 \mathrm{pm}\)
Diameter = 2r = 2 x 144 = 288 Pm
Chemistry MCQs Solid State NEET
Question 33. AB crystallizes in a body-centred cubic lattice with edge length ‘a’ equal to 387 pm. The distance between two oppositely charged ions in the lattice is
- 335 pm
- 250 pm
- 200 pm
- 300 pm
Answer: 1. 335 pm
For a bcc latlice, 2(r++r–)= √3a
where r+ = radius of cation, r– = radius of anion
a = edge length
∴ \(\left(r_{+}+r_{-}\right)=\frac{\sqrt{3} \times 387}{2}=335.15 \mathrm{pm}\) = 335 Pm
Question 34. Lithium metal crystallises in a body-centred cubic crystal. If the length of the side of the unit cell of lithium is 351 pm, the atomic radius of lithium will be
- 151.8 pm
- 75.5 pm
- 300.5 pm
- 240.8 pm
Answer: 1. 151.8 pm
Lithium metal crystallises in a body-centred cubic crystal. If the length of the side of the unit cell of lithium is 351 pm
Since Li crystallises in body-centred cubic crystal atomic radius,
r = \(\frac{\sqrt{3} a}{4} \quad(a=\text { edge length })\)
∴ r = \(\frac{\sqrt{3}}{4} \times 351=151.8 \mathrm{pm}\)
Given: a=351 pm
Question 35. Copper crystallises in a face-centred cubic lattice with a unit cell length of 361 pm. What is the radius of a copper atom in pm?
- 157
- 181
- 108
- 128
Answer: 4. 128
Since Cu crystallises in a face-centred cubic lattice.
Atomic radius r = \(\frac{a}{2 \sqrt{2}}\) (a = edge length = 361 pm)
∴ r = \(\frac{361}{2 \sqrt{2}}=127.6=128 \mathrm{pm}\)
Question 36. Which of the following statements is not correct?
- The number of carbon atoms in a unit cell of diamond is 8.
- The number of Bravais lattices in which a crystal can be categorized is 14.
- The fraction of the total volume occupied by the atoms in a primitive cell is 0.48.
- Molecular solids are generally volatile.
Answer: 3. The fraction of the total volume occupied by the atoms in a primitive cell is 0.48.
The packing fraction for a cubic unit cell is given by f = \(\frac{Z \times \frac{4}{3} \pi r^3}{a^3}\).
Solid State quiz for NEET
Question 37. If a stands for the edge length of the cubic systems: simple cubic, body-centred cubic and face-centred cubic, then the ratio of radii of the spheres in these systems will be respectively
- \(\frac{1}{2} a: \frac{\sqrt{3}}{2} a: \frac{\sqrt{2}}{2} a\)
- \(1 a: \sqrt{3} a: \sqrt{2} a\)
- \(\frac{1}{2} a: \frac{\sqrt{3}}{4} a: \frac{1}{2 \sqrt{2}} a\)
- \(\frac{1}{2} a: \sqrt{3} a: \frac{1}{\sqrt{2}} a\)
Answer: 3. \(\frac{1}{2} a: \frac{\sqrt{3}}{4} a: \frac{1}{2 \sqrt{2}} a\)
For simple cubic: r = a/2
For body centred: r = \(a \sqrt{3} / 4\)
For face-centred: r = \(\frac{a}{2 \sqrt{2}}\)
where a = edge length, r = radius
∴ The ratio of radii of the three will be \(\frac{a}{2}: \frac{a \sqrt{3}}{4}: \frac{a}{2 \sqrt{2}}\)
Question 38. The fraction of the total volume occupied by the atoms present in a simple cube is
- \(\frac{\pi}{3 \sqrt{2}}\)
- \(\frac{\pi}{4 \sqrt{2}}\)
- \(\frac{\pi}{4}\)
- \(\frac{\pi}{6}\)
Answer: 4. \(\frac{\pi}{6}\)
Question 39. The pyknometric density of sodium chloride crystal is 2.165 x 103 kg m-3 while its X-ray density is 2.178 x 103 kg m-3. The fraction of unoccupied sites in sodium chloride crystals is
- 5.96
- 5.96 x 10-2
- 5.96 x 10-1
- 5.96 x 10-3
Answer: 4. 5.96 x 10-3
The pyknometric density of sodium chloride crystal is 2.165 x 103 kg m-3 while its X-ray density is 2.178 x 103 kg m-3.
Molar volume from psychometric density = \(\frac{M}{2.165 \times 10^3} \mathrm{~m}^3\)
Molar volume from X-ray density = \(\frac{M}{2.178 \times 10^3} \mathrm{~m}^3\)
Volume occupied = \(\frac{M}{10^3}\left(\frac{1}{2.165}-\frac{1}{2.178}\right) \mathrm{m}^3\)
Fraction unoccupied
= \(\left(\frac{0.013 M \times 10^{-3}}{2.165 \times 2.178}\right) /\left(\frac{M \times 10^{-3}}{2.165}\right)=5.96 \times 10^{-3}\)
Question 40. The edge length of face-centred unit cubic cells is 508 pm. If the radius of the cation is 110 pm, the radius of the anion is
- 144 pm
- 398 pm
- 288 pm
- 618 pm
Answer: 1. 144 pm
The edge length of face-centred unit cubic cells is 508 pm. If the radius of the cation is 110 pm
In the face-centred cubic lattice, the unit cell, a = r + 2R + r
where r = Radius of cation, R = Radius of anion
⇒ 508 = 2 x 110 + 2R
∴ R = 144Pm
NEET MCQs on Solid State
Question 41. Copper crystallises in a fcc unit cell with a cell edge length of 3.608 x 10-8 cm. The density of copper is 8.92 g cm-3. Calculate the atomic mass of copper.
- 63.1 u
- 31.55 u
- 60 u
- 65 u
Answer: 1. 63.1 u
Copper crystallises in a fcc unit cell with a cell edge length of 3.608 x 10-8 cm. The density of copper is 8.92 g cm-3.
Density of unit cell
d = \(\frac{Z \times M}{N_0 \times a^3}\)
Given, a = \(3.608 \times 10^{-8} \mathrm{~cm}\)
d = \(8.92 \mathrm{~g} / \mathrm{cm}^3\)
Z = 4(for fcc)
M = \(\frac{N_0 \times a^3 \times d}{Z}=\frac{6.023 \times 10^{23} \times\left(3.608 \times 10^{-8}\right)^3 \times 8.92}{4}\)
= \(63.08 \mathrm{u} \approx 63.1 \mathrm{u}^4\)
Question 42. Iron exhibits bee structure at room temperature. Above 900°C, it transforms to fcc structure. The ratio of the density of iron at room temperature to that at 900°C (assuming molar mass and atomic radii of iron remain constant with temperature) is
- \(\frac{\sqrt{3}}{\sqrt{2}}\)
- \(\frac{4 \sqrt{3}}{3 \sqrt{2}}\)
- \(\frac{3 \sqrt{3}}{4 \sqrt{2}}\)
- \(\frac{1}{2}\)
Answer: 3. \(\frac{3 \sqrt{3}}{4 \sqrt{2}}\)
For bcc lattice: \(Z=2, a=\frac{4 r}{\sqrt{3}}\)
For fcc lattice : Z=4, a = \(2 \sqrt{2}r\)
∴ \(\frac{d_{R T}}{d_{900^{\circ} \mathrm{C}}}=\frac{\left(\frac{Z M}{N_A a^3}\right)_{b c c}}{\left(\frac{Z M}{N_A a^3}\right)_{f c c}}\)
Given, molar mass and atomic radii are constant.
= \(\frac{2}{4}\left(\frac{2 \sqrt{2} r}{\frac{4 r}{\sqrt{3}}}\right)^3=\frac{3 \sqrt{3}}{4 \sqrt{2}}\)
NEET MCQs on Solid State
Question 43. Lithium has a bcc structure. Its density is 530 kg m-3 and its atomic mass is 6.94 g mol-1. Calculate the edge length of a unit cell of lithium metal. (NA = 6.02 x 1023 mol-1)
- 527 pm
- 264 pm
- 154 pm
- 352 pm
Answer: 4. 352 pm
Lithium has a bcc structure. Its density is 530 kg m-3 and its atomic mass is 6.94 g mol-1.
For bcc, Z = 2, ρ = 530 kg m-3,
at, mass of Li = 6.94 g mol-1, NA = 6.02 x 1023 mol-1
⇒ \(\rho=530 \mathrm{~kg} \mathrm{~m}^{-3}=\frac{530 \times 1000 \mathrm{~g}}{1 \times(100)^3 \mathrm{~cm}^3}=0.53 \mathrm{~g} \mathrm{~cm}^{-3}\)
⇒ \(\rho=\frac{Z \times \text { At. mass }}{N_A \times a^3}\)
⇒ \(a^3=\frac{Z \times \text { At. mass }}{N_A \times \rho}=\frac{2 \times 6.94}{6.02 \times 10^{23} \times 0.53}\)
= \(43.5 \times 10^{-24} \mathrm{~cm}^3\)
a = \(352 \times 10^{-10} \mathrm{~cm}=352 \mathrm{pm}\)
Question 44. A metal has a fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g cm-3. The molar mass of the metal is (NA Avogadro’s constant = 6.02 x 1023 mol-1)
- 27 g mol-1
- 20 g mol-1
- 40 g mol-1
- 30 g mol-1
Answer: 1. 27 g mol-1
A metal has a fcc lattice. The edge length of the unit cell is 404 pm. The density of the metal is 2.72 g cm-3.
d = \(\frac{Z M}{N_A a^3}(Z=4 \text { for } f c c)\)
M = \(\frac{d \times N_A \times a^3}{Z}=\frac{2.72 \times 6.023 \times 10^{23} \times\left(404 \times 10^{-10}\right)^3}{4}\)
M = \(27 \mathrm{~g} \mathrm{~mol}^{-1}\)
Question 45. CsBr crystallises in a body-centred cubic lattice. The unit cell length is 436.6 pm. Given that the atomic mass of Cs = 133 and that of Br = 80 amu and Avogadro number being 6.02 x 1023 mol-1, the density of CsBr is
- 4.25 g/cm³
- 42.5 g/cm³
- 0.425 g/cm³
- 8.25 g/cm³
Answer: 1. 4.25 g/cm³
CsBr crystallises in a body-centred cubic lattice. The unit cell length is 436.6 pm. Given that the atomic mass of Cs = 133 and that of Br = 80 amu and Avogadro number being 6.02 x 1023 mol-1,
Density of \(\mathrm{CsBr}=\frac{Z \times M}{a^3 \times N_A}\)
= \(\frac{1 \times 213}{\left(436.6 \times 10^{-10}\right)^3 \times 6.023 \times 10^{23}}=4.25 \mathrm{~g} / \mathrm{cm}^3\)
(It has one formula unit in the unit cell, so Z=1.)
Question 46. An element (atomic mass =100 g/mol) having bcc structure has a unit cell edge of 400 pm. The density of the element is
- 7.289 g/cm³
- 2.144 g/cm³
- 10.376 g/cm³
- 5.188 g/cm³
Answer: 4. 5.188 g/cm³
Cell edge = 400 pm; number of atoms in bcc (Z) = 2
and atomic mass = 100 g/mol.
Since atomic mass is 100 g/mol, therefore the mass of each atom (m) = \(\frac{100}{6.023 \times 10^{23}}=16.6 \times 10^{-23} \mathrm{~g}\)
We know that volume of unit cell = (400 pm)³
= (64 x 106)pm³ = 64 x 10-24 cm³ and
mass of unit cell= Z x m =2x (16.6 x 10-23) = 33.2 x 10-23 g
Therefore, density = \(\frac{\text { Mass of unit cell }}{\text { Volume of unit cell }}\)
= \(\frac{33.2 \times 10^{-23}}{64 \times 10^{-24}}=5.188 \mathrm{~g} / \mathrm{cm}^3\)
Solid State NEET question bank
Question 47. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): In a particular point defect, an ionic solid is electrically neutral, even if a few of its cations are missing from its unit cell.
Reason (R): In an ionic solid, Frenkel defect arises due to the dislocation of a cation from its lattice site to the interstitial site, maintaining overall electrical neutrality.
In the light of the above statements, choose the most appropriate answer from the options given below:
- Both (A) and (R) are correct and (R) is the correct explanation of (A).
- Both (A) and (R) are correct but (R) is not the correct explanation of (A).
- (A) is correct but (R) is not correct.
- (A) is not correct but (R) is correct
Answer: 2. Both (A) and (R) are correct but (R) is not the correct explanation of (A).
Frenkel defect is shown by ionic solids. The smaller ion (usually a cation) is dislocated from its normal site to an interstitial site. It creates a vacancy defect at its original site and an interstitial defect at its new location.
Question 48. The formula of nickel oxide with metal deficiency defect in its crystal is Ni0-98O. The crystal contains Ni2+ and Ni3+ ions. The fraction of nickel existing as Ni2+ ions in the crystal is
- 0.96
- 0.04
- 0.50
- 0.3
Answer: 1. 0.96
The formula of nickel oxide with metal deficiency defect in its crystal is Ni0-98O. The crystal contains Ni2+ and Ni3+ ions.
Let the fraction of metal which exists as Ni2+ ion be x.
Then the fraction of metal as Ni3+ = 0.98 – x
∴ 2x + 3(0.98 – x) = 2
⇒ 2x+2.94-3x=2
⇒ x=0.94=0.96
Question 49. The correct statement regarding defects in crystalline solids is
- Frenkel defects decrease the density of crystalline solids
- Frenkel defect is a dislocation defect
- Frenkel defect is found in halides of alkaline metals
Answer: 2. Frenkel defect is a dislocation defect
Frenkel defect is a dislocation defect as smaller ions (usually cations) are dislocated from normal sites to interstitial sites. Frenkel defect is shown by compounds having large differences in the size of cations and anions hence, alkali metal halides do not show Frenkel defect.
Also, Schottky’s defect decreases the density of the crystal while Frenkel’s defect has no effect on the density of crystals.
Question 50. The appearance of colour in solid alkali metal halides is generally due to
- Interstitial positions
- F-centres
- Schottky defect
- Frenkel defect.
Answer: 2. F-centres
F-centres are the sites where anions are missing and instead, electrons are present. They are responsible for colours.
Solid State NEET question bank
Question 51. Schottky defect in crystals is observed when
- The density of the crystal is increased
- Unequal number of cations and anions are missing from the lattice
- An ion leaves its normal site and occupies an interstitial site
- An equal number of cations and anions are missing from the lattice.
Answer: 4. Equal number of cations and anions are missing from the lattice.
In the Schottky defect, an equal no. of cations and anions are missing from the lattice. So, the crystal remains neutral. Such defect is more common in highly ionic compounds of similar cationic and anionic size, i.e. NaCl
Question 52. Ionic solids, with Schottky defects, contain in their structure
- Cation vacancies only
- Cation vacancies and interstitial cations
- An equal number of cation and anion vacancies
- Anion vacancies and interstitial anions.
Answer: 3. Equal number of cation and anion vacancies
When an atom is missing from its normal lattice site, a lattice vacancy is created. Such a defect, which involves an equal number of cation and anion vacancies in the crystal lattice is called a Schottky defect
Question 53. Which is the incorrect statement?
- Density decreases in the case of crystals with Schottky defect.
- NaCl(s) is an insulator, silicon is a semiconductor, silver is a conductor, and quartz is a piezoelectric crystal.
- Frenkel defect is favoured in those ionic compounds in which sizes of cations and anions are almost equal.
- FeO0.98 has a non-stoichiometric metal deficiency defect.
Answer: 3. Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal. and
4. FeO0.98 has a non-stoichiometric metal deficiency defect.
Frenkel defect is favoured in those ionic compounds in which there is a large difference in the size of cations and anions.
Non-stoichiometric defects due to metal deficiency are shown by FexO where x = 0.93 to 0.96.
Question 54. With which one of the following elements silicon should be doped so as to give a p-type of semiconductor?
- Selenium
- Boron
- Germanium
- Arsenic
Answer: 2. Boron
If silicon is doped with any of the elements of group 13 (B, Al, Ga, In, Tl) of the periodic table, the p-type of the semiconductor will be obtained.
Solid State NEET question bank
Question 55. If NaCl is doped with 10-4 mol % of SrCl2, the concentration of cation vacancies will be (NA = 6.02 x 1023 mol-1)
- 6.02 x 1016 mol-1
- 6.02 x 1017 mol-1
- 6.02 x 1014 mol-1
- 6.02 x 1015 mol-1
Answer: 2. 6.02 x 1017 mol-1
As each Sr2+ ion introduces one cation vacancy, therefore, the concentration of cation vacancies = mole % of SrCI2 added.
∴ The concentration of cation vacancies = 10-4 mole%
= \(\frac{10^{-4}}{100} \times 6.023 \times 10^{23}=6.023 \times 10^{17}p\)
Question 56. If we mix a pentavalent impurity in a crystal lattice of germanium, what type of semiconductor formation will occur?
- n-type semiconductor
- p-type semiconductor
- Both (1) and (2)
- None of these
Answer: 1. n-type semiconductor
When an impurity atom with 5 valence electrons (as arsenic) is introduced in a germanium crystal, it replaces one of the germanium atoms. Four of the five valence electrons of the impurity atom form covalent bonds with each valence electron of four geranium atoms and the fifth valence electron becomes free to move in the crystal structure.
This free electron acts as a charge carrier. Such as an impure germanium crystal is called an n-type semiconductor because in it charge carriers are negative (free electrons)
Solid State NEET question bank
Question 57. On doping Ge metal with a little of In or Ga, one gets
- p-type semiconductor
- n-type semiconductor
- Insulator
- Rectifier.
Answer: 1. p-type semiconductor
p-type of semiconductors are produced
Due to metal deficiency defects
By adding impurities containing fewer electrons (i.e. atoms of group 13). Ge belongs to Group 14 and In or Ga to Group 13. Hence on doping-type semiconductor is obtained. This doping of Ge with In increases the electrical conductivity of the Ge crystal.
