Thermal Properties Of Matter MCQs
NEET Physics For Thermal Properties Of Matter Multiple Choice Questions
Question 1. Mercury thermometer can be used to measure temperature up to:
- 260°C
- 100°C
- 360°C
- 500°C
Answer: 1. 260°C
A mercury thermometer is a liquid thermometer that uses the homogeneous variation in volume of a liquid as a temperature. Mercury is opaque and bright, making it easy to see in the glass tube, and it is a good heat conductor, soon reaching the temperature of the hot bath. A mercury thermometer can be used to measure temperatures up to 300°C or so because mercury begins to evaporate at 367°C.
Question 2. A Centigrade and a Fahrenheit thermometer are dipped in boiling water. The water temperature is lowered until the Fahrenheit thermometer registers 140°. What is the fall in temperature as registered by the Centigrade thermometer?
- 80°
- 60°
- 40°
- 30°
Answer: 3. 40°
Given
A Centigrade and a Fahrenheit thermometer are dipped in boiling water. The water temperature is lowered until the Fahrenheit thermometer registers 140°.
The relation between the Celsius scale and the Fahrenheit scale is \(\frac{C}{100}=\frac{F-32}{180}\)
Putting value of F=\(140^{\circ}\), we get
⇒ \(\frac{C}{100}=\frac{140-32}{180}\)=0.6
C = 60°
Hence, Fall in temperature = Temperature of boiling water – final temperature
100°C – 60°C = 40°C
Question 3. A copper rod of 88 cm and an aluminium rod of unknown length have their increase in length independent of increase in length independent of increase in temperature. The length of aluminium rod is: \(\alpha_{\mathrm{Cu}}=1.7\times10^{-5}\mathrm{~K}^{-1},\alpha_{\mathrm{Al}}=2.2 \times 10^{-5} \mathrm{~K}^{-1}\)
- 113.9 cm
- 88 cm
- 68 cm
- 6.8 cm
Answer: 3. 68 cm
Given
A copper rod of 88 cm and an aluminium rod of unknown length have their increase in length independent of increase in length independent of increase in temperature.
According to the question,
⇒ \(L_w=l_w\left(1+\alpha_w \Delta T\right)\) → Equation 1
⇒ \(L_{\mathrm{cu}}=l_{\mathrm{cu}}\left(l+\alpha_{\mathrm{cu}} \Delta t\right)\) → Equation 2
⇒ \(L_{\mathrm{A} l}=l_{\mathrm{A} l}\left(A+\alpha_{\mathrm{A} l} \Delta T\right)\) → Equation 3
From equation. (1) and equation (3),
Read and Learn More NEET Physics MCQs
⇒ \(L_{\mathrm{A} l}-L_{\mathrm{C} u}=L_{\mathrm{A} l}-L_{\mathrm{C} u}+\left(l_{\mathrm{A} l} \alpha_{\mathrm{A} l}-l_{\mathrm{C} u} \alpha_{\mathrm{Cu}}\right) \Delta T\)
Since, \(l_{\mathrm{A} l} \alpha_{\mathrm{A} l}=l_{\mathrm{C} u} \alpha_{C u}\)
⇒ \(l_{\mathrm{A} l}=\frac{l_{C u} \alpha_{\mathrm{Cu}}}{\alpha_{\mathrm{A} l}}\)
= \(\frac{8.8 \times 1.7 \times 10^{-5}}{2.2 \times 10^{-5}}=68 \mathrm{~cm}\)
∴ \(l_{\mathrm{Al}}=68 \mathrm{~cm}\)
Thermal Properties of Matter MCQs
Question 4. Coefficient of linear expansion of brass and steel rods and \(\alpha_I \text { and } \alpha_2\). Length of brass and steel rods are \(\mathrm{l}_1\text { and } I_2\) respectively. If I1-I2 is maintained the same at all temperatures, which one of the following relations holds good?
- \(\alpha_1 l_2^2=\alpha_1{ }^2 l_2^2\)
- \(\alpha_1{ }^2 l_2=\alpha_2^2 l_1\)
- \(\alpha_1 l_1=\alpha_2 l_2\)
- \(\alpha_1 l_2=\alpha_2 l_1\)
Answer: 3. \(\alpha_1 l_1=\alpha_2 l_2\)
Given
Coefficient of linear expansion of brass and steel rods and \(\alpha_I \text { and } \alpha_2\). Length of brass and steel rods are \(\mathrm{l}_1\text { and } I_2\) respectively. If I1-I2 is maintained the same at all temperatures
The change in length for both rods should be the same,
⇒ \(\Delta l_1 =\Delta l_2\)
⇒ \(l_1 \alpha_1 \Delta T =l_2 \alpha_2 \Delta T\)
∴ \(l_1 \alpha_1 =l_2 \alpha_2\)
Question 5. The value of the coefficient of volume expansion of glycerin is \(5 \times 10^{-4} \mathrm{~K}^{-1}\). The fractional change in the density of glycerin for a rise of 40°C in its temperature is:
- 0.015
- 0.020
- 0.025
- 0.010
Answer: 2. 0.020
The value of coefficient of volume expansion of glycerin is =\(5 \times 10^{-4} \mathrm{~K}^{-1}\)
We know that, \(d_f=\frac{d_i}{(1+V \Delta \mathrm{T})}\)
Fractional change =\(\frac{d_i-d_f}{d_f}=1-\frac{d_f}{d_i}\)
= \(1-(1+V \Delta T)^{-1}=1-(1-V \Delta T)\)
⇒ \(\left\{\mathrm{Q}(1+x)^n \approx 1+n x\right\}\)
= V \(\Delta T=5 \times 10^{-4} \times 40\)=0.020
MCQs on Thermal Properties of Matter for NEET
Question 6. The density of water at 20°C is 998 kg/m³ and at 40°C 992 kg/m³. The coefficient of volume expansion of water is:
- 3 x 10-4/°C
- 2 x 10-4/°C
- 6 x 10-4/°C
- 10-3/°C
Answer: 1. 3 x 10-4/°C
According to the question,
⇒ \(T_1=20^{\circ} \mathrm{C}, T_2=40^{\circ} \mathrm{C}\)
⇒ \(\rho_{20}=998 \mathrm{~kg} / \mathrm{m}^3 \rho_{40}=992 \mathrm{~kg} / \mathrm{m}^3\)
So, \(\rho_{\mathrm{T}_2}=\frac{\rho_{\mathrm{T}_1}}{(1+v \Delta T)}=\frac{\rho_{\mathrm{T}_1}}{1+v\left(T_2-T_1\right)}\)
Putting the values we get, 992=\(\frac{998}{1+\gamma(40-20)}\)
992=\(\frac{998}{1+20 \gamma}\)
⇒ \(992(1+20 \gamma)\) =998
1+20\( \gamma =\frac{998}{992} \)
⇒ \(20 \gamma =\frac{6}{992} \)
=\(\frac{6}{992} \times \frac{1}{20} =3 \times 10^{-1 \circ} \mathrm{C}\)
Thermal Properties Questions for NEET
Question 7. (A)The weight of the sphere in the air is 50 g. Its weight is 40 g in a liquid, at a temperature of 20°C. When the temperature increases to 70°C, its weight becomes 45 g. Find: (1) the ratio of densities of liquid at given two temperatures is. (2) the coefficient of cubical expansion of liquid assuming that there is no expansion of the volume of a sphere.
Answer: 3.
Given
The weight of the sphere in the air is 50 g. Its weight is 40 g in a liquid, at a temperature of 20°C. When the temperature increases to 70°C, its weight becomes 45 g.
(1) \(W_{\text {apparent }}=W_{\text {air }}-\rho V g\)
Let \(\rho_1=\text { density of liquid at } 20^{\circ} \mathrm{C}\)
40= \(50-V \rho_1 g \)
10= \(V \rho_1 g\)
Again, Let,\(\rho_2\)=density of liquid at \(70^{\circ} \mathrm{C}\)
45= \(50-V \rho_2 g \)
5= \(V \rho_2 g \)
From eq. (1) and (2)
∴ \(\frac{\rho_1}{\rho_2}=\frac{2}{1}\)
(2) Because \(\rho_2=\frac{M}{V_1(1+\gamma \Delta \theta)}=\frac{\rho_1}{(1+\gamma \Delta \theta)}\)
⇒ \(\frac{\rho_1}{\rho_2}=1+\gamma \Delta \theta\)
Since, \(\frac{\rho_1}{\rho_2}=2 \)
⇒ \(2=1+\gamma \Delta \theta \)
⇒ \(\gamma \Delta \theta=1\)
⇒ \(\gamma=\frac{1}{\Delta \theta}\)
∴ \(\gamma=\frac{1}{70-20}=\frac{1}{50}\)=0.02
Thermal Properties Questions for NEET
Question 8. The quantities of heat required to raise the temperature of two solid copper spheres of radii \(r_1 \) and \(r_2\left(r_1=1.5 r_2\right)\) through 1 K are in the ratio of:
- \(\frac{9}{4}\)
- \(\frac{3}{2}\)
- \(\frac{5}{3}\)
- \(\frac{27}{8}\)
Answer: 4. \(\frac{27}{8}\)
Heat required
Q = \(m c \Delta \mathrm{T}\)
= \(\left(\frac{4}{3} \pi r^3 \cdot \rho\right) c \Delta \mathrm{T}\)
Since \(\pi, \rho, c, \Delta\) t are constant.
⇒ \(\mathrm{Q} \propto r^3 \).
or, \(\frac{\mathrm{Q}_1}{\mathrm{Q}_2} =\frac{r_1^3}{r_2{ }^3}=\left(\frac{r_1}{r_2}\right)^3=\left(\frac{1.5 r_2}{r_2}\right)^3 \)
=\(\frac{27}{8}\)
Question 9. The thermal capacity of 40 g of aluminium (s-0.2 cal/g-K) is:
- 168 J/K
- 672 J/K
- 840 J/K
- 33.6 J/K
Answer: 4. 33.6 J/K
The thermal capacity of a body is defined as the amount of heat required to raise the temperature of the (whole) body through 1°C or 1 K.
The amount of heat energy required (ΔQ) to raise the temperature of mass m of a body through temperature range (ΔT) is, ΔQ = ms (ΔT)
where, s is the specific heat of the body, when ΔT = 1K,
ΔQ = thermal capacity
Thermal capacity = s x m x 1 = ms
Here,m = 40 g, s = 0.2 cal/g K
Thermal capacity = 40 x 0.2 = 8 cal/K
= 4.2 x 8 J/K = 33.6 J/K
Class 11 Thermal Properties of Matter MCQs
Question 10. Steam at 100°C is passed into 20 g of water at 10°C. When water acquires a temperature of 80°C, the mass of water present will be [Take specific heat of water = 1 cal g-1 C-1latent heat of steam = 540 cal g-1]:
- 24 g
- 31.5 g
- 42.5 g
- 22.5 g
Answer: 4. 22.5 g
Heat gain by water = heal loss of steam
20 x 1 x (80 – 10) = m x 540 + m x 1 x (100 – 80) 1400 = 560 m
m = 2.5 g
Total mass of water = 20 + 2.5 = 22.5 g
Question 11. A piece of ice falls from a height h so that it melts completely. Only one quarter of the heat produced is absorbed by the ice and all energy of ice gets converted into heat during its fall. The value of h is [Latent heat of ice is 3.4 x 105 J/kg andg= 10 N/kg]:
- 544 km
- 136 km
- 38 km
- 34 km
Answer: 2. 136 km
Given that:
A piece of ice falls from a height h so that it melts completely. Only one quarter of the heat produced is absorbed by the ice and all energy of ice gets converted into heat during its fall.
mgH = ml
H = \(\frac{4 L}{g}=\frac{4 \times 3.4 \times 10^5}{10}\)
=136 km
Class 11 Thermal Properties of Matter MCQs
Question 12. If 1 g of steam is mixed with 1 g of ice, then the resultant temperature of the mixture is:
- 270°C
- 230°C
- 100°C
- 50°C
Answer: 3. 100°C
The heat required by 1 g of ice at 0°C to melt into 1g of water at 0°c,
⇒ \(Q_1 \)=m L (L =latent heat of fusion )
=\(1 \times 80=80 \mathrm{cal}(L=80 \mathrm{cal} / \mathrm{g})\)
Heat required by 1 g of water at 0°C to boil at 100°C,
⇒ \(Q_2 =m c \Delta \mathrm{T}\) (c = specific heat of water) =
= 1 x 1(100-0)
⇒ \((c \left.=1 \mathrm{cal} / \mathrm{g}^{\circ} \mathrm{C}\right)\)
=100 cal
Thus, the total heat required by 1 g of ice to reach a temperature of 100°C,
⇒ \(Q=Q_1+Q_2=80+100=180 \mathrm{cal}\)
Heat available with 1 g of steam to condense into 1 g of water at 100°C,
⇒ \(Q^{\prime}= m L^{\prime}\)
⇒ \(\left(L^{\prime}\)= latent heat of vaporisation
= 1 x 536 cal ( L’ =536 call/g)
= 536 cal
The whole steam will not be condensed and ice will attain a temperature of 100°C. Thus, the temperature of the mixture is 100°C.
Question 13. The energy that will be ideally radiated by a 100kw transmitter in 1 hour is:
- 36 x 107J
- 36 x 104J
- 36 x 103J
- 36 x 105J
Answer: 1. 36 x 107J
Power, \(\mathrm{P}=100 \mathrm{kw}=100 \times 10^3 \mathrm{~W}\) ;
Time, t = 1 hour =\(3600 \mathrm{~s}\)
Energy, \(\mathrm{E}=\mathrm{P} \times \mathrm{t} =100 \times 10^3 \times 3600\)
= \(36 \times 10^7 \mathrm{~J}\)
Important MCQs on Thermal Properties of Matter
Question 14. A deep rectangular pond of surface area A, containing water (density = p specific heat capacity = 5), is located in a region where the outside air temperature is steady value at – 26°C. The thickness of the ice layer in the pond, at a certain instant x. Taking the thermal conductivity of ice as K, and its specific latent heat of fusion is L, 1 the rate of increase of the thickness of the ice layer, at this instant would be given by:
- \(\frac{26 K}{\rho \mathrm{x}(L-4 s)}\)
- \(\frac{26 K}{\left(\rho x^2 L\right)}\)
- \(\frac{26 K}{(\rho x L)}\)
- \(\frac{26 K}{\rho \mathrm{x}(L+4 s)}\)
Answer: 3. \(\frac{26 K}{(\rho x L)}\)
Given
A deep rectangular pond of surface area A, containing water (density = p specific heat capacity = 5), is located in a region where the outside air temperature is steady value at – 26°C. The thickness of the ice layer in the pond, at a certain instant x. Taking the thermal conductivity of ice as K, and its specific latent heat of fusion is L,
If the area of the cross-section of a surface is not uniform or if the steady state condition is not reached the heat flow equation can be applied to a thin layer of material perpendicular to the direction of heat flow.
The rate of heat flow by conduction for the growth of ice is given by,\(\frac{d \theta}{d t}=\frac{K A\left(\theta_0-\theta_1\right)}{x}\) → Equation 1
where,\(\mathrm{d} \theta=\rho \mathrm{A} d x L, \theta_0=0 \text { and } \theta_1=-\theta \)
⇒ \(\theta_0=0^{\circ} \mathrm{C} \Rightarrow \theta_1=-26^{\circ} \mathrm{C}\) .
Given that the rate of increase in thickness can be calculated from the equation. (1),
⇒ \(\frac{d \theta}{d t} =\frac{K A\left(\theta_0-\theta_1\right)}{x}\)
⇒ \(\frac{\rho A d x L}{d t} =\frac{K A\left(\theta_0-\theta_1\right)}{x}\)
⇒ \(\frac{d x}{d t} =\frac{K A\left(\theta_0-\theta_1\right)}{\rho A x L}\)
∴ \(\frac{K[0-(-26)]}{\rho x L} =\frac{26 K}{\rho x L}\)
Question 15. Two rods A and B of different materials are welded together as shown in the figure. Their thermal conductivities are K1 and K2. The thermal conductivity of the composite rod will be:
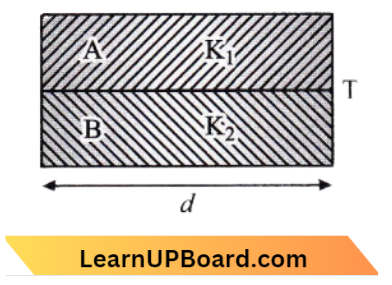
- \(\frac{K_1+K_2}{2}\)
- \(\frac{3\left(K_1+K_2\right)}{2}\)
- \(K_1+K_2\)
- \(2\left(K_1+K_2\right)\)
Answer: 1. \(\frac{K_1+K_2}{2}\)
Heat flow rate, \(H=H_1+H_2\)
H =\(\frac{K_1 A\left(T_1-T_2\right)}{d}+\frac{K_2 A\left(T_1-T_2\right)}{d}\)
⇒ \(\frac{K_{e g}\left(T_1-T_2\right)}{d} =\frac{A\left(T_1-T_2\right)}{d}\left[K_1+K_2\right]\)
∴ \(K_{\text {eg. }} =\left[\frac{K_1+K_2}{2}\right]\)
Important MCQs on Thermal Properties of Matter
Question 16. A spherical black body with a radius of 12 cm radiates 450-watt power at 500 J. If the radius were halved and the temperature doubled, the power radiated in watts would be:
- 225
- 450
- 1000
- 1800
Answer: 4. 1800
From Stefan’s law, Here radiated power of the black body P=\(\sigma \mathrm{AT}^4\) becomes half then a new area
⇒ \(A^{\prime}=\frac{A}{4}\)
Power radiation, \(\mathrm{P}^{\prime}=\sigma\left(\frac{A}{4}\right)(2 T)^4\)
Question 17. A black body is at a temperature of 5760 K. The energy of radiation emitted by the body at wavelength 250 nm is U1, at wavelength 500 nm is U2 and that at 1000 nm is u3. Wein’s constant, b = 2.88 x 106 nm. Which of the following is correct?
- U3 = 0
- U1 > U2
- U2 > U1
- U1 = 0
Answer: 3. U2 > U1
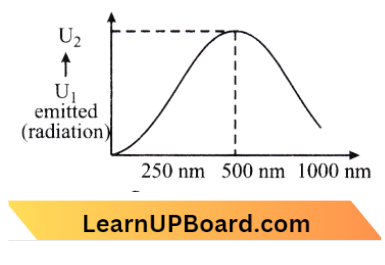
Given
A black body is at a temperature of 5760 K. The energy of radiation emitted by the body at wavelength 250 nm is U1, at wavelength 500 nm is U2 and that at 1000 nm is u3. Wein’s constant, b = 2.88 x 106 nm.
Temperature, T_\(1=5760 \mathrm{~K}\) (Given)
Using Wien’s law,
⇒ \(\lambda_m T=b\) → Equation 1
b= Wien’s constant =\(2.86 \times 10^6 \mathrm{nmK}\)
From (1) \(\lambda_m=\frac{b}{T}\)
⇒ \(\lambda_m=\frac{2.88 \times 10^6 \mathrm{nmK}}{5760 \mathrm{~K}}=500 \mathrm{~nm}\)
∴ \(U_2>U_1\)
Important MCQs on Thermal Properties of Matter
Question 18. Two metal rods 1 and 2 of the same length have the same temperature difference between their ends. Their thermal conductivities are K1 and K2 and cross-sectional areas A1 and A2 respectively. If the rate of heat conduction in 1 is four times that in 2, then:
- K1A1 = 4K2A2
- K1A1 = 2K2A2
- 4K1A1 = K2A2
- K1A1 = K2A2
Answer: 1. K1A1 = 4K2A2
Given
Two metal rods 1 and 2 of the same length have the same temperature difference between their ends. Their thermal conductivities are K1 and K2 and cross-sectional areas A1 and A2 respectively.
Let, L= length of each rod Temperature difference = \(\Delta T\)
The rate of flow of rod (1) is,
⇒ \(H_1=\frac{K_1 A_1 \Delta \mathrm{T}}{L}\)
and Rate of flow of rod (2) is, \(H_2=\frac{K_2 A_2 \Delta T}{L}\)
from the equation, \(H_1 =4 H_2\)
⇒ \(\frac{K_1 A_1 \Delta \mathrm{T}}{L} =4 \frac{K_2 A_2 \Delta T}{L}\)
∴ \(K_1 A_1 =4 K_2 A_2\)
Question 19. If the radius of a star is R and it acts as a black body, what would be the temperature of the star, in which the rate of energy production is Q?
- \(\frac{Q}{4 \pi R^2 \sigma}\)
- \(\left(\frac{Q}{4 \pi R^2 \sigma}\right)^{-\frac{1}{2}}\)
- \(\left(\frac{4 \pi R^2 Q}{\sigma}\right)^{\frac{1}{4}}\)
- \(\left(\frac{Q}{4 \pi R^2 \sigma}\right)^{\frac{1}{4}}\)
Answer: 4. \(\left(\frac{Q}{4 \pi R^2 \sigma}\right)^{\frac{1}{4}}\)
From Stefan’s law, we can write,
E=\(\sigma T^4\)
and rate of energy production, Q =\(E \times A \)
= \(\sigma \mathrm{T}^4 A =\sigma T^{4 \pi} 4 \pi \mathrm{R}^2\)
T =\(\left(\frac{Q}{4 \pi \mathrm{R}^2 \sigma}\right)^{1 / 4}\)
Important MCQs on Thermal Properties of Matter
Question 20. A slab of stone with an area of 0.36 m2 and thickness of 0.1 m is exposed on the lower surface to steam at 100°. A block of ice at 0°C rests on the upper surface of the slab. In one hour 4.8 kg of ice is melted. The thermal conductivity of the slab is:(Given latent heat of fusion of ice = 3.36 x 105 J kg-1)
- 1.24 J/m/s/°C
- 1.29J/m/s/°C
- 2.05 J/m/s/°C
- 1.02J/m/s/°C
Answer: 1. 1.24 J/m/s/°C
Given
A slab of stone with an area of 0.36 m2 and thickness of 0.1 m is exposed on the lower surface to steam at 100°. A block of ice at 0°C rests on the upper surface of the slab. In one hour 4.8 kg of ice is melted.
We know that,
⇒ \(\frac{d Q}{d t} =\frac{K A}{L}\left(T_1-T_2\right)\)
Q =\(\frac{K A}{L}\left(T_1-T_2\right) t\)
Q = \( m L_f\)
⇒ \(\frac{K A}{L}\left(T_1-T_2\right) t =m L_f\)
K =\(\frac{m \mathrm{~L}_f(\mathrm{~L})}{\mathrm{A}\left(\mathrm{T}_1-\mathrm{T}_2\right) t}\)
= \(\frac{4.8 \times 3.36 \times 10^5 \times 0.1}{0.36 \times 100 \times 3600} \mathrm{~J} / \mathrm{m} / \mathrm{S} /{ }^{\circ} \mathrm{C}\)
= \(\frac{4.8 \times 3.36}{0.36 \times 36}=1.24 \mathrm{~J} / \mathrm{m} / \mathrm{S} /{ }^{\circ} \mathrm{C}\)
Question 21. A cylinder metallic rod is in thermal contact with two reservoirs of heat at its two ends and conducts an amount of heat Q in time t. The metallic rod is melted and the material is formed into a rod of half the radius of the original rod. What is the amount of heat conducted by the new rod when placed in thermal contact with the two reservoirs in time ft?
- \(\frac{Q}{4}\)
- \(\frac{Q}{16}\)
- 2Q
- \(\frac{Q}{2}\)
Answer: 2. \(\frac{Q}{16}\)
Given
A cylinder metallic rod is in thermal contact with two reservoirs of heat at its two ends and conducts an amount of heat Q in time t. The metallic rod is melted and the material is formed into a rod of half the radius of the original rod.
We know that, Amount of heat, Q=\(\frac{K A\left(\theta_1-\theta_2\right) t}{t}\)
Where, K= coefficient of thermal conduction
⇒ \(\frac{Q}{t} \propto \frac{A}{l} \propto \frac{r^2}{l}\) → Equation 1
As the metallic rod is melted and the material is formed into a rod of half the radius,
⇒ \(V_1 =V_2\)
⇒ \(\pi r_1^2 l_1 =\pi r_2^2 l_2 \)
⇒ \(l_1 =\frac{l_2}{4}\) → Equation 2
From eq. (1) and eq. (2),
⇒ \(\frac{Q_1}{Q_2}=\frac{r_1^2}{l_2} \times \frac{l_2}{r_2^2}=\frac{r_1^2}{l_2} \times \frac{4 l_1}{\left(r_2 / 2\right)^2}\)
∴ \(Q_1=16 Q_2 \)
Question 22. The total radiant energy per unit area, normal to the direction of incidence, received at a distance R from the centre of a star radius r, whose outer surface radiates as a black body at a temperature T K is given by:
- \(\frac{\sigma r^2 T^4}{R^2}\)
- \(\frac{\sigma r^2 T^4}{4 \pi r^2}\)
- \(\frac{\sigma r^4 T^4}{r^4}\)
- \(\frac{4 \pi \sigma r^4 T^4}{R^2}\)
Answer: 1. \(\frac{\sigma r^2 T^4}{R^2}\)
Using Stefan’s law A \(\sigma T^4=4 \pi r^2 \sigma T^4\)
∴ \(T_{\mathrm{K}}=\sigma r^2 T^4 / \mathrm{R}^2\)
Question 23. A black body is at 727°C. It emits energy at a rate which is proportional to:
- (1000)4
- (1000)2
- (727)4
- (727)2
Answer: 1. (1000)4
According to Stefan’s law,
Rate of radiated energy, \(E \propto T^4\)
where, T= absolute temperature of a black body.
⇒ \(E \propto(727+273)^4\)
or \(E \propto[1000]^4\)
Specific Heat Capacity MCQs for NEET
Question 24. Assuming the sun to have a spherical outer surface of radius r, radiating like a black body at temperature fC, the power received by a unit surface, (normal to the incident rays) at a distance R from the centre of the sun is where σ is the Stefan’s constant:
- \(\frac{r^2 \sigma(t+273)^4}{4 \pi R^2}\)
- \(\frac{16 \pi^2 r^2 \sigma t^4}{R^2}\)
- \(\frac{r^2 \sigma(t+273)^4}{R^2}\)
- \(\frac{4 \pi r^2 \sigma t^4}{R^2}\)
Answer: 3. \(\frac{r^2 \sigma(t+273)^4}{R^2}\)
Solar constant =\(\frac{\sigma\left(4 \pi r^2\right) T^4}{\left(4 \pi R^2\right)}\)
= \(\frac{\sigma r^2(t+273)^4}{R^2}\)
Question 25. A black body at 1227°C emits radiations with maximum intensity at a wavelength of 5000 A. If the temperature of the body is increased by 1000°C, the maximum intensity will be observed at:
- 3000 A
- 4000 A
- 5000 A
- 6000 A
Answer: 1. 3000 A
By Wein’s displacement law, \(\lambda_m T \)= Constant
we have, \(\frac{\lambda_{m 1}}{\lambda_{m 2}}=\frac{T_2}{T_1}\)
or \(\lambda_{m 2}=\frac{\lambda_{m 1} \times T_1}{T_2}\)
∴ \(\lambda_{m 2}=\frac{5000 \times 1500}{2500}=3000\) .
Question 26. Which of the following rods, (given radius r and length l) each made of the same material and whose ends are maintained at the same temperature will conduct the most heat?
- r=\(r_0, l=l_0\)
- r=\(2 r_0, l=l_0\)
- r=\(r_0, l=2 l_0\)
- r=\(2 r_0, l=2 l_0\)
Answer: 2. r=\(2 r_0, l=l_0\)
Heat conducted =\(\frac{K A\left(T_1-T_2\right) t}{l}=\frac{K \pi r^2\left(T_1-T_2\right) t}{l}\)
The rod with a maximum ratio of \(\frac{A}{l}\) will conduct the most.
Here the rod r= \( 2 r_0 and l=l_0 \) will conduct most.
Specific Heat Capacity MCQs for NEET
Question 27. If km denotes the wavelength at which the radioactive emission from a black body at a temperature TK is maximum, then:
- \(\lambda_m \propto T^1\)
- \(\lambda_m\) is independent of T
- \(\lambda_m \propto T\)
- \(\lambda_m \propto T^{-1}\)
Ans wer: 4. \(\lambda_m \propto T^{-1}\)
According to Wein’s displacement law
⇒ \(\lambda_m T\)= constant
∴ \(\lambda_m \propto T^{-1}\)
Question 28. Consider a compound slab consisting of two different materials having equal thickness and thermal conductivities K and 2K, respectively. The equivalent thermal conductivity of the slab is:
- \(\frac{2}{3} K\)
- \(\sqrt{2} K\)
- 3 K
- \(\frac{2}{3} K\)
Answer: 1. \(\frac{2}{3} K\)
The slabs are in series.
Total resistance, \(\mathrm{R}=\mathrm{R}_1+\mathrm{R}_2\)
⇒ \(\frac{l}{K_{e f f}(A)}=\frac{l}{2 K A}+\frac{l}{K A}\)
∴ \(K_{\text {eff }}=\frac{2}{3} K\)
Question 29. The Wein’s displacement law expresses the relation between:
- wavelength corresponding to maximum energy and temperature.
- relation energy and wavelength.
- temperature and wavelength.
- colour of light and temperature.
Answer: 1. relation energy and wavelength.
According to Wein’s displacement law, the wavelength corresponding to maximum energy is inversely proportional to the absolute temperature of the body \(\lambda_m \propto \frac{1}{T}\)
∴ \(\lambda_m T=\text { constant }\)
Specific Heat Capacity MCQs for NEET
Question 30. Which of the following is best close to an ideal black body?
- Black lamp.
- Cavity maintained at a constant temperature.
- Platinum black.
- A lamp of charcoal heated to high temperature.
Answer: 2. Cavity maintained at a constant temperature.
A perfect black body absorbs all incident radiation without reflecting or transmitting any of it. A black-light absorbs around 96% of incoming radiation.
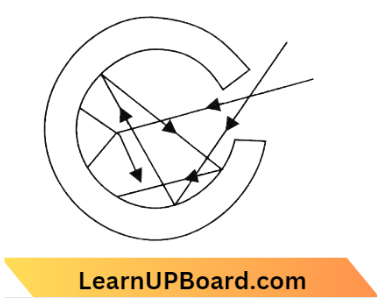
In reality, a perfect black body may be achieved via a tiny hole in the wall of a uniformly heated hollow body (as indicated in the image). Any radiation that enters the hollow body through the perforations is reflected several times before being absorbed. This can be aided by painting the inside surface with black, absorbing around 96% of the energy at each reflection. The section of the inner surface opposite the hole is conical to prevent the reflected beam from escaping after one reflection.
Question 31. For a black body at a temperature of 727°C, its radiating power is 60 W and the temperature of the surrounding is 227°C. If the temperature of the black body is changed to 1227°C, then its radiating power will be:
- 304 W
- 320 W
- 240 W
- 120 W
Answer: 2. 320 W
We can use the formula,
⇒ \(P\left(T_4-T_0{ }^4\right)\)
⇒ \(\frac{P_2}{P_1} =\frac{(1500)^4-(500)^4}{(1000)^4-(500)^4}\)
=\(\frac{500^4\left(3^4-1\right)}{500^4\left(2^4-1\right)}\)
⇒ \(\frac{P_2}{60} =\frac{80}{15}\)
∴ \(P_2 =320 \mathrm{~W}\)
Chapter-Wise Physics MCQs for NEET
Question 32. A cup of coffee cools from 90°C to 80°C in two minutes, when the room temperature is 20°C. The time taken by a similar cup of coffee to cool from 80°C to 60°C at a room temperature same at 20°C is:
- \(\frac{13}{10} t\)
- \(\frac{13}{5} t\)
- \(\frac{10}{13} t\)
- \(\frac{5}{13} t\)
Answer: 2. \(\frac{13}{5} t\)
Given, Initial temperature \(\left(\mathrm{T}_{\mathrm{i}}\right)=90^{\circ} \mathrm{C}\)
Final temperature \(\left(T_f\right)=80^{\circ} \mathrm{C}\)
Room temperature \(\left(\mathrm{T}_{\mathrm{o}}\right)=20^{\circ} \mathrm{C}\)
Let the time taken be t minutes
According to Newton’s law of cooling,
Rate of cooling,
⇒ \(\frac{d T}{d t} =K\left[\frac{\mathrm{T}_i+\mathrm{T}_f}{2}-\mathrm{T}_o\right]\)
∴ \(\left(\frac{\mathrm{T}_f-\mathrm{T}_i}{t}\right) =K\left[\frac{90+80}{2}-20\right]=K[65]\)
K =\(\frac{10}{65 t}\) → Equation 1
In \(2^{\text {nd }}\)condition,
Initial temperature \(\mathrm{T}_{\mathrm{i}}=80^{\circ} \mathrm{C}\)
Final temperature \(\mathrm{T}_{\mathrm{f}}=60^{\circ} \mathrm{C}\)
Let the time taken be t minutes
Then,\(\frac{(80-60)}{t^{\prime}} =\frac{10}{65 t}\left[\frac{(80+60)}{2}-20\right] \frac{20}{t^{\prime}} 10\)
=\(\frac{10}{65 t}(50)\) → Equation 2
From equation (1) and (2),
∴ \(\mathrm{t}^{\prime}=\frac{13}{5} t\)
Chapter-Wise Physics MCQs for NEET
Question 33. An object kept in a large room having an air temperature of 25° C takes 12 minutes to cool from 80° C to 70° C. The time taken to cool the same object from 70° C to 60° C would be nearly :
- 10 min
- 12 min
- 20 min
- 15 min
Answer: 4. 15 min
From Newton’s law of cooling the expression for the time taken by a body to cool from \(T_1 \text { to } T_2\) when placed in a medium of temperature to is given by :
⇒ \(\frac{T_1-T_2}{t}=\frac{1}{K}\left(\frac{T_1+T_2}{2}-T_0\right)\)
The first condition, When the object cools from \(80^{\circ} \mathrm{C} to 70^{\circ}\) in 12 minutes then from equation 1
⇒ \(\frac{80-70}{12}=\frac{1}{K}\left(\frac{80+70}{2}-25\right)\)
Since, \(T_0\) = 25°C is given
K = 60 → Equation 2
Again when the object cools from \(70^{\circ} \mathrm{C} to 60^{\circ} \mathrm{C}\) we have
⇒ \(\frac{70-60}{t} =\frac{1}{K}\left(\frac{70+60}{2}-25\right)\)
=\(\frac{10}{t} =\frac{40}{\mathrm{~K}}\)
⇒ \(\frac{10}{t} =\frac{40}{60}\) From Equation 2
t =15 min
Question 34. A body cools from a temperature of 3T to 2T in 10 minutes. The room temperature is T. Assume that Newton’s law of cooling is applicable. The temperature of the body at the end of next 10 minutes will be:
- \(\frac{7}{4} T\)
- \(\frac{3}{2} T\)
- \(\frac{4}{3} T\)
- T
Answer: 2. \(\frac{3}{2} T\)
According to the formula from Newton’s law of cooling
In \(\frac{3 T-T}{2 T^{\prime}-T}=K t\)
In(\(2)=K(10)\)
for the next 10 minutes
In \(\frac{2 \mathrm{~T}-\mathrm{T}}{\mathrm{T}^{\prime}-\mathrm{T}} =\mathrm{K}(10)\)
⇒ \(\frac{2 \mathrm{~T}-\mathrm{T}}{\mathrm{T}^{\prime}-\mathrm{T}}\) =2
∴ \(\mathrm{~T}^{\prime} =\frac{3}{2}\)
Heat Transfer MCQs for NEET
Question 35. A certain quantity of water cools from 70°C to 60°C in the first 5 min and to 54°C in the next 5 min. The temperature of the surroundings is:
- 45°C
- 20°C
- 42°C
- 10°C
Answer: 1. 45°C
The question is based on Newton’s law of cooling According to Wien displacement law,
⇒ \(\frac{\theta_1-\theta_2}{\Delta t}=K\left[\frac{\theta_1+\theta_2}{2}-\theta_0\right]\)
Where, \(\theta_0=q\)
⇒ \(\frac{70-60}{5} =K\left[\frac{70+60}{2}-\theta_0\right] \)
=\(K\left[65-\theta_0\right]\) → Equation 1
⇒ \(\frac{60-54}{5} =K\left[\frac{60+54}{2}-\theta_0\right]=\mathrm{K}\left[57-\theta_0\right]\) → Equation 2
From (1) and (2),
⇒ \(\frac{2 \times 5}{6} =\left(\frac{65-\theta_0}{57-\theta_0}\right)\)
⇒ \(\frac{5}{3} =\frac{65-\theta_0}{57-\theta_0}\)
2 \(\theta_0 =90 \)
∴ \(\theta_0 =45^{\circ} \mathrm{C}\) .
Question 36. A piece of iron is heated in a flame. If first becomes dull red then becomes reddish yellow and finally turns to white hot. The correct explanation for the above observation is possible by using:
- Stefan’s law
- Wein’s displacement law
- KirchofFs law
- Newton’s law of cooling
Answer: 2. Wein’s displacement law
We can explain this observation by using \(\lambda_m T=b\)
Which is Wien’s displacement law
Heat Transfer MCQs for NEET
Question 37. The two ends of a rod of length L and a uniform cross-sectional area A are kept at two temperatures T1 and T2(T1 > T2). The rate of heat transfer, through the rod in a steady state is given by:
- \(\frac{d \mathrm{Q}}{d t}=\frac{K L\left(T_1-T_2\right)}{A}\)
- \(\frac{d \mathrm{Q}}{d t}=\frac{K\left(T_1-T_2\right)}{L A}\)
- \(\frac{d \mathrm{Q}}{d t}=K L A\left(T_1-T_2\right)\)
- \(\frac{d \mathrm{Q}}{d t}=\frac{K A\left(T_1-T_2\right)}{L}\)
Answer: 4. \(\frac{d \mathrm{Q}}{d t}=\frac{K A\left(T_1-T_2\right)}{L}\)
The rate of heat transfer \((\mathrm{dQ} / \mathrm{dt})\) is given by:
For a rod of length land area of cross-section A whose faces are maintained at temperature T1 and T2 respectively then in steady-state the rate of heat flowing from one face to the other in time T is given by Here temperature difference \((\Delta \mathrm{T})=\left(\mathrm{T}_1-\mathrm{T}_2\right)\)
The rate of heat transfer (dQ/dt) is given by
Also, \(\frac{d Q}{d t} =\frac{\Delta T}{R}\)
⇒ \(\mathrm{R} =\frac{L}{k A}\)
∴ \(\frac{d \mathrm{Q}}{d t} =\frac{K A\left(T_1-T_2\right)}{L}\)
