NEET Questions On d And f Block Elements
NEET Chemistry For d And f Block Elements Multiple Choice Questions
Question 1. Sc(Z=21) is a transition element but Zn (Z = 30) is not because
- Both Sc3+ and Zn2+ ions are colourless and form white compounds
- In the case of Sc, 3d orbitals are partially filled but in Zn, these are filled
- The last electron is assumed to be added to the 4s level in the case of Zn
- Both Sc and Zn do not exhibit variable gn oxidation states.
Answer: 2. In the case of Sc, 3d orbitals are partially filled but in Zn, these are filled
Sc (Z = 21) has incompletely filled 3d-orbitals in its ground state (3d1), it is considered as a transition element but Zn (Z = 30) has completely filled d-orbitals (3d10) in its ground state and its common oxidation state (+2), thus, it is not considered as a transition element.
Question 2. Which of the following ions has electronic configuration [Ar] 3d6?
- \(\mathrm{Ni}^{3+}\)
- \(\mathrm{Mn}^{3+}\)
- \(\mathrm{Fe}^{3+}\)
- \(\mathrm{Co}^{3+}\)
(Atomic nunbers Mn = 25, Fe = 26, Co = 27, Ni = 28)
Answer: 4. \(\mathrm{Co}^{3+}\)
The electronic configuration ofthe given ions is \(\mathrm{Ni}^{3+}:[\mathrm{Ar}] 3 d^0 4 s^0, \mathrm{Mn}^{3+}:[\mathrm{Ar}] 3 d^4 4 s^0\); \(\mathrm{Fe}^{3+}:[\mathrm{Ar}] 3 d^5 4 s^0, \mathrm{Co}^{3+}:[\mathrm{Ar}] 3 d^6 4 s^0\)
Question 3. Among the following series of transition metal ions, the one where all metal ions have 3d² electronic configuration is
[Atomic number Ti = 22, V = 23, Cr = 24, Mn = 25]
- \(\mathrm{Ti}^{3+}, \mathrm{V}^{2+}, \mathrm{Cr}^{3+}, \mathrm{Mn}^{4+}\)
- (\(\mathrm{Ti}^{+}, \mathrm{V}^{4+}, \mathrm{Cr}^{6+}, \mathrm{Mn}^{7+}\)
- \(\mathrm{Ti}^{4+}, \mathrm{V}^{3+}, \mathrm{Cr}^{2+}, \mathrm{Mn}^{3+}\)
- \(\mathrm{Ti}^{2+}, \mathrm{V}^{3+}, \mathrm{Cr}^{4+}, \mathrm{Mn}^{5+}\)
Answer: 4. \(\mathrm{Ti}^{2+}, \mathrm{V}^{3+}, \mathrm{Cr}^{4+}, \mathrm{Mn}^{5+}\)
⇒ \({ }_{22} \mathrm{Ti}: 3 d^2 4 s^2 ; \mathrm{Ti}^{2+}: 3 d^2\)
⇒ \({ }_{23} \mathrm{~V}: 3 d^3 4 s^2 ; \mathrm{V}^{3+}: 3 d^2\)
⇒ \({ }_{24} \mathrm{Cr}: 3 d^4 4 s^2 ; \mathrm{Cr}^{4+}: 3 d^2\)
⇒ \({ }_{25} \mathrm{Mn}: 3 d^5 4 s^2 ; \mathrm{Mn}^{5+}: 3 d^2\)
Read and Learn More NEET MCQs with Answers
NEET questions on d and f Block Elements
Question 4. Which of the following configurations is correct for iron?
- \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^7\)
- \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^5\)
- \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^5\)
- \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^6\)
Answer: 4. \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 4 s^2 3 d^6\)
Question 5. Which of the following has more unpaired d-electrons?
- \(\mathrm{N}^{3+}\)
- \(\mathrm{Fe}^{2+}\)
- \(\mathrm{Zn}^{+}\)
- \(\mathrm{Cu}^{+}\)
Answer: 2. \(\mathrm{Fe}^{2+}\)
Question 6. The electronic configuration of transition elements is exhibited by
- \(n s^1\)
- \(n s^2 n p^5\)
- \(n s^2(n-1) d^{1-10}\)
- \(n s^2(n-1) d^{10}\)
Answer: 3. \(n s^2(n-1) d^{1-10}\)
The general electronic configuration of transition elements is ns2 (n – 1)d1-10.
Question 7. The electronic configurations of four elements are given below. Which element does not belong to the same family as others?
- \([\mathrm{Xe}] 4 f^{14} 5 d^{10} 6 s^2\)
- \([\mathrm{Kr}] 4 d^{10} 5 s^2\)
- \([\mathrm{Ne}] 3 s^2 3 p^5\)
- \([\mathrm{Ar}] 3 d^{10} 4 s^2\)
Anwer: 3. \([\mathrm{Ne}] 3 s^2 3 p^5\)
It is the electronic configuration of a p-block element whereas other configurations are those of d-block elements
Question 8. The stability of Cu2+ is more than Cu+ salts in an aqueous solution due to
- Hydration energy
- Second ionisation enthalpy
- First ionisation enthalpy
- Enthalpy of atomization.
Answer: 1. Hydration energy
The stability of \(\mathrm{Cu}_{(a q)}^{2+}\) rather than \(\mathrm{Cu}_{(\mathrm{aq})}^{+}\) is due to the much more negative \(\Delta_{\text {ind }} H^{\circ}\) of \(\mathrm{Cu}_{(\text {app }}^{2+}\) than \(\mathrm{Cu}_{(\text {aqp })}^{+}\), which more than compensates for the second ionisation enthalpy of \(\mathrm{Cu}\). \(\mathrm{V}_2 \mathrm{O}_4\) dissolves in acids to give \(\mathrm{VO}^{2+}\) salts.
Question 9. Zr (Z = 40) and Hf (Z = 72) have similar atomic and ionic radii because of
- Having similar chemical properties
- Belonging to the same group
- Diagonal relationship
- Lanthanoid contraction.
Answer: 4. Lanthanoid contraction.
The atomic and ionic radii of Zt and Hf are almost identical due to the poor shielding effect of 4f-electrons, which leads to lanthanoid contraction.
NEET questions on d and f Block Elements
Question 10. Identify the incorrect statement.
- \(\mathrm{Cr}^{2+}\left(d^4\right)\) is a stronger reducing agent than \(\mathrm{Fe}^{2+}\left(d^6\right)\) in water.
- Transition metals and their compounds are known for their catalytic activity due to their ability to adopt multiple oxidation states and to form complexes.
- Interstitial compounds are those that are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals.
- The oxidation states of chromium in \(\mathrm{CrO}_4^{2-}\) and \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) are not the same.
Answer: 4. The oxidation states of chromium in \(\mathrm{CrO}_4^{2-}\) and \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) are not the same.
The oxidation states of Cr in \(\mathrm{CrO}_4^{2-}\) and \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\) is same i.e., +6.
Question 11. The calculated spin-only magnetic moment of Cr2+ ion is
- 3.87 BM
- 4.90 BM
- 5.92 BM
- 2.84 BM
Answer: 2. 4.90 BM
Cr: \(3 d^5 4 s^1, \mathrm{Cr}^{2+}: 3 d^4\) has four unpaired electrons.
μ \(=\sqrt{n(n+2)}=\sqrt{4(4+2)}=\sqrt{24} \approx 4.90\) B.M.
Question 12. Match the metal ions given in Column A with the spin magnetic moments of the ions given in Column B and assign the correct code:
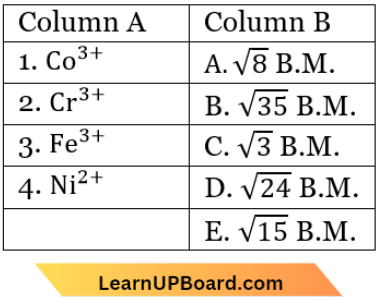
- 1-D, 2-E, 3-B, 4-A
- 1-A, 2-B, 3-C, 4-D
- 1-D, 2-A, 3-B, 4-C
- 1-C, 2-E, 3-A, 4-B
Answer: 1. 1-D, 2-E, 3-B, 4-A
⇒ \(\mathrm{Co}^{3+}:[\mathrm{Ar}] 3 d^6\), unpaired \(e^{-}(n)=4\)
Spin magnetic moment \((\mu)=\sqrt{4(4+2)}=\sqrt{24}\) B.M.
⇒ \(\mathrm{Cr}^{3+}:[\mathrm{Ar}] 3 d^3\), unpaired \(e^{-}(n)=3\)
Spin magnetic moment \((\mu)=\sqrt{3(3+2)}=\sqrt{15}\) B.M.
⇒ \(\mathrm{Fe}^{3+}:[\mathrm{Ar}] 3 d^5\), unpaired \(e^{-}(n)=5\)
Spin magnetic moment \((\mu)=\sqrt{5(5+2)}=\sqrt{35}\) B.M.
⇒ \(\mathrm{Ni}^{2+}:[\mathrm{Ar}] 3 d^s\), unpaired \(e^{-}(n)=2\)
Spin magnetic moment \((\mu)=\sqrt{2(2+2)}=\sqrt{8}\) B.M.
Transition Elements multiple choice NEET
Question 13. Magnetic moment 2.84 B.M. is given by (Atomic number Ni = 28, Ti = 22, Cr = 24, Co = 27)
- \(\mathrm{Cr}^{2+}\)
- \(\mathrm{Co}^{2+}\)
- \(\mathrm{Ni}^{2+}\)
- \(\mathrm{Ti}^{3+}\)
Answer: 3. \(\mathrm{Ni}^{2+}\)
Magnetic moment \((\mu)=\sqrt{n(n+2)}\)
2.84 B.M. corresponds to 2 unpaired electrons.
Cr2+; 3d4, 4 unpaired electrons
⇒ \(\mathrm{Co}^{2+}: 3 d^p, 3\) unpaired electrons
⇒ \(\mathrm{Ni}^{2+}: 3 d^8, 2\) unpaired electrons
⇒ \(\mathrm{Ti}^{3+}: 3 d^1, 1\) unpaired electron
Question 14. Which of the following processes does not involve the oxidation of iron?
- Formation of Fe(CO)5 from Fe.
- Liberation of H2 from steam by iron at high temperature.
- Rusting of iron sheets.
- Decolourisation of blue CuSO4 solution by iron.
Answer: 1. Formation of Fe(CO)5 from Fe.
The oxidation number of Fe in Fe(CO)5 is zero.
Question 15. Which of the following statements about the interstitial compounds is incorrect?
- They are much harder than the pure metal.
- They have higher melting points than the pure metal.
- They retain metallic conductivity.
- They are chemically reactive.
Answer: 4. They are chemically reactive.
Interstitial compounds are generally chemically inert.
Question 16. Identify the alloy containing a non-metal as a constituent in it.
- Invar
- Steel
- Bell metal
- Bronze
Answer: 2. Steel
Invar ⇒ Ni(metal) + Fe(metal)
Steel ⇒ C(non-metal) + Fe(metal)
Betrl ⇒ Cu(metal) + Sn(metal) + F
Bronze ⇒ Cu(rnetal) + Sn(metal)
Question 17. The catalytic activity of transition metals and their compounds is ascribed mainly to
- Their magnetic behaviour
- Their unfilled d-orbitals
- Their ability to adopt variable oxidation states
- Their chemical reactivity.
Answer: 3. Their ability to adopt variable oxidation states
Question 18. Which one of the following does not correctly represent the correct order of the property indicated against it?
- Ti < V < Cr < Mn; increasing number of oxidation states
- Ti3+ < V3+ < Cr3+ < Mn3+; increasing magnetic moment
- Ti < V < Cr < Mn; increasing melting points
- Ti < V < Mn < Cr; increasing 2nd ionization enthalpy
Answer: 3. Ti < V < Cr < Mn; increasing melting points

Hence, the given order is correct.
Magnetic moment \((\mu)=\sqrt{n(n+2)}\) B.M.
For \(\mathrm{Ti}^{3+} n=1, \mu=\sqrt{1(1+2)}=\sqrt{3}\) B.M.
For \(\mathrm{V}^{3+} n=2, \mu=\sqrt{2(2+2)}=\sqrt{8}\) B.M.
For \(\mathrm{Cr}^{3+} n=3, \mu=\sqrt{3(3+2)}=\sqrt{15}\) B.M.
For \(\mathrm{Mn}^{3+} n=4, \mu=\sqrt{4(4+2)}=\sqrt{24}\) B.M.
Thus, magnetic moment order : \(\mathrm{Ti}^{3+}<\mathrm{V}^{3+}<\mathrm{Cr}^{3+}<\mathrm{Mn}^{3+}\)
Transition Elements multiple choice NEET
Question 19. Four successive members of the first series of transition metals are listed below. For which one of them does the standard potential (E°M²/M) value have a positive sign?
- Co (Z = 27)
- Ni (Z = 28)
- Cu(Z=29)
- Fe (Z = 26)
Asnwer: 3. Cu(Z=29)
Question 20. For the four successive transition elements (Cr, Mn, Fe and Co), the stability of +2 oxidation state will be there in which of the following order?
- Mn > Fe > Cr > Co
- Fe > Mn > Co > Cr
- Co > Mn > Fe > Cr
- Cr > Mn > Co > Fe
(Atomic numbers Cr = 24, Mn = 25, Fe = 26, Co = 27)
Answer: 1. Mn > Fe > Cr > Co
Spin correlation and exchange energy give an electronic configuration special stability which is greatest for half-filled electronic configurations. Mn2+ (d5) gets stabilisation due to half-filled configuration’ In Fe2+ (d6) the placing of one extra electron in a subshell destabilises. Placing 2 electrons in Co2+ (d7) destabilises it more. Cr2+ (d6) has one vacant subshell. Fe2+ gets more -stabilisation compared to Cr2+ through exchange energy So, the order is as follows: Mn > Fe > Cr > Co.
Question 21. Which of the following ions will exhibit colour in aqueous solutions?
- \(\mathrm{La}^{3+}(Z=57)\)
- \(\mathrm{Ti}^{3+}(Z=22)\)
- \(\mathrm{Lu}^{3+}(Z=71)\)
- \(\mathrm{Sc}^{3+}(Z=21)\)
Answer: 2. \(\mathrm{Ti}^{3+}(Z=22)\)
Ions which have unpaired electrons exhibit colour in aqueous solution. Ti3+ has an outer electronic configuration of 4s03d1, i.e., 1 unpaired electron. Thus, its solution will be coloured. Others are colourless due to empty or completely filled outermost orbitals.
Question 22. Which of the following pairs has the same size?
- \(\mathrm{Fe}^{2+}, \mathrm{Ni}^{2+}\)
- \(\mathrm{Zr}^{4+}, \mathrm{Ti}^{4+}\)
- \(\mathrm{Zr}^{4+}, \mathrm{Hf}^{4+}\)
- \(\mathrm{Zn}^{2+}, \mathrm{Hf}^{4+}\)
Answer: 3. \(\mathrm{Zr}^{4+}, \mathrm{Hf}^{4+}\)
Hf+ and Zr++ belong to group 4B. However, Hf4+ has the same size as Zr4+ due to the addition of 14 lanthanide elements before it in which electrons are added into the f subshell which poorly shields the outer electrons and contraction in size occurs.
NEET practice questions d and f Block
Question 23. Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states?
- \(3 d^5 4 s^1\)
- \(3 d^5 4 s^2\)
- \(3 d^2 4 s^2\)
- \(3 d^3 4 s^2\)
Answer: 2. \(3 d^5 4 s^2\)
The greater the number of valence electrons, the more will be the number of oxidation states exhibited by the element.
3d54s1 can show a maximum of 6 oxidation states.
3d54s2, carr shows a maximum of 7 oxidation states.
3d54s2 can show a maximum of 4 oxidation states.
3d34s2 can show a maximum of 5 oxidation states
Question 24. The correct order of decreasing second ionisation enthalpy of Ti(22), V(23), Cr(24) and Mn(25) is
- Mn > Cr > Ti > V
- Ti > V > Cr > Mn
- Cr > Mn > V > Ti
- V > Mn > Cr > Ti
Answer: 3. Cr > Mn > V > Ti
The electronic configuration of the given elements are
Mn: \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^5 4 s^2\)
Cr: \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^5 4 s^1\)
Ti: \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^2 4 s^2\)
V: \(1 s^2 2 s^2 2 p^6 3 s^2 3 p^6 3 d^3 4 s^2\)
In general, ionization potential (both lst and 2nd) increases from left to right across the period due to an increase in effective nuclear charge. On this basis, the second IP values should exhibit the trend: Mn>Cr>V>Ti
But the actual observed order is: Cr > Mn > V > Ti Practically, only chromium is exceptional and the rest others show the normal trend. This exceptional behaviour of chromium is due to the stable configuration (3d5) that it achieves after the loss of the first electron.
Question 25. In which of the following pairs are both the ions coloured in aqueous solution?
(Atomic number Sc = 21, Ti = 22, Ni = 28, Cu = 29,Co = 27)
- \(\mathrm{Ni}^{2+}, \mathrm{Cu}^{+}\)
- \(\mathrm{Ni}^{2+}, \mathrm{Ti}^{3+}\)
- \(\mathrm{Sc}^{3+}, \mathrm{Ti}^{3+}\)
- \(\mathrm{Sc}^{3+}, \mathrm{Co}^{2+}\)
Answer: 2. \(\mathrm{Ni}^{2+}, \mathrm{Ti}^{3+}\)
Sc: \([\mathrm{Ar}] 3 d^1 4 s^2, \mathrm{Sc}^{3+}:[\mathrm{Ar}]\) Colourless
Ti: \([\mathrm{Ar}] 3 d^2 4 s^2, \mathrm{Ti}^{3+}:[\mathrm{Ar}] 3 d^1\) Coloured
Ni: \([\mathrm{Ar}] 3 d^3 4 s^2, \mathrm{Ni}^{2+}:[\mathrm{Ar}] 3 d^8\) Coloured
Cu: \([\mathrm{Ar}] 3 d^{10} 4 s^1, \mathrm{Cu}^{+}:[\mathrm{Ar}] 3 d^{10}\) Colourless
Co: \([\mathrm{Ar}] 3 d^7 4 s^2, \mathrm{Co}^{2+}:[\mathrm{Ar}] 3 d^7\) Coloured
⇒ \(\mathrm{Ti}^{3+}, \mathrm{Ni}^{2+}\) and \(\mathrm{Co}^{2+}\) are coloured due to presence of unpaired electrons.
NEET practice questions d and f Block
Question 26. Four successive members of the first-row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy?
- Vanadium (Z = 23)
- Chromium (Z = 24)
- Manganese (Z = 25)
- Iron (Z = 26)
Answer: 3. Manganese (Z = 25)
- \(\mathrm{V}^{2+}(23):[\mathrm{Ar}] 3 d^3 4 s^0\)
- \(\mathrm{Cr}^{2+}(24):[\mathrm{Ar}] 3 d^4 4 s^0\)
- \(\mathrm{Mn}^{2+}(25):[\mathrm{Ar}] 3 d^5 4 s^0\)
- \(\mathrm{Fe}^{2+}(26):[\mathrm{Ar}] 3 d^5 4 s^1\)
Question 27. The aqueous solution containing which one of the following ions will be colourless?
(Atomic number : Sc = 21, Fe = 26,Ti = 22, Mn = 25)
- \(\mathrm{Sc}^{3+}\)
- \(\mathrm{Fe}^{2+}\)
- \(\mathrm{Ti}^{3+}\)
- \(\mathrm{Mn}^{2+}\)
Answer: 1. \(\mathrm{Sc}^{3+}\)
- If the transition metal ion has an unpaired electron then it shows colour.\(\mathrm{Sc}^{3+}:[\mathrm{Ar}] 3 d^0 4 s^0\)
- \(\mathrm{Fe}^{2+}:[\mathrm{Ar}] 3 d^5 4 s^1\)
- \(\mathrm{Ti}^{3+}:[\mathrm{Ar}] 3 d^1 4 s^0\)
- \(\mathrm{Mn}^{2+}:[\mathrm{Ar}] 3 d^5 4 s^0\)
Sc3+ does not contain unpaired electrons, hence it will not undergo d – d transition and will not show colour.
Question 28. Which one of the following characteristics of the transition metals is associated with their catalytic activity?
- High enthalpy of atomization
- Paramagnetic behaviour
- Colour of hydrated ions
- Variable oxidation states
Answer: 4. Variable oxidation states
The transition elements, on account of their variable valency, are able to form unstable intermediate compounds very readily.
Question 29. The basic character of the transition metal monoxides follows the order
(Atomic numbers Ti = 22, V = 23, Cr = 24, Fe = 26)
- VO > CrO > TiO > FeO
- CrO > VO > FeO > TiO
- TiO > FeO > VO > CrO
- TiO > VO > CrO > FeO
Answer: 4. TiO > VO > CrO > FeO
The order of basicity of transition metal monoxides is, TiO > VO > CrO > FeO.
NEET practice questions d and f Block
Question 30. Which of the following shows a maximum number of oxidation states?
- Cr
- Fe
- Mn
- V
Answer: 3. Mn
Each of the elements in groups 3 B to 7 B can show the maximum oxidation state equal to its group number. Mn in group seven and shows a maximum oxidation state of +7 in KMnO4.
Question 31. Which ion is colourless?
- \(\mathrm{Cr}^{4+}\)
- \(\mathrm{Sc}^{3+}\)
- \(\mathrm{Ti}^{3+}\)
- \(\mathrm{V}^{3+}\)
Answer: 2. \(\mathrm{Sc}^{3+}\)
⇒ \({ }_{21} \mathrm{Sc}:[\mathrm{Ar}] 3 d^1 4 s^2\)
In Sc3+ there are no unpaired ‘d’ electrons, therefore it is colourless in its solution.
Question 32. Bell metal is an alloy of
- Cu + Zn
- Cu + Sn
- Cu + Pb
- Cu + Ni
Answer: 2. Cu + Sn
Bell metal ⇒ Cu = 80%, Sn = 20%
It is used for making belts, utensils, etc.
Question 33. In which of the following compounds does transition metal have a zero oxidation state?
- \(\mathrm{NOClO}_4\)
- \(\mathrm{NH}_2 \mathrm{NH}_2\)
- \(\mathrm{CrO}_5\)
- \(\left[\mathrm{Fe}(\mathrm{CO})_5\right]\)
Answer: 4. \(\left[\mathrm{Fe}(\mathrm{CO})_5\right]\)
In iron carbonyl, the oxidation number of ‘Fe’ is zero.
⇒ \(\left[\mathrm{Fe}(\mathrm{CO})_5\right]: x+5 \times 0=0 \Rightarrow x=0\)
Question 34. Which one of the following ionic species will impart colour to an aqueous solution?
- \(\mathrm{Zn}^{2+}\)
- \(\mathrm{Cu}^{+}\)
- \(\mathrm{Ti}^{4+}\)
- \(\mathrm{Cr}^{3+}\)
Answer: 4. \(\mathrm{Cr}^{3+}\)
⇒ \(\mathrm{Cr}^{3+}(24): 1 s^2, 2 s^2, 2 p^6, 3 s^2, 3 p^6, 3 d^3\)
As Cr3+ ion has three unpaired electrons in its valence shell, so it imparts colour to an aqueous solution.
Chemistry MCQs d and f Block NEET
Question 35. A transition element 10 has a configuration [Ar]3d4 in its +3 oxidation state. Its atomic number is
- 22
- 19
- 25
- 26
Answer: 3. 25
The metal atom will have three more electrons.
Therefore, the atomic number of the metal = 18 + 4 + 3 =25
Question 36. Amongst \(\mathrm{TiF}_6^{2-}, \mathrm{CoF}_6^{3-}, \mathrm{Cu}_2 \mathrm{Cl}_2 \text { and } \mathrm{NiCl}_4^{2-} \text {, }\) which are the colourless species? (Atomic number of Ti = 22, Co = 27, Cu = 29, Ni = 28)
- \(\mathrm{CoF}_6^{3-}\) and \(\mathrm{NiCl}_4^{2-}\)
- \(\mathrm{TiF}_6^{2-}\) and \(\mathrm{Cu}_2 \mathrm{Cl}_2\)
- \(\mathrm{Cu}_2 \mathrm{Cl}_2\) and \(\mathrm{NiCl}_4^{2-}\)
- \(\mathrm{TiF}_6^{2-}\) and \(\mathrm{CoF}_6^{3-}\)
Answer: 2. \(\mathrm{TiF}_6^{2-}\) and \(\mathrm{Cu}_2 \mathrm{Cl}_2\)
In TiF62- titanium is in +4 oxidation state. In Cu2Cl2, the copper is in +1 state. Thus, in both cases, the transition from one d-orbital to another is not possible.
Ti: \([\mathrm{Ar}] 3 d^2 4 s^2 \rightarrow \mathrm{Ti}^{4+}:[\mathrm{Ar}] 3 d^0 4 s^0\)
Cu:\([\mathrm{Ar}] 3 d^{10} 4 s^1 \rightarrow \mathrm{Cu}^{+}:[\mathrm{Ar}] 3 d^{10} 4 s^0\)
Question 37. The mercury is the only metal which is liquid at 0°C. This is due to its
- High Vapour Pressure
- Weak Metallic Bond
- High Ionization Energy
- Both (2) And (3).
Answer: 4. Both (2) And (3).
The very high ionisation energy of Hg makes it difficult for electrons to participate in metallic bonding.
Question 38. Which of the following statements is incorrect?
- All the transition metals except scandium form MO oxides which are ionic.
- The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7
- Basic character increases from V2O3 to V2O4 to V2O5
- V2O4 dissolves in acids to give VO43- salts.
- CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below:
- 3 and 4 only
- 2 and 3 only
- 1 and 5 only
- 2 and 4 only
Answer: 1. 3 and 4 only
In vanadium, there is a gradual change of basic character from the basic V2O3 to less basic V2O4 and to amphoteric V2O5
Chemistry MCQs d and f Block NEET
Question 39. In the neutral or faintly alkaline medium, KMnO4 oxidises iodide into iodate. The change in the oxidation state of manganese in this reaction is from
- +7 to +4
- +6 to +4
- +7 to +3
- +6 to +5
Answer: 1. +7 to +4
In the neutral or faintly alkaline medium, KMnO4 oxidises iodide into iodate.
Reaction of MnO4– with I in neutral or faintly alkaline solution: \(\stackrel{+7}{2 \mathrm{MnO}_4^{-}}+\mathrm{H}_2 \mathrm{O}+\stackrel{-1}{\mathrm{I}^{-}} \longrightarrow \stackrel{+4}{\mathrm{MnO}_2}+2 \mathrm{OH}^{-}+\stackrel{+5}{\mathrm{IO}_3^{-}}\)
Question 40. The manganate and permanganate ions are tetrahedral, due to
- The π-bonding involves the overlap of d-orbitals of oxygen with d- d-orbitals of manganese
- The π-bonding involves the overlap of p-orbitals of oxygen with d-orbitals of manganese
- There is no π-bonding
- The π-bonding involves the overlap of the p-orbitals of oxygen with the p-orbitals of manganese.
Answer: 2. The π-bonding involves the overlap of p-orbitals of oxygen with d-orbitals of manganese
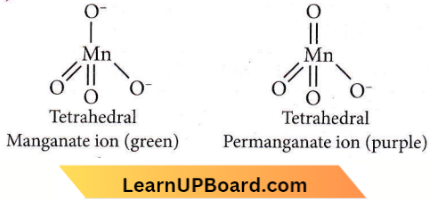
In manganate and permanganate ions, π-bonding takes place by the overlap of p -p-orbitals of oxygen with the d-orbitals of manganese.
Question 41. When neutral or faintly alkaline KMnO4 is treated with potassium iodide, the iodide ion is converted into ‘X’. ‘X’ is
- \(\mathrm{I}_2\)
- \(\mathrm{IO}_4^{-}\)
- \(\mathrm{IO}_3^{-}\)
- \(\mathrm{IO}^{-}\)
Answer: 3. \(\mathrm{IO}_3^{-}\)
In neutral or faintly alkaline solutions: \(2 \mathrm{MnO}_4^{-}+\mathrm{H}_2 \mathrm{O}+\mathrm{I}^{-} \longrightarrow 2 \mathrm{MnO}_2+2 \mathrm{OH}^{-}+\mathrm{IO}_3^{-}\)
Question 42. Which one of the following ions exhibits d-d transition and paramagnetism as well?
- \(\mathrm{CrO}_4^{2-}\)
- \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\)
- \(\mathrm{MnO}_4^{-}\)
- \(\mathrm{MnO}_4^{2-}\)
Answer: 4. \(\mathrm{MnO}_4^{2-}\)
In \(\mathrm{CrO}_4^{2-}, \mathrm{Cr}^{6+}(n=0)\) diamagnetic
In \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}, \mathrm{Cr}^{6+}(n=0)\) diamagnetic
In \(\mathrm{MnO}_4^{-}, \mathrm{Mn}^{7+}(n=0)\) diamagnetic
In \(\mathrm{MnO}_4^{2-}, \mathrm{Mn}^{6+}(n=1)\) paramagnetic
In \(\mathrm{MnO}_4^{2-}\), one unpaired electron (n) is present in the d-orbital so, the d-d transition is possible.
d and f Block quiz for NEET
Question 43. Name the gas that can readily decolourise acidified KMnO4 solution.
- \(\mathrm{SO}_2\)
- \(\mathrm{NO}_2\)
- \(\mathrm{P}_2 \mathrm{O}_5\)
- \(\mathrm{CO}_2\)
Answer: 1. \(\mathrm{SO}_2\)
SO, readily decolourises the pink-violet colour of acidified KMnO4 solution.
⇒ \({2 \mathrm{KMnO}_4}+5 \mathrm{SO}_2+2 \mathrm{H}_2 \mathrm{O} \longrightarrow \mathrm{K}_2 \mathrm{SO}_4+\underset{\mathrm{(Colourless)}}{2 \mathrm{MnSO}_4}+2 \mathrm{H}_2 \mathrm{SO}_4\)
Question 44. Which one of the following statements is correct when SO2 is passed through acidified K2Cr3O73 solution?
- SO2 is reduced.
- Green Cr2(SO4)3 is formed.
- The solution turns blue.
- The solution is decolourised.
Answer: 2. Green Cr2(SO4)3 is formed.
⇒ \(\begin{aligned}
\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+\mathrm{H}_2 \mathrm{SO}_4+3 \mathrm{SO}_2 \longrightarrow \mathrm{K}_2 \mathrm{SO}_4 \\
+\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3+\mathrm{H}_2 \mathrm{O} \\
(\text { Green) }
\end{aligned}\)
Question 45. Assuming complete ionisation, same moles of which of the following compounds will require the least amount of acidified KMnO4 for complete oxidation?
- \(\mathrm{FeSO}_3\)
- \(\mathrm{FeC}_2 \mathrm{O}_4\)
- \(\mathrm{Fe}\left(\mathrm{NO}_2\right)_2\)
- \(\mathrm{FeSO}_4\)
Answer: 4. \(\mathrm{FeSO}_4\)
⇒ \(\mathrm{KMnO}_4\left(\mathrm{Mn}^{7+}\right)\) changes to \(\mathrm{Mn}^{2+}\) i.e., number of electrons involved per mole of \(\mathrm{KMnO}_4\) is 5
1. For \(\mathrm{FeSO}_3\), \(\mathrm{Fe}^{2+} \longrightarrow \mathrm{Fe}^{3+} \quad(\mathrm{No}\), of \(e^{-}\)s involved =1)
⇒ \(\mathrm{SO}_3^{2-} \longrightarrow \mathrm{SO}_4^{2-}\) (No. of electrons involved=2)
Total number of \(e^{-} s\) involved =1+2=3
2. For \(\mathrm{FeC}_2 \mathrm{O}_4\), \(\mathrm{Fe}^{2+} \longrightarrow \mathrm{Fe}^{3+}\)(No. of es involved =1)
⇒ \(\mathrm{C}_2 \mathrm{O}_4^{2-} \longrightarrow 2 \mathrm{CO}_2(\mathrm{No}\), of \(e^{-}s\) involved =2)
Total number of \(e^{-} \mathrm{s}\) involved =1+2=3
3. For \(\mathrm{Fe}\left(\mathrm{NO}_2\right)_2\), \(\mathrm{Fe}^{2+} \longrightarrow \mathrm{Fe}^{3+}(\) No. of \(e^{-}\)s involved=1)
⇒ \(2 \mathrm{NO}_2^{-} \longrightarrow 2 \mathrm{NO}_3^{-}\)(No. of \(e^{-}\)s involved =4)
Total number of \(e^{-}\)s involved =1+4=5
For \(\mathrm{FeSO}_4\), \(\mathrm{Fe}^{2+} \longrightarrow \mathrm{Fe}^{3+}\) (Number of \(e^{-}\)s involved =1)
Total number of \(e^{-}\)s involved =1
⇒ As \(\mathrm{FeSO}_4\) requires the least number of electrons thus, it will require the least amount of \(\mathrm{KMnO}_4\).
d and f Block quiz for NEET
Question 46. The reaction of aqueous KMnO4 with TT2O2 in acidic conditions gives
- \(\mathrm{Mn}^{4+}\) and \(\mathrm{O}_2\)
- \(\mathrm{Mn}^{2+}\) and \(\mathrm{O}_2\)
- \(\mathrm{Mn}^{2+}\) and \(\mathrm{O}_3\)
- \(\mathrm{Mn}^{4+}\) and \(\mathrm{MnO}_2\)
Answer: 2. \(\mathrm{Mn}^{2+}\) and \(\mathrm{O}_2\)
Hydrogen peroxide is oxidised to H2O and O2
Hydrogen peroxide is oxidised to \(\mathrm{H}_2 \mathrm{O}\) and \(\mathrm{O}_2\)
\(2 \mathrm{KMnO}_4+3 \mathrm{H}_2 \mathrm{SO}_4+5 \mathrm{H}_2 \mathrm{O}_2 \longrightarrow \mathrm{K}_2 \mathrm{SO}_4+2 \mathrm{MnSO}_4+8 \mathrm{H}_2 \mathrm{O}+5 \mathrm{O}_2\)
or, \(2 \mathrm{MnO}_4^{-}+5 \mathrm{H}_2 \mathrm{O}_2+6 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Mn}^{2+}+8 \mathrm{H}_2 \mathrm{O}+5 \mathrm{O}_2\)
Question 47. Which of the statements is not true?
- On passing H2S through an acidified K2Cr2O7 solution, a milky colour is observed.
- Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis.
- K2Cr2O7 solution in an acidic medium is orange.
- K2Cr2O7 solution becomes yellow MB on increasing the pH beyond 7.
Solution: 2. Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis.
Potassium dichromate is preferred over sodium dichromate in volumetric analysis, primarily because the latter is hygroscopic in nature and therefore, accurate weighing is not possible in a normal atmosphere.
Question 48. The acidified K2Cr2O7 solution turns green when Na2SO3 is added to it. This is due to the formation of
- \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3\)
- \(\mathrm{CrO}_4^{2-}\)
- \(\mathrm{Cr}_2\left(\mathrm{SO}_3\right)_3\)
- \(\mathrm{CrSO}_4\)
Answer: 1. \(\mathrm{Cr}_2\left(\mathrm{SO}_4\right)_3\)
The acidified K2Cr2O7 solution turns green when Na2SO3 is added to it.
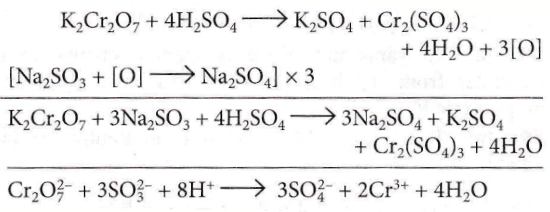
Question 49. The number of moles of KMnO4 reduced by one mole of KI in an alkaline medium is
- One
- Two
- Five
- One Fifth.
Answer: 2. Two

NEET MCQs on Transition Elements
Question 50. K2Cr2O7 on heating with aqueous NaOH gives
- \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}\)
- \(\mathrm{Cr}(\mathrm{OH})_2\)
- \(\mathrm{CrO}_4^{2-}\)
- \(\mathrm{Cr}(\mathrm{OH})_3\)
Answer: 3. \(\mathrm{CrO}_4^{2-}\)
⇒ \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7+2 \mathrm{NaOH} \rightarrow \mathrm{K}_2 \mathrm{CrO}_4+\mathrm{Na}_2 \mathrm{CrO}_4+\mathrm{H}_2 \mathrm{O}\) or \(\mathrm{Cr}_2 \mathrm{O}_7^{2-}+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{CrO}_4^{2-}+\mathrm{H}_2 \mathrm{O}\)
Question 51. KMnO4 reacts with oxalic acid according to the equation \(2 \mathrm{MnO}_4^{-}+5 \mathrm{C}_2 \mathrm{O}_4{ }^{2-}+16 \mathrm{H}^{+} \longrightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}\), Here 20 mL of 0.1 M KMnO4 is equivalent to
- 50 mL of 0.5 M C2H2O4
- 20 mL of 0.1 M C2H2O4
- 20 mL of 0.5 M C2H2O4
- 50 mL of 0.1 M C2H2O4
Answer: 4. 50 mL of 0.1 M C2H2O4
⇒ \(2 \mathrm{MnO}_4^{-}+5 \mathrm{C}_2 \mathrm{O}_4^{2-}+16 \mathrm{H}^{+} \rightarrow 2 \mathrm{Mn}^{2+}+10 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}\)
∴ 2 moles of MnO–4 = 5 moles of C2O2-4–
20 mL of 0.1 M KMnO4 = 2 mmol of KMnO4
Also, 50 mL of 0.1 M C2H2O4 = 5 mmol of C2O2-4
Therefore, these are equivalent
Question 52. The oxidation state of Cr in K2Cr2O7 is
- +5
- +3
- +6
- +7
Answer: 3. +6
Let, the oxidation state of Cr in K2Cr2O7 is x. Then
2+2x-14=0
⇒ 2x=12
∴ x=+6
Question 53. Gadolinium has a low value of third ionisation, enthalpy because of
- Small size
- High exchange enthalpy
- High electronegativity
- High basic character.
Answer: 2. High exchange enthalpy
Due to high exchange enthalpy Gd3+ (4f7) acquires extra stability and has low third ionisation enthalpy.
Question 54. Which one of the following statements related to lanthanons is incorrect?
- Europium shows a +2 oxidation state.
- The basicity decreases as the ionic radius decreases from Pr to Lu.
- All the lanthanoids are much more reactive than aluminium.
- Ce(+4) solutions are widely used as (H) oxidizing agents in volumetric analysis.
Answer: 3. All lanthanons are much more reactive than aluminium.
The first few members of the lanthanoid series are quite reactive, almost like calcium. However, with increasing atomic numbers, their behaviour becomes similar to that of aluminium.
NEET MCQs on Transition Elements
Question 55. The electronic configurations of Eu (Atomic Number 63), Gd (Atomic Number 64) and Tb (Atomic Number 65) are
- \([\mathrm{Xe}] 4 f^6 5 d^1 6 s^2,[\mathrm{Xe}] 4 f^7 5 d^1 6 s^2\) and \([\mathrm{Xe}] 4 f^8 5 d^1 6 s^2\)
- \([\mathrm{Xe}] 4 f^7 6 s^2,[\mathrm{Xe}] 4 f^7 5 d^1 6 s^2\) and \([\mathrm{Xe}] 4 f^9 6 s^2\)
- \([\mathrm{Xe}] 4 f^7 6 s^2,[\mathrm{Xe}] 4 f^8 6 s^2\) and \([\mathrm{Xe}] 4 f^8 5 d^1 6 s^2\)
- \([\mathrm{Xe}] 4 f^6 5 d^1 6 s^2,[\mathrm{Xe}] 4 f^7 5 d^1 6 s^2\) and \([\mathrm{Xe}] 4 f^9 6 s^2\)
Answer: 2. \([\mathrm{Xe}] 4 f^7 6 s^2,[\mathrm{Xe}] 4 f^7 5 d^1 6 s^2\) and \([\mathrm{Xe}] 4 f^9 6 s^2\)
Question 56. Gadolinium belongs to 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
- \([\mathrm{Xe}] 4 f^3 5 s^1\)
- \([\mathrm{Xe}] 4 f^7 5 d^1 6 s^2\)
- \([\mathrm{Xe}] 4 f^6 5 d^2 6 s^2\)
- \([\mathrm{Xe}] 4 f^8 6 d^2\)
Answer: 2. \([\mathrm{Xe}] 4 f^7 5 d^1 6 s^2\)
Question 57. Because of lanthanoid contraction, which of the following pairs of elements have nearly the same atomic radii? (Numbers in the parenthesis are atomic numbers)
- Zr(40) and Hf(72)
- Zr(40) and Ta(73)
- Ti(22) and Zr(40)
- Zr(40) and Nb(41)
Answer: 1. Zr(40) and Hf(72)
Zr and Hf have nearly the same radii due to lanthanoid contraction.
Question 58. The reason for lanthanoid contraction is
- Negligible screening effect of ‘f’ -orbitals
- Increasing nuclear charge
- Decreasing nuclear charge
- Decreasing screening effect.
Answer: 1. Negligible screening effect of ‘f’-orbitals
Due to the poor shielding effect of 4f orbitals nucleus will exert a strong attraction and the size of the atom or ion will decrease as moves in the series with an increase in atomic number.
d and f Block NEET question bank
Question 59. Which of the following lanthanoid ions is diamagnetic?
(Atomic numbers Ce = 58, Sm = 62, Eu = 63, Yb = 70)
- \(\mathrm{Eu}^{2+}\)
- \(\mathrm{Yb}^{2+}\)
- \(\mathrm{Ce}^{2+}\)
- \(\mathrm{Sm}^{2+}\)
Answer: 2. \(\mathrm{Yb}^{2+}\)
- \(\mathrm{Sm}^{2+}(Z=62):[\mathrm{Xe}] 4 f^6\)
- \(\mathrm{Eu}^{2+}(Z=63):[\mathrm{Xe}] 4 f^7\)
- \(\mathrm{Yb}^{2+}(Z=70):[\mathrm{Xe}] 4 f^{14}\)
- \(\mathrm{Ce}^{2+}(Z=58):[\mathrm{Xe}] 4 f^2\)
Only Yb2+ is diamagnetic
Question 60. Which of the following oxidation states is the most common among the lanthanoids?
- 4
- 2
- 5
- 3
Answer: 4. 3
The common stable oxidation state of all the lanthanoids is +3. The oxidation states of +2 and +4 are also exhibited by some of the elements. These oxidation states are only stable in those cases where stable 4f0, 4f7 or 4f14 configurations are achieved.
Question 61. Identify the incorrect statement among the following:
- Lanthanoid contraction is the accumulation of successive shrinkages.
- As a result of lanthanoid contraction, the properties of the 4d series of transition elements have no similarities with the 5d series of elements.
- The shielding power of 4f electrons is quite weak.
- There is a decrease in the radii of the atoms or ions as one proceeds from La to Lu.
Answer: 2. As a result of lanthanoid contraction, the properties of 4d series of the transition elements have no similarities with the 5d series of elements.
In each vertical column of transition elements, the elements of the second and third transition series resemble each other more closely than the elements of the first and second transition series on account of lanthanide contraction. Hence the properties of elements of 4d series of transition elements resemble the properties of the elements of 5d series of transition elements.
Question 62. Lanthanoids are
- 14 elements in the sixth period (atomic number 90 to 103) that are filling 4f sublevel
- 14 elements in the seventh period (atomic number = 90 to 103) that are filling 5f sublevel
- 14 elements in the sixth period (atomic number = 58 to 71) that are filling the 4f sublevel
- 14 elements in the seventh period (atomic number = 58 to 71) that are filling 4f sublevel.
Answer: 3. 14 elements in the sixth period (atomic number = 58 to 71) that are filling the 4f sublevel
As the sixth period can accommodate only 18 elements in the table, 14 members of 4f series (atomic numbers 58 to 71) are separately accommodated in a horizontal row below the periodic table. These are called lanthanides.
Question 63. The correct order of ionic radii of Y3+, La3+, Eu3+ and Lu3+is (Atomic number Y = 39, La = 57, Eu = 63, Lu = 71)
- \(\mathrm{Y}^{3+}<\mathrm{La}^{3+}<\mathrm{Eu}^{3+}<\mathrm{Lu}^{3+}\)
- \(\mathrm{Y}^{3+}<\mathrm{Lu}^{3+}<\mathrm{Eu}^{3+}<\mathrm{La}^{3+}\)
- \(\mathrm{Lu}^{3+}<\mathrm{Eu}^{3+}<\mathrm{La}^{3+}<\mathrm{Y}^{3+}\)
- \(\mathrm{La}^{3+}<\mathrm{Eu}^{3+}<\mathrm{Lu}^{3+}<\mathrm{Y}^{3+}\)
Answer: 2. \(\mathrm{Y}^{3+}<\mathrm{Lu}^{3+}<\mathrm{Eu}^{3+}<\mathrm{La}^{3+}\)
Ongoing from La3+ to Lu3+, the ionic radius shrinks from 1.15 Å to 0.93 Å (lanthanide contraction) The radius of La3+ is also larger than that of Y3+ ion which lies immediately above it in the periodic table.
d and f Block NEET question bank
Question 64. The general electronic configuration of lanthanides is
- \((n-2) f^{1-14}(n-1) s^2 p^6 d^{0-1} n s^2\)
- \((n-2) f^{10-14}(n-1) d^{0-1} n s^2\)
- \((n-2) f^{0-14}(n-1) d^{10} n s^2\)
- \((n-2) d^{0-1}(n-1) f^{-14} n s^2\)
Answer: 1. \((n-2) f^{1-14}(n-1) s^2 p^6 d^{0-1} n s^2\)
The general electronic structure of lanthanides is: \((n-2) f^{1-14}(n-1) s^2 p^6 d^{0-1} n s^2 \text {. }\)
Question 65. Which of the following statements is not correct?
- La(OH)3 is less basic than Lu(OH)3.
- In the lanthanide series, the ionic radius of Ln3+ ion decreases.
- La is actually an element of transition series rather than lanthanides.
- Atomic radii of Zr and Hf are the same because of lanthanide contraction.
Answer: 1. La(OH)3 is less basic than Lu(OH)3
La(OH)3 is more basic than Lu(OH)3 In lanthanides the basic character of hydroxides decreases as the ionic radius decreases.
Question 66. The lanthanide contraction is responsible for the fact that
- Zr and Hf have about the same radius
- Zr and Zn have the same oxidation state
- Zr and Y have about the same radius
- Zr and Nb have similar oxidation states.
Answer: 1. Zr and Hf have about the same radius
Due to lanthanide contraction, the elements of the second and third transition series i.e., Zr and Hf resemble more with each other tiran the elements of the first and second transition series.
Question 67. Which of the following statements concerning lanthanide elements is false?
- All lanthanides are highly dense metals.
- The more characteristic oxidation state of lanthanide elements is +3.
- Lanthanides are separated from one another by the ion exchange method.
- Ionic radii of trivalent lanthanides steadily increase with the increase in the atomic number
Answer: 4. Ionic radii of trivalent lanthanides steadily increase with increase in the atomic number
Ionic radii of trivalent lanthanides decrease with an increase in atomic number.
Question 68. The incorrect statement among the following is
- Actinoids are highly reactive metals, especially when finely divided
- Actinoid contraction is greater for element to element-than lanthanoid contraction
- Most of the trivalent lanthanoid ions are colorless in the solid state
- Lanthanoids are good conductors of heat and electricity.
Answer: 3. Most of the trivalent lanthanoid ions are colourless in the solid state
Question 69. The reason for the greater range of oxidation states in actinoids is attributed to
- Actinoid contraction
- 5F 6d and 7s levels having comparable energies
- 4F and 5d levels are close in energies
- The radioactive nature of actinoids.
Answer: 2. 5F 6d and 7s levels having comparable energies
Actinoids have a greater range of oxidation states due to comparable energies if 5f, 6d and 7s orbitals. Hence all their electrons can take part in bond formation.
d and f Block NEET question bank
Question 70. Which of the following exhibits only +3 oxidation state?
- U
- Th
- Ac
- Pa
Answer: 3. Ac
U exhibits + 3,+ 4,+ 5, +6
Th exhibits + 3, + 4 ; Ac exhibits + 3 only
Pa exhibits + 3, + 4, + 5
Question 71. More number of oxidation states are exhibited by the actinoids than by the lanthanoids. The main reason for this is
- The more active nature of the actinoids
- More energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals
- Lesser energy difference between 5f and 6d orbitals than that between 4f and 5d orbitals
- Greater metallic character of the lanthanoids than that of the corresponding actinoids.
Answer: 3. More active nature of the actinoids
The 5f-orbitals extend into space beyond the 6s and 6p-orbitals and participate in bonding. This is in direct contrast to the lanthanides where the 4f orbitals are buried deep inside the atom, totally shielded by outer orbitals and thus unable to take part in bonding.
Question 72. Which one of the following elements shows a maximum number of different oxidation states in its compounds?
- Gd
- La
- Eu
- Am
Answer: 4. Am
La forms compounds in which the oxidation number is +3.
‘Eu’ and ‘Gd’ exhibit +2 as well as +3 oxidation states and are not higher than that, due to stable (f) configuration. Whereas All exhibit the oxidation states +3, +4, +5, +6′ etc’ due to extremely large size and low ionisation energy.
Question 73. Match the catalyst with the process
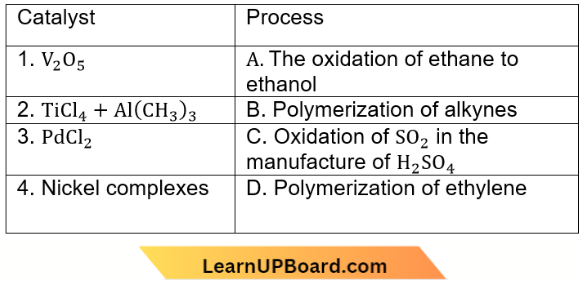
Which of the following is the correct option?
- 1-C, 2-D, 3-A, 4-B
- 1-A, 2-B, 3-C, 4-D
- 1-A, 2-C, 3-B, 4-D
- 1-C, 2-A, 3-D, 4-B
Answer: 1. 1-C, 2-D, 3-A, 4-B
d and f Block NEET question bank
Question 74. HgCl2 and I2 both when dissolved in water containing L ions, the pair of species formed is
- \(\mathrm{HgI}_2, \mathrm{I}^{-}\)
- \(\mathrm{HgI}_4^{2-}, \mathrm{I}_3^{-}\)
- \(\mathrm{Hg}_2 \mathrm{I}_2, \mathrm{I}^{-}\)
- \(\mathrm{HgI}_2, \mathrm{I}_3^{-}\)
Answer: 2. \(\mathrm{HgI}_4^{2-}, \mathrm{I}_3^{-}\)
⇒ \(\mathrm{HgCl}_{2(\mathrm{mq})}+4 \mathrm{I}_{(\mathrm{aq})}^{-} \longrightarrow \mathrm{HgI}_4^{2-}(\mathrm{aq})+2 \mathrm{Cl}_{(\mathrm{aq})}^{-}\)
⇒ \(\mathrm{I}_{2(\mathrm{~s})}+\mathrm{I}_{(\mathrm{aq})} \longrightarrow{\mathrm{imq}} \mathrm{I}_{3(\mathrm{aq})}^{-}\)
Question 75. Which of the following elements is responsible for the oxidation of water to O2 in biological processes?
- Cu
- Mo
- Fe
- Mm
Answer: 3. Fe
Question 76. When calomel reacts with NH4OH, we get
- \(\mathrm{Hg}_2 \mathrm{O}\)
- \(\mathrm{HgO}\)
- \(\mathrm{HgNH}_2 \mathrm{Cl}\)
- \(\mathrm{NH}_2-\mathrm{Hg}-\mathrm{Hg}-\mathrm{Cl}\)
Answer: 3. \(\mathrm{HgNH}_2 \mathrm{Cl}\)
When calomel reacts with NH4OH it turns black due to the formation of a mixture of mercury and ammonium basic mercury (2) chloride.
⇒ \(\underset{\text{ Calmole }}{\mathrm{Hg}_2 \mathrm{Cl}_2}+2 \mathrm{NH}_4 \mathrm{OH} \rightarrow \mathrm{NH}_4 \mathrm{Cl}+2 \mathrm{H}_2 \mathrm{O}+\mathrm{Hg}+\mathrm{HgNH}_2 \mathrm{Cl}\)
d and f Block NEET question bank
Question 77. Photographic films and plates have essential ingredients of
- Silver nitrate
- Silver bromide
- Sodium chloride
- Oleic acid.
Asnwer: 2. Silver bromide
AgBr is highly photosensitive and is used as an ingredient for photographic films and plates.
