Thermodynamics MCQs
NEET Physics For Thermodynamics Multiple Choice Questions
Question 1. A sample of 0.1 g of water at 100° C and normal pressure (1.013 x 105 Nm-2) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample is:
- 42.2 J
- 208.7 J
- 104.3 J
- 84.5 J
Answer: 2. 208.7 J
Given
A sample of 0.1 g of water at 100° C and normal pressure (1.013 x 105 Nm-2) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc,
From the question, Heat spent during the conversion of a sample of water at \(100^{\circ}\) to steam is,
⇒ \(\Delta \mathrm{Q} =54 \mathrm{cal}=54 \times 4.18 \mathrm{~J}=225.72 \mathrm{~J}\)
⇒ \(P =1.013 \times 10^5 \mathrm{Nm}^{-2}\)
Work done \(\Delta W=P \Delta V=P\left[V_{\text {steam }}-V_{\text {water }}\right]\)
Here, \(V_{\text {steam }}=|167| c c=1 \times 10^{-6} \mathrm{~m}^3\)
⇒ \(V_{\text {water }} =0.1 \mathrm{~g}=0.1 c c=0.1 \times 10^{-6} \mathrm{~m}\)
⇒ \(\Delta W =1.013 \times 10^5\left[(167.1-0.1) \times 10^{-6}\right]\)
=\(1.013 \times 167 \times 10^{-1}=(16.917)\)
We also know that,
⇒ \(\Delta Q =\Delta U+\Delta W\)
⇒ \(\Delta U =\Delta Q-\Delta W=2.25 .72-16.917\)
=208.75
Question 2. Liquid oxygen at 50 K is heated to 300 K at a constant pressure of 1 atm. The rate of heating is constant. Which one of the following graphs represents the variation of temperature with time?
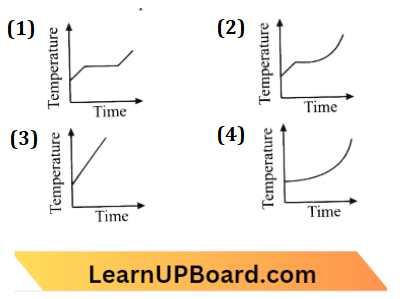
Answer: 1.
The given graph in option (1) shows the variation of temperature with time.
Thermodynamics MCQs
Question 3. The internal energy change in a system that has absorbed 2 KCal of heat and done 500 J of work is:
- 8900 J
- 6400 J
- 5400 J
- 7900 J
Answer: 4. 7900 J
Using the first law of thermodynamics, \(\Delta U=\Delta Q-\Delta W\)
ΔU = ΔQ-ΔW
= 2 x 4.2 x 1000-500 = 8400 – 500 = 7900 J
Read and Learn More NEET Physics MCQs
Question 4. 110 J of heat is added to a gaseous system, whose internal energy is 40 J, then the amount of external work done is:
- 150 J
- 70 J
- 110 J
- 40 J
Answer: 2. 70 J
From the first law of thermodynamics,
⇒ \(\Delta Q=\Delta U+\Delta W\)
where, \(\Delta Q\)= heat given
⇒ \(\Delta U\)= change in internal energy
⇒ \(\Delta W\)= work done
Here,\(\Delta Q = 110 \mathrm{~J}\)
⇒ \(\Delta U =40 \mathrm{~J}\)
⇒ \(\Delta W =\Delta Q-\Delta U\)
=110-40=70 \(\mathrm{~J}\)
NEET Physics MCQs on Thermodynamics
Question 5. The first law of thermodynamics is a consequence of the conservation of:
- work
- energy
- heat
- All of these
Answer: 2. energy
According to the first law of thermodynamics, when some amount of heat (dQ) is supplied to a system capable of doing external work, the amount of heat absorbed by the system (dQ) equals the sum of the increase in the internal energy of the system (dU) due to temperature rise and the external work done by the system (dW) in expansion.
dQ =dU + dW
This law is the law of conservation of energy that applies to every process in nature.
Question 6. One mode of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure. The change in internal energy of the gas during the transition is:
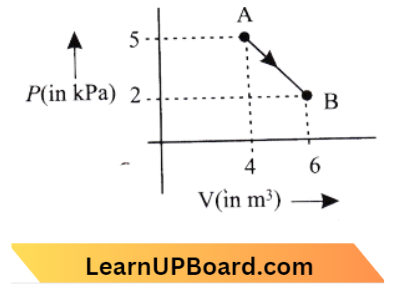
- 20 J
- -12 kJ
- 20 kJ
- -20 kJ
Answer: 4. -20 kJ
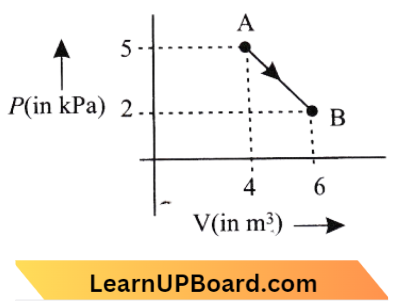
We know, \(\Delta U=n C_V+\Delta T\)
= \(\mathrm{n}\left(\frac{5 \mathrm{R}}{2}\right)\left(\mathrm{T}_{\mathrm{B}}-\mathrm{T}_{\mathrm{A}}\right) \quad\left[\text { for diatomic, } \mathrm{CV}=\frac{5 \mathrm{R}}{2}\right]\)
= \( \frac{5 \mathrm{nR}}{2}\left(\frac{\mathrm{P}_{\mathrm{B}} \mathrm{V}_{\mathrm{B}}}{\mathrm{nR}}-\frac{\mathrm{P}_{\mathrm{A}} \mathrm{V}_{\mathrm{A}}}{\mathrm{nR}}\right)\mathrm{PV}=\mathrm{nRT}]\)
= \(\frac{5}{2}\left(\mathrm{P}_{\mathrm{B}} \mathrm{V}_{\mathrm{B}}-\mathrm{P}_{\mathrm{A}}-\mathrm{V}_{\mathrm{A}}\right)=\frac{5}{2}\left(2 \times 10^3 \times 6-5 \times 10^3 \times 4\right)\)
=\(\frac{5}{2}\left(-8 \times 10^3\right)=20 \mathrm{~kJ}\)
=\(n\left(\frac{5 R}{2}\right)\left(T_B-T_A\right)\) [for diatomic gas, \(\mathrm{Cv}\)=0
NEET Physics MCQs on Thermodynamics
Question 7. If \(C_P\) and \(C_V\) denote the specific heats per unit mass of and ideal gas of molecular weight M, then
- \(C_{P-} C_{V=R / M}{ }^2\)
- \(C_P-C_{V=R}\)
- \(C_{P-} C_{V=R / M}\)
- \(C_{P-} C_{V=M R}\)
Where R is the molar gas constant.
Answer: 3. \(C_{P-} C_{V=R / M}\)
Let \(C_V\) and \(C_P \)be molar-specific heats of the ideal gas at constant volume and constant pressure, respectively, and then
⇒ \(\mathrm{C}_{\mathrm{P}}=\mathrm{MC}_{\mathrm{p}} \text { and } \mathrm{C}_{\mathrm{V}}=\mathrm{MC}_{\mathrm{V}}\)
⇒ \(\mathrm{C}_{\mathrm{P}}-\mathrm{C}_{\mathrm{V}}=\mathrm{R}\)
⇒ \(\mathrm{MC}_{\mathrm{P}}-\mathrm{MC}_{\mathrm{V}}=\mathrm{R}\)
∴ \(\mathrm{C}_{\mathrm{P}}-\mathrm{C}_{\mathrm{V}}=\mathrm{R} / \mathrm{M}\)
Question 8. If the ratio of the specific heat of a gas at constant pressure to that of the constant volume is y, the change in internal energy of a mass of gas when the volume changes from V to 2V at constant pressure P is:
- \(\frac{R}{(\gamma-1)}\)
- PV
- \(\frac{P V}{(\gamma-1)}\)
- \(\frac{\gamma P V}{(\gamma-1)}\)
Answer: 3. \(\frac{P V}{(\gamma-1)}\)
Change in internal energy of a gas having atomicity \(\gamma\) is given by,
⇒ \(\Delta U=\frac{1}{(\gamma-1)}\left(p_2 V_2-p_1 V_1\right)\)
Given,\(V_1=V, V_2=2 V\)
So,\(\Delta U =\frac{1}{\gamma-1}[p \times 2 V-p x V]\)
= \(\frac{1}{\gamma-1} p V=\frac{p V}{\gamma-1}\)
Thermodynamics Questions for NEET
Question 9. One mole of an ideal gas requires 207 J heat to raise the temperature by 10 K when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by the same 1 K, the heat required is:
- 198.7 J
- 29 J
- 215.3 J
- 124 J
Answer: 4. 124 J
Using \(C_p-C_v=R,\)
⇒ \(\mathrm{C}_{\mathrm{p}}\) is heat needed for raising by 10 \(\mathrm{~K}\).
⇒ \(\mathrm{C}_{\mathrm{p}}=20.7 \mathrm{~J} / \mathrm{mole} \mathrm{K}\)
Given R = 8.3 J/mole K
For raising by 10 K = 124 J
Question 10. A cylinder contains hydrogen gas at a pressure of 249 kPa and temperature 27°C. Its density is (R = 8.3 J mol-1 k-1):
- 0.5 kg / m3
- 0.2 kg / m3
- 0.1 kg / m3
- 0.2 kg / m3
Answer: 2. 0.2 kg / m3
Given: \(\mathrm{P}=249 \mathrm{kPa} ; \mathrm{T}=27^{\circ} \mathrm{C}, \mathrm{M}=2 \times 10^{-3} \mathrm{~kg}\)
Equation of state,
P V=\(\eta R T or P M=\delta \mathrm{RT} \quad\left[\eta=\frac{m}{M}\right]\)
⇒ \(\delta =\frac{P M}{R T}\)
= \(\frac{\left(249 \times 10^3\right)\left(2 \times 10^{-3}\right)}{8.3 \times 300}=0.2 \mathrm{~kg} / \mathrm{m}^3\)
Question 11. Which of the following is not a thermodynamical function?
- Enthalpy
- Work done
- Gibb’s energy
- Internal energy
Answer: 2. Work done
- Certain specific thermodynamic variables, such as pressure p, volume V, temperature T, and entropy S, can be used to describe the thermodynamic state of a homogeneous system.
- Any two of these four variables are independent and once they’re known, the others can be calculated. As a result, there are just two independent variables, with the others working as functions.
- Certain relationships are essential for a thorough understanding of the system, and we introduce some thermodynamic functions, which are functions of variables p, V, T, and S. Thermodynamic functions are classified into four categories.
- Internal energy (U)
- Helmholtz tunction (F)
- Enthalpy (H)
- Gibb’s energy (G)
Hence, the work done is not a thermodynamic function
Thermodynamics Questions for NEET
Question 12. An ideal gas undergoes four different processes at the same initial state as shown in the figure below. These processes are adiabatic, isothermal, isobaric, and isochoric. The curve which represents the adiabatic process among 1,2, 3, and 4 is:
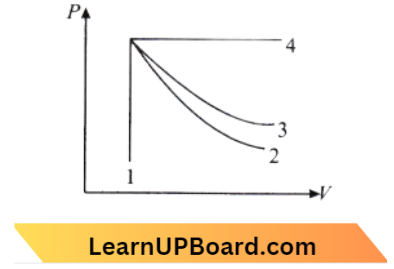
- 1
- 2
- 3
- 4
Answer: 2. 2
Curve 4 is an isobaric process because the pressure is constant. Curve I is isochoric because the volume is constant. Curve 3 is smaller than curve 2.
Question 13. In which of the following processes, heat is neither absorbed nor released by a system?
- Adiabatic
- Isobaric
- Isochoric
- Isothermal
Answer: 1. Adiabatic
In an adiabatic process, there is no exchange of heat of the system with its surroundings. Thus in the adiabatic process P1V1 and T change but ΔQ = 0 or entropy remains a constant process, I – Isochoric, volume is constant\(\left(\Delta S=\frac{\Delta Q}{T}\right)\)
Question 14. Thermodynamic processes are indicated in the following diagram:
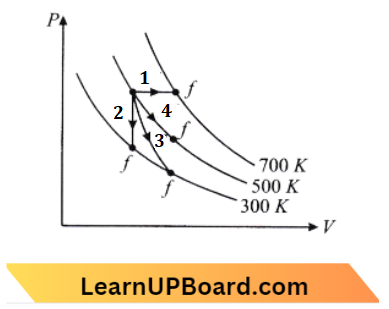
Match The Following:
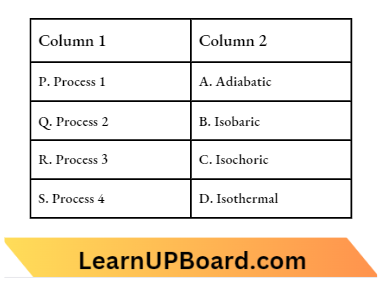
- P → A, Q → C, R → D, S → B
- P → C, Q → A, R → D, S → B
- P → C, Q → D, R → B, S → A
- P → D ,Q → B, R → A, S → C
Answer: 1. P → A, Q → C, R → D, S→ B
Process, 1 – Isochoric, volume is constant.
2: Adiabatic, the slope of curve II is more than the slope of curve 3
3: Isothermal
4: Isobaric, Pressure is constant. repeat
Thermodynamics Questions for NEET
Question 15. The volume (V) of a monatomic gas varies with its temperature (7), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is:
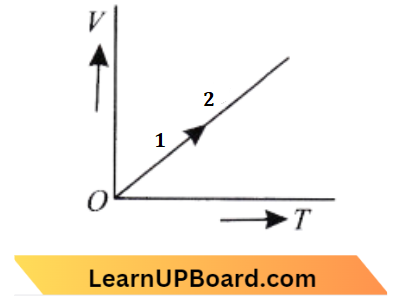
- \(\frac{2}{5}\)
- \(\frac{2}{3}\)
- \(\frac{1}{3}\)
- \(\frac{2}{7}\)
Answer: 1. \(\frac{2}{5}\)
In the process 1, volume is constant.
Process 1 → Isochoric; P → C
As slope of curve 2 is more than the slope of curve III.
Process 2 → Adiabatic and
Process 3 -→ Isothermal
Q → A, R→D
In process 4, pressure is constant.
Process 4 → Isobaric; S → B
Class 11 Thermodynamics MCQs
Question 16. A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then:
- compressing the gas through adiabatic.
- compressing the gas isothermally or adiabatically will require the same amount of work.
- which of the cases (whether compression through . isothermal or through an adiabatic process) requires more work will depend upon the atomicity of the gas.
- compressing the gas isothermally will require more to be done.
Answer: 2. compressing the gas isothermally or adiabatically will require the same amount of work.
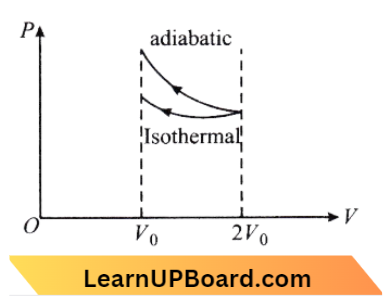
⇒ \(\mathrm{W}_{\text {ext }} =\text { negative of area with volume }- \text { axis }\)
∴ \(W(\text { adiabatic }) >W \text { (isothermal })\)
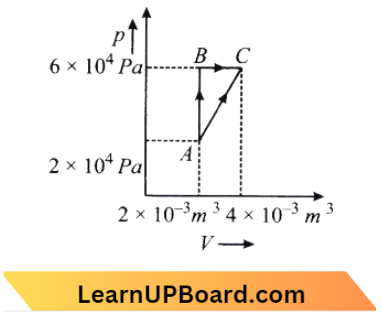
Question 17. The figure below shows two paths that may be taken by a gas to go from a state A to a state C. In process AB, 400 J of heat is added to the system, and in process, BC, 100 J of heat is added to the system. The heat absorbed by the system in process AC will be:
- 380 J
- 500 J
- 460 J
- 300 J
Answer: 3. 460 J
Given
The figure below shows two paths that may be taken by a gas to go from a state A to a state C. In process AB, 400 J of heat is added to the system, and in process, BC, 100 J of heat is added to the system.
From the diagram, We see that the initial and final points are the same.
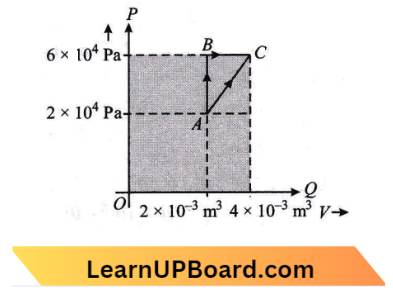
Case 1 :
So, \(\Delta U_{A \rightarrow B \rightarrow C}=\Delta U_{A \rightarrow C}\) → Equation 1
A \(\rightarrow B \)(Isochoric process)
⇒ \(d \mathrm{~W}_{\mathrm{A} \rightarrow \mathrm{B}}\)=0 and
⇒ \(d \mathrm{Q}=d \mathrm{U}=d \mathrm{~W}\)
∴ \(d \mathrm{Q}_{\mathrm{A} \rightarrow \mathrm{B}}=d \mathrm{U}_{\mathrm{A} \rightarrow \mathrm{B}}=400 \mathrm{~J}\)
Case 2 : \(\mathrm{B} \rightarrow \mathrm{C}\) is isobaric process
⇒ \(d \mathrm{Q}_{\mathrm{B} \rightarrow \mathrm{C}} =d \mathrm{U}_{\mathrm{B} \rightarrow \mathrm{C}}+d \mathrm{~W}_{\mathrm{B} \rightarrow \mathrm{C}}\)
= \(d \mathrm{U}_{\mathrm{B} \rightarrow \mathrm{C}}+\mathrm{P} \Delta \mathrm{V}_{\mathrm{B} \rightarrow \mathrm{C}}\)
⇒ \(100 =d \mathrm{U}_{\mathrm{B} \rightarrow \mathrm{C}}+6 \times 10^4\left(2 \times 10^{-3}\right)\)
∴ \(d \mathrm{U}_{\mathrm{B} \rightarrow \mathrm{C}} =100-120=-20 \mathrm{~J}\)
Using eq. (1),
⇒ \(\Delta \mathrm{U}_{\mathrm{A} \rightarrow \mathrm{B} \rightarrow \mathrm{C}}=\Delta \mathrm{U}_{\mathrm{A} \rightarrow \mathrm{C}}\)
⇒ \(\Delta \mathrm{U}_{\mathrm{A} \rightarrow \mathrm{B}}+\Delta \mathrm{U}_{\mathrm{B} \rightarrow \mathrm{C}}\)
= \(d \mathrm{Q}_{\mathrm{A} \rightarrow \mathrm{C}}-\mathrm{dW}_{\mathrm{A} \rightarrow \mathrm{C}}\)
⇒ \(400+(-20)=d \mathrm{Q}_{\mathrm{A} \rightarrow \mathrm{C}}-\left(\mathrm{P} \Delta \mathrm{U}_{\mathrm{A}}+\text { Area } \Delta_{\mathrm{A} \rightarrow \mathrm{B} \rightarrow \mathrm{C}}\right)\)
⇒ \(d \mathrm{Q}_{\mathrm{A} \rightarrow \mathrm{C}}=380[2 \times 10^4 \times 2 \times 10^{-3}+\).
⇒ \(\left.\frac{1}{2} \times 2 \times 10^{-3} \times 4 \times 10^4\right]\)
∴ \(d Q_{\mathrm{A} \rightarrow \mathrm{C}}=460 \mathrm{~J}\)
Class 11 Thermodynamics MCQs
Question 18. A thermodynamic system undergoes the cyclic process ABCD as shown in the figure. The work done by the system in the cycle is:
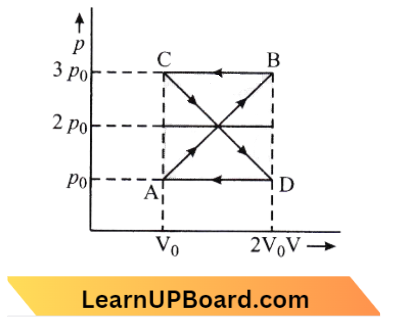
- \(p_0 V_0\)
- \(2 p_0 V_0\)
- \(\frac{p_0 V_0}{2}\)
- zero
Answer: 4. zero
According to the question,
Work done in the cyclic process: Area bound by the closed configuration
Area of closed configuration \(\left\{2\left[\frac{1}{2}\left(\frac{\mathrm{V}_0}{2}\right) \times \mathrm{P}_0\right]+\left[-2\left\{\frac{1}{2}\left(\frac{\mathrm{V}_0}{2}\right) \times \mathrm{P}_0\right\}\right]\right\}=0\)
Important MCQs on Thermodynamics for NEET
Question 19. An ideal gas is compressed to half its initial volume using several processes. Which of the processes results in the maximum work done on the gas?
- Adiabatic
- Isobaric
- Isochoric
- Isothermal
Answer: 1. Adiabatic
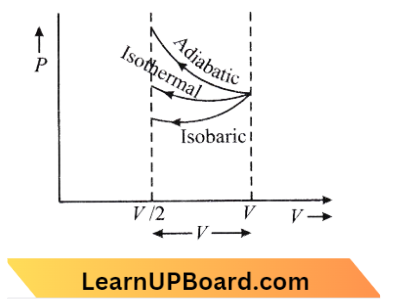
Work done = Area under P-V graph So large work is in adiabatic Here,
∴ \(W_{\text {adiabatic }}>W_{\text {isothermal }}>W_{\text {isobaric }}\)
Question 20. A Monoatomic gas at a pressure p, having a volume V expands isothermally to a volume 2 V and then adiabatically to a volume 16 V. The final pressure of the gas is (take \(\lambda=\frac{5}{3}\)):
- 64p
- 32p
- \(\frac{P}{64}\)
- 16p
Answer: 3. \(\frac{P}{64}\)
Using Ideal Gas Equation, P =\(\frac{n R T}{V}\)
⇒ \(P_1 =\frac{n R T}{2 V}=\frac{P}{2}\)
For isothermal expansion, \(P V =P_1 \times 2 V \)
⇒ \(P_1 =\frac{P}{2}\)
For adiabatic expansion, \(P V^r\) = Constant
⇒ \(P_1 V_1^r= P_2 V_2^r\)
⇒ \(P_2 =[2 V]^{5 / 3}=P_2[16]^{5 / 3} \)
⇒ \(P^2 =\frac{P}{2}\left[\frac{2 V}{16 V}\right]^{\frac{5}{3}}=\frac{P}{2}\left[\frac{1}{8}\right]^{\frac{5}{3}}\)
= \(\frac{P}{2} \times 0.0312=0.0156 P\)
= \(\frac{P}{64}\)
Important MCQs on Thermodynamics for NEET
Question 21. A gas is taken through the cycle A → B → C → A as shown. What is the net work done by the gas?
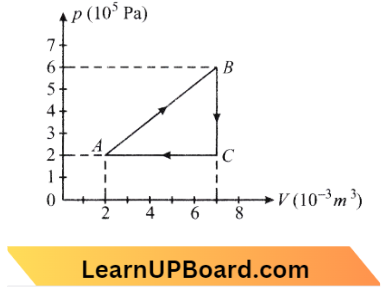
- 200J
- 1000J
- Zero
- -200J
Answer: 2. 1000J
Net work done = Area of triangle
= \(\frac{1}{2} \times\left[(7-2) \times 10^{-3}\right]\left[(6-2) \times 10^5\right] =1000 \mathrm{~J}\)
Important MCQs on Thermodynamics for NEET
Question 22. During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature.
The ratio of \(\frac{C_P}{C_V}\) for the gas is:
- \(\frac{4}{3}\)
- 2
- \(\frac{5}{3}\)
- \(\frac{3}{2}\)
Answer: 4. \(\frac{3}{2}\)
According to the question, \(P T^{-3}\)= Constant → Equation 1
For an adiabatic process, \(PT \|-r\)= constant → Equation 2
compare equation (1) and (2), we get
⇒ \(\frac{r}{1-r}=-3\)
r =\(-3+3 r \text { or } r=\frac{3}{2}\)
As, r =\(\frac{C_P}{C_V}\):
∴ \(\mathrm{C}_{\mathrm{P}} / \mathrm{C}_{\mathrm{V}} =\frac{3}{2}\)
Question 23. A system is taken from state a to state c by two paths act and abc as shown in the figure. The internal energy a is U2 – 10 J. Along the path adc the amount of heat absorbed is δQ1 = 50 J, and the work obtained δW1 = 20 J whereas, along the path ABC, the heat absorbed δQ2 = 36 J. The amount of work along the path ABC is:
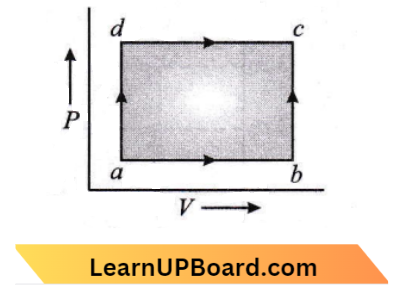
- 10 J
- 12 J
- 36 J
- 6 J
Answer: 4. 6 J
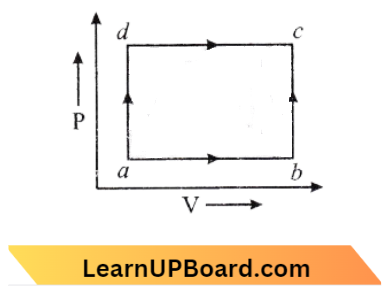
Given
A system is taken from state a to state c by two paths act and abc as shown in the figure. The internal energy a is U2 – 10 J. Along the path adc the amount of heat absorbed is δQ1 = 50 J, and the work obtained δW1 = 20 J whereas, along the path ABC, the heat absorbed δQ2 = 36 J.
According to \(1^{\text {st }}\) law of thermodynamics
⇒ \(\delta Q=\delta V+\delta W\)
Along the path adc, \(\delta U_1=\delta Q_1-\delta W_1\)
=50-20
= 30 \(\mathrm{~J}\)
then path ABC , \(\delta U_2=36-\delta W_2\)
Since the change in Internal energy in path independence
So,\(\delta U_1 =\delta U_2\)
⇒ \(30 \mathrm{~J} =36 \mathrm{~J}-\delta W_2\)
⇒ \(\delta W_2 =36 \mathrm{~J}-30 \mathrm{~J} \)
=6 \(\mathrm{~J}\)
Important MCQs on Thermodynamics for NEET
Question 24. Which of the following relations does not give the equation of an adiabatic process, where terms have their usual meaning?
- \(P^{1-\gamma} T^\gamma\)= constant
- \(P V^\gamma\)= constant
- \(T V^{\gamma-1}\)= constant
- \(P^\gamma T^{1-\gamma}\)= constant
Answer: 4. \(P^\gamma T^{1-\gamma}\)= constant
Adiabatic process \(\rightarrow \)Heat does not exchange between system surrounding
⇒ \(P V^\gamma\)= constant → Equation 1
Ideal gas equation P V=n R T
P =\(\frac{n R T}{V}=\frac{n R T}{V} \quad V^\gamma\)= constant
⇒ \(T V^\gamma-1\) = constant → Equation 2
PV = nRT
V =\(\frac{n R T}{P}\) { Put in eq. (1) }
Again PV=n R T
⇒ \(P\left(\frac{n R T}{P}\right)^\gamma\) = constant
⇒ \(P^{1-\gamma} T^\gamma\) = constant
So, option (d) does not give the relation.
Question 25. A thermodynamic system is taken through the cycle ABCD as shown in the figure. Heat rejected by the gas during the cycle is:
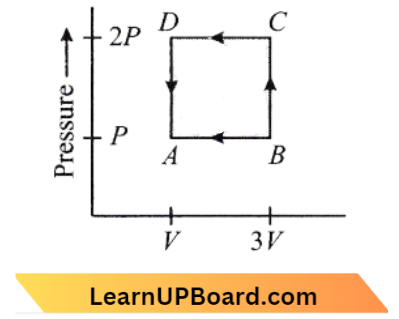
- 2 PV
- 4 PV
- \(\frac{1}{2} P V\)
- PV
Answer: 1. 2 PV
From the question,
For a given cyclic process AV= 0
Q = W
W =- Area enclosed in the curve
= AB AD
= (2P-P) x (3V- V)
=-p x 2V
Heat rejected = 2 PV
Physics MCQs for NEET with Answers
Question 26. An ideal gas goes from state A to state B via three different processes as indicated in the p-V diagram. If Q1, Q2, Q3 indicate the heat absorbed by the gas along the three processes and ΔU1, ΔU2, ΔU2 indicate the change in internal energy along the three processes respectively, then:
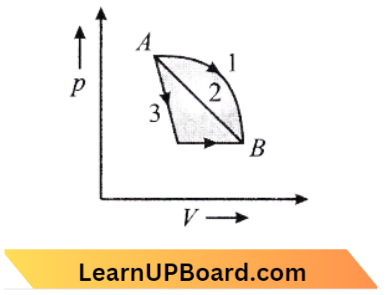
- Q1 > Q2 > Q3 and ΔU1 = ΔU2 = ΔU3
- Q3 > Q2 > Q1 and ΔU1 = ΔU2 = ΔU3
- Q1 = Q2 = Q3 and ΔU1 > ΔU2 > ΔU3
- Q3 > Q2 > Q1 and ΔU1 > ΔU2 > ΔU3
Answer: 1. Q1 > Q2 > Q3 and ΔU1 = ΔU2 = ΔU3
Given
An ideal gas goes from state A to state B via three different processes as indicated in the p-V diagram. If Q1, Q2, Q3 indicate the heat absorbed by the gas along the three processes and ΔU1, ΔU2, ΔU2 indicate the change in internal energy along the three processes respectively,
According to the question,
⇒ \(\Delta U_1=\Delta U_2=\Delta U_3\)
⇒ \(Q_1>Q_2>Q_3\)
because, \(\Delta Q=\Delta W+d U\)
change in internal energy is independent of the path.
Question 27. One mole of an ideal gas goes from an initial state A to a final state B via two processes: It first undergoes isothermal expansion from volume V to 3 V and then its volume is reduced from 3V to V to constant pressure. The correct PL-V diagram representing the two processes is
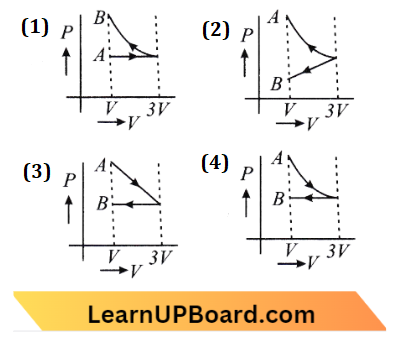
Answer: 4.
Given that, gas A → B
firstly isothermal expansion = V to 3 V
then volume reduced from 3 V to V at constant pressure
(1) For isothermal expansion,
T= constant
PV= NRT = constant
PV= constant
Hence hyperbolic curve
(2) Now for isobaric compression,
P = constant
PV= nRT
∴ straight line. Hence option (D) is correct.
Physics MCQs for NEET with Answers
Question 28. During an isothermal expansion, a confined ideal gas does – 150 J of work against its surroundings. This implies that:
- 150 J of heat has been removed from the gas.
- 300 J of heat has been added to the gas
- No heat is transferred because the process is isothermal.
- 150 J of heat has been added to the gas.
Answer: 3. No heat is transferred because the process is isothermal
From the first law of thermodynamics
⇒ \(\Delta U=\Delta \mathrm{Q}+\Delta W\)
for isothermal process \(\Delta U\)=0
⇒ \(\Delta \mathrm{Q}=-\Delta W \)
⇒ \(\Delta Q\)=-(-150)=150
∴ Here \(\Delta\) Q is positive means heat is added to gas.
Laws of Thermodynamics MCQs for NEET
Question 29. A mass of diatomic gas (γ = 1.4) at a pressure of 2 atmospheres is compressed adiabatically so that its temperature rises from 27°C to 927°C. The pressure of the gas in the final state is:
- 8 at
- 28 atm
- 68.7 atm
- 256 atm
Answer: 4. 256 atm
For an adiabatic process, \(\frac{T^\gamma}{P^{\gamma-1}}\)= constant
⇒ \(\left(\frac{T_i}{T_f}\right)^\gamma=\left(\frac{P_i}{P_f}\right)^{\gamma-1}\)
Here,\( P_f=P_i\left(\frac{T_i}{T_f}\right)^{\frac{\gamma}{\gamma-1}}\)
⇒ \(\mathrm{~T}_{\mathrm{i}}=27^{\circ} \mathrm{C}=300 \mathrm{~K}\),
⇒ \(\mathrm{~T}_{\mathrm{f}}=927^{\circ} \mathrm{C}=1200 \mathrm{~K}\)
⇒ \(\mathrm{P}_{\mathrm{i}}=2 \mathrm{~atm}, \gamma\) = 1.4
Substituting these values in eqn (i), we get
⇒ \(P_f =(2)\left(\frac{1200}{300}\right)^{\frac{1.4}{1.4-1}}=(2)(4)^{1.4 / 0.4}\)
= \(2\left(2^2\right)^{7 / 2}=(2)(2)^7=2^8=256 \mathrm{~atm}\)
Question 30. If AU and AW represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true?
- ΔU = – ΔW, in adiabatic process
- ΔU= ΔW, in an isothermal process
- ΔU = ΔW, in a adiabatic process
- ΔU = -ΔW, in an isothermal process
Answer: 1. ΔU = – ΔW, in adiabatic process
From the first law of thermodynamics,
⇒ \(\Delta Q=\Delta U+\Delta W\)
for adiabatic process, \(\Delta \mathrm{Q}\)=0
∴ \(\Delta U=-\Delta W\)
Question 31. In a thermodynamic process which of the following statements is not true?
- In an adiabatic process, the system is insulated from the surroundings
- In an isochoric process, pressure remains constant
- In an isothermal process, the temperature remains constant
- In an adiabatic process PV1 = constant
Answer: 2. In an isochoric process pressure remains constant
In an isochoric process, volume remains constant.
Laws of Thermodynamics MCQs for NEET
Question 32. If Q, E, and W denote respectively the heat added, change in internal energy, and the work done in a closed cycle process, then:
- W= 0
- Q=W=0
- E = 0
- g = 0
Answer: 3. E = 0
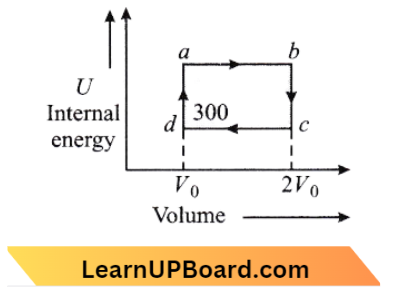
In the cyclic process, since initial and final states are of the same internal energy is a state function therefore initial and final internal energies are also the same. So, the charge in internal energy is the same, hence E = 0
Question 33. Two moles of an ideal gas are taken in a cyclic process abcda. During the process ab and cd temperatures are 500 K and 300 K respectively. Calculate heat absorbed by the system (in 2 = 0.69 and R = 8.3 J/mol-K).
- 2290.3 J
- 2209.3 J
- 2029.3 J
- 2293.3 J
Answer: 1. 2290.3 J
From the diagram, it is clear that:
⇒ \(Q_{a b c d a}=Q_{a b}+Q_{b c}+Q_{c d}+Q_{d a}\)
Here a b, b c, c d, and d a represent isothermal expansion, isochoric compression, isothermal compression, and isochoric expansion respectively,
⇒ \(Q_{b c} =Q_{d a}\)
⇒ \(Q_{a b c d a} =Q_{a b}+Q_{c d}\)
⇒ \(Q_{a b c d a} =Q_{a b}+Q_{c d}\)
= \(\eta R T_1 \log \left[\frac{2 V_0}{V_0}\right]+\mu R T_2 \log \left[\frac{V_0}{2 V_0}\right]\)
= \(2 \times 8.3 \times 0.09(500-300)\)
= 2290.3 J
Laws of Thermodynamics MCQs for NEET
Question 34. A sample of gas expands from volume V1 to V2. The amount of work done by the gas is greatest when the expansion is:
- adiabatic
- isobaric
- isothermal
- equal in all the above cases
Answer: 2. isobaric
The P-V diagram for the isobaric, isothermal, and adiabatic processes of an ideal gas is shown in the graph below,
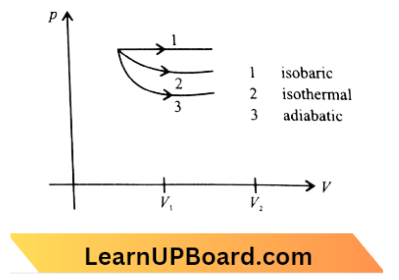
In thermodynamics, for some volume change, the work done is maximum for the curve having maximum area enclosed with the volume axis.
The area enclosed by the curve,
⇒ (Slope of curve ) alpha
⇒ \((\text { slope })_{\text {isobaric }}<(\text { slope })_{\text {isothermal }}<(\text { slope })_{\text {adiabatic }}\)
⇒ \((\text { Area })_{\text {isobaric }}>(\text { Area })_{\text {isothermal }}>(\text { Area })_{\text {adiabatic }}\)
Hence, the work done is maximum in the isobaric process.
⇒ \((\text { Slope })_{\text {adiabatic }} =-\gamma\left(\frac{P}{V}\right)\)
⇒ \(\text { and }(\text { Slope })_{\text {isothermal }} =-\frac{P}{V} \)
⇒ \((\text { Slope })_{\text {adiabatic }} =\gamma \times(\text { slope })_{\text {isothermal }}\)
The slope of the adiabatic curve is always steeper than that of the isothermal curve
Question 35. An ideal gas A and a real gas B have their volumes increased from V to 2V under isothermal conditions. The increase in internal energy:
- will be the same in both A and B
- will be zero in both the gases
- of B will be more than that of A
- of A will be more than that of B
Answer: 2. will be zero in both the gases
An isothermal change occurs when the pressure and volume of a gas change without the temperature changing. There is a free exchange of heat between the gas and its surroundings during such a transition.
T= constant, \(\Delta\)= 0
∴ So, internal energy (U) remains constant.
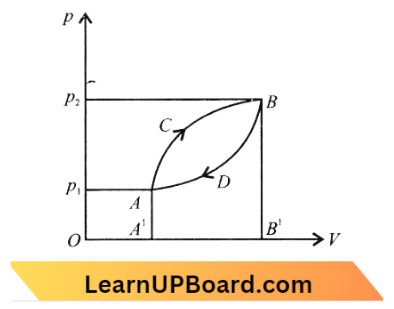
Heat and Thermodynamics NEET Questions
Question 36. A thermodynamic system is taken from state A to B along ACB and is brought back to A along BDA as shown in the diagram. The net work done during the complete cycle is given by the area:
- \(p_1 A C B p_2 p_1\)
- \(A C B B^{\prime} A^{\prime} A\)
- A C B D A
- \(A D B B^{\prime} A^{\prime} A\)
Answer: 3. A C B D A
Work done during path ACB = area ACBB’ A’ A
Work done during going from ACB and then to BDA path is = area ACB B’A’A – area BDAA’ B’ B
= area ACBDA
Question 37. A thermodynamic process is shown in the figure. The pressure and volumes corresponding to some points in the figure are:
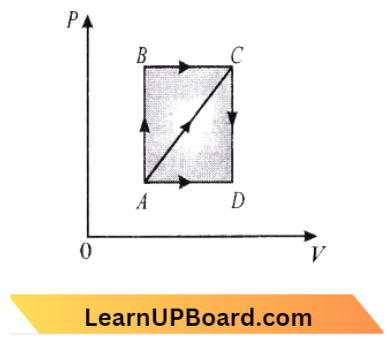
⇒ \(P_A=3 \times 10^4 \mathrm{~Pa} ; V_A=2 \times 10^{-3} \mathrm{~m}^3 \)
⇒ \(P_B=8 \times 10^4 \mathrm{~Pa} ; V_D=5 \times 10^{-3} \mathrm{~m}^3\)
In the process, AB, 600 J of heat is added to the system. The change in internal energy of the system is process AC would be
- 560 J
- 800 J
- 600 J
- 640 J
Answer: 1. 560 J
Since AB is an isochoric process. So no work is done. \mathrm{BC} is isobaric process
⇒ \(\mathrm{W}=\mathrm{P}_{\mathrm{B}} \times\left(\mathrm{V}_{\mathrm{D}}-\mathrm{V}_{\mathrm{A}}\right)=240 \mathrm{~J}\)
⇒ \(\Delta \mathrm{Q}=600+200=800 \mathrm{~J}\)
Using \(\Delta \mathrm{Q}=\Delta \mathrm{U}+\Delta \mathrm{W}\)
∴ \(\Delta \mathrm{U}=\Delta \mathrm{Q}-\Delta \mathrm{W}=800-240=560 \mathrm{~J}\)
Heat and Thermodynamics NEET Questions
Question 38. The efficiency of an ideal heat engine working between the freezing point and boiling point of water is :
- 6.25%
- 20%
- 26.8%
- 12.5%
Answer: 3. 26.8%
We know that the efficiency of an ideal heat engine \(\eta=\left(1-\frac{T_2}{T_1}\right)\)
Where, \(T_1=100+273=373 \mathrm{~K}\)
⇒ \(T_2=0.273=273 \mathrm{~K}\)
Putting the value of \(T_1 and T_2\) in eq. (1), We get,
⇒ \(\eta =1-\frac{273}{373}=\frac{373-273}{373}=\frac{100}{373}\)
=0.268
Now, % \(\eta\) =0.268 x 100=26.8 %
Question 39. A heat engine has a source at a temperature of 527°C and a sink at a temperature of 127°C. If the useful work is required to be done by the engine at the rate of 800 W, then find the amount of heat absorbed by the sink per second from the source in calories. Also, find the efficiency of the heat engine.
- \(381 \mathrm{cal} \mathrm{s}^{-1}, 50 \%\)
- \(318 \mathrm{cal} \mathrm{s}^{-1}, 50 \%\)
- \(381 \mathrm{cal} \mathrm{s}^{-1}, 45 \%\)
- \(318 \mathrm{cal} \mathrm{s}^{-1}, 45 \%\)
Answer: 1. \(381 \mathrm{cal} \mathrm{s}^{-1}, 50 \%\)
Given
A heat engine has a source at a temperature of 527°C and a sink at a temperature of 127°C. If the useful work is required to be done by the engine at the rate of 800 W, then find the amount of heat absorbed by the sink per second from the source in calories.
From question, \(T_1=800 \mathrm{~K}, T_2=400 \mathrm{~K}\) and \(W=a_1-a_2=800 \mathrm{~W}\)
Work done per unit amount of heat absorbed is:
⇒ \(\frac{W}{Q_1} =\frac{T_2-T_2}{T_1}=\frac{800-900}{800}=\frac{1}{2}\)
⇒ \(\frac{800}{Q_1} =\frac{1}{2}\)
⇒ \(Q_1=1600 \mathrm{~W}\)
⇒ \(Q_1=\frac{1600}{4.2}=381 \mathrm{cal} \mathrm{s}^{-1}\)
∴ Efficiency, \(\eta=\frac{T_1-T_2}{T_1}=\frac{800-400}{800}=\frac{1}{2}\)= 50%
Question 40. The temperatures of the source and sink of a heat engine are 127°C and 27°C respectively. An inventor claims its efficiency to be 26%, then:
- it is impossible
- it is possible with a high probability
- it is possible with a low probability
- Data is insufficient
Answer: 1. it is impossible
Efficiency of heat engine is, \(\eta =1-\frac{T_2}{T_1}\) { or } \(\eta=\frac{T_1-T_2}{T_1}\)
⇒ T1 = temperature of sink
⇒ T2=Temperature of source
Given, T1=273+127=400 K
⇒ T2=273+27=300 K
⇒ \(\eta =\frac{400-300}{400}=\frac{100}{400}\)
=0.25 = 25 %
Hence, 26% efficiency is impossible for a given heat engine
Heat and Thermodynamics NEET Questions
Question 41. A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second to keep the temperature of the refrigerated space constant. The power required is (Take, 1 cal = 4.2 Joules):
- 23.65 W
- 236.5 W
- 2365 W
- 2.365 W
Answer: 2. 236.5 W
According to the question, Temperature of source, \(T_1=30^{\circ} \mathrm{C}=30+273\)
⇒ \(T_1=303 \mathrm{~K}\)
Temperature of sink, \(T_2=4^{\circ} \mathrm{C}=4+273\)
⇒ \(T_2=277 \mathrm{~K}\)
We know that \(\frac{Q_1}{Q_2} =\frac{T_1}{T_2}\)
⇒ \(\frac{Q_2+W}{Q_2} =\frac{T_1}{T_2}\left\{ W=\mathrm{Q}_1-\mathrm{Q}_2\right\}\)
⇒ \(W T_2+T_2 Q_2 =T_1 Q_2 \)
⇒ \(W T_2 =T_1 Q_2-T_2 Q=Q_2\left(T_1-T_2\right)\)
W =\(\frac{Q_2}{T_2}\left(T_1-T_2\right)=Q_2\left(\frac{T_1}{T_2}-1\right)\)
=600 \(\times 4.2\left(\frac{303}{277}-1\right)\)
=600 \(\times 4.2 \times \frac{26}{277} \)
=236.5 \(\mathrm{~J}=\text { Retain }\)
Power =\(\frac{\text { Work done }}{\text { time }}\)
= \(\frac{236.5}{1}=236.5 \mathrm{~W}\)
Question 42. The temperature inside a refrigerator is t2°C and the room temperature is t1°C. The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be:
- \(\frac{t_1}{t_1-t_2}\)
- \(\frac{t_1+273}{t_1-t_2}\)
- \(\frac{t_2+273}{t_1-t_2}\)
- \(\frac{t_1+t_2}{t_1+273}\)
Answer: 2. \(\frac{t_1+273}{t_1-t_2}\)
If \(Q_1\)= amount of heat delivered to the room
W = electrical energy consumed
⇒ \(T_1 \)= room temperature =\(t_1+273 \)
⇒ \(T_2 \)= temp. of sink =\(t_2+273\)
Then, for the refrigerator,
⇒ \(\frac{Q_1}{W} =\frac{Q_1}{Q_1-Q_2}=\frac{T_1}{T_1-T_2}\)
⇒ \(\frac{Q_1}{1} =\frac{t_1+273}{\left(t_1+273\right)-\left(t_2+273\right)}\)
∴ \(Q_1 =\frac{t_1+273}{\left(t_1-t_2\right)}\)
Heat and Thermodynamics NEET Questions
Question 43. The coefficient of performance of a refrigerator is 5. If the temperature inside the freezer is – 20°C, the temperature of the surroundings to which it rejects heat is:
- 31°C
- 41°C
- 11°C
- 21°C
Answer: 1. 31°C
Coefficient of performance of the refrigerator
C.O.P =\(\frac{T_L}{T_H-T_L}\)
Where\( T_L\)= Lower temperature
⇒ \(T_H\)= Higher temperature
5 =\(\frac{T_L}{T_H-T_L}\)
⇒ \(T_H =\frac{6}{5} T_L\)
= \(\frac{6}{5} \times 253=303.6 \mathrm{~K}\)
= 31 \(\dot{\mathrm{C}}\)
Question 44. Which of the following processes is reversible?
- Transfer of heat by conduction.
- Transfer of heat by radiation.
- Isothermal compression.
- Electrical heating of a nichrome wire.
Answer: 3. Isothermal compression.
Isothermal compression is reversible. for example; Carnot cycle, heat engine.
Heat and Thermodynamics NEET Questions
Question 45. The efficiency of a Carnot engine depends upon:
- the temperature of the sink only
- the temperature of the source and sink
- the volume of the cylinder of the engine
- the temperature of the source only.
Answer: 2. the temperature of the source and sink
The efficiency of a camot engine is given by :\(\eta=1-\frac{T_2}{T_1}\)
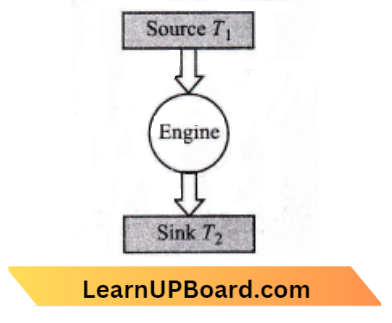
Where, \(T_1\) = Temperature of source
⇒ \(T_2=\)= temperature of sink
This confirms that the efficiency of the Carnot engine depends upon the temperature of the source and also the temperature of the sink
Question 46. A Carnot engine having the efficiency of a heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at a lower temperature is:
- 1 J
- 90 J
- 99 J
- 100 J
Answer: 2. 90 J
In case of carnot engine, efficiency =\(\frac{\text { Work }}{\text { heat absorbed }}\)=\(\frac{W}{q_1}\)
⇒ \(\frac{W}{q_1} =\frac{1}{10}\)
⇒ \(\frac{10 \mathrm{~J}}{q_1} =\frac{1}{10}\)
⇒ \(q_1 =100 \mathrm{~J}\)
When the engine is reversed then\( W+q_2 =q_1 \)
⇒ \(10+q_2 \)=100
∴ \(q_2 =90 \mathrm{~J}\)
Heat and Thermodynamics NEET Questions
Question 47. Two Carnot engines A and B are operated in series. Engine A receives heat from the source at temperature T1 and rejects the heat to the sink at temperature T. The second engine B receives the heat at temperature T and rejects to its sink at temperature T2. For what value of T the efficiencies of the two engines are equal:
- \(\frac{T_2-T_2}{2}\)
- \(T_1 T_2\)
- \(\sqrt{T_1 T_2}\)
- \(\frac{T_1+T_2}{2}\)
Answer: 3. \(\sqrt{T_1 T_2}\)
Given
Two Carnot engines A and B are operated in series. Engine A receives heat from the source at temperature T1 and rejects the heat to the sink at temperature T. The second engine B receives the heat at temperature T and rejects to its sink at temperature T2.
We know that the efficiency of the Carnot engine,
⇒ \(\eta=1-\frac{T_2}{T_1}\)
Where, \(T_1\)= temperature of source
⇒ \(T_2\)= temp of sink
For engine A, \(\eta_{\mathrm{A}}=1-\frac{T}{T_1}\)
engine B, \(\eta_{\mathrm{B}}=1-\frac{T_2}{T}\)
From question, \(\eta_A =\eta_B\)
⇒ \(1-\frac{T}{T_1} =1-\frac{T_2}{T}\)
⇒ \(\frac{T}{T_1}=\frac{T_2}{T} =T^2\)
⇒ \(T^2 =T_1 T_2 \)
T =\(\sqrt{T_1 T_2}\)
Question 48. An engine has an efficiency of 1/6. When the temperature of the sink is reduced by 62°C, its efficiency is doubled. Temperatures of the source are:
- 37°C
- 62°C
- 99°C
- 124°C
Answer: 3. 99°C
Using the formula for efficiency, \(\eta =1-\frac{T_2}{T_1} \)
⇒ \(\frac{1}{6} =1-\frac{T_2}{T_1}\)
⇒ \(\frac{T_2}{T_1} =\frac{5}{6}\)
Since the temp. of the source remains unchanged,
⇒ \(2 \times \frac{1}{6} =1-\frac{T_2-60}{T_1}\)
⇒ \(\frac{1}{3} =1-\frac{\left(T_2-62\right)}{T_1}\)
⇒ \(\frac{2}{3} =\left[\frac{5}{2}-2\right] T_1 \)
⇒ \(T_1 =3 T_2-186\)
⇒ \({\left[\frac{5}{2}-2\right] T_1}\) =186
∴ \(T_1 =372 \mathrm{~K}=99^{\circ} \mathrm{C}\)
Heat and Thermodynamics NEET Questions
Question 49. A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of the source be increased to increase its efficiency by 50% of the original efficiency?
- 380 K
- 275 K
- 325 K
- 250 K
Answer: 4. 250 K
Efficiency of Carnot engine,
⇒ \(\eta =1-\frac{T_2}{T_1}\)
⇒ \(\frac{T_2}{T_1} =1-\eta\)
⇒ \(\frac{300}{T_1} =1-\frac{40}{100}=\frac{3}{5}\)
⇒ \(\mathrm{~T}_1 =500 \mathrm{~K}\) or
New efficiency, \(\eta^{\prime}=\eta+\frac{50}{100} \eta=\frac{3}{2} \eta=60 \%\)
⇒ \(\frac{T_2}{T_1}=1-\frac{60}{100}=\frac{2}{5} \)
⇒ \(T_1^{\prime}=\frac{5}{2} \times T_2=\frac{5}{2} \times 300=750 \mathrm{~K}\)
Increase in temperature of the source,
⇒ \(\Delta \mathrm{T}=T_1^{\prime}-T_1 \)
∴ \(\Delta \mathrm{T}=750-500=250 \mathrm{~K}\) .
Question 50. An ideal gas heat engine operates in a Carnot cycle between 227°C and 127°C. It absorbs 6 x 104 cal of heat at higher temperatures. The amount of heat converted into work is:
- 4.8 x 104cals
- 6 x 104cals
- 2.4 x 104cals
- 1.2 x 104cals
Answer: 4. 1.2 x 104cals
For a Carnot engine efficiency, \(\eta =1-\frac{T_2}{T_1}\)
= \(1-\frac{127+273}{227+273}\)
= \(1-\frac{400}{500}=\frac{1}{5}\)
Now, \( \eta =\frac{\text { Work Output }}{\text { Heat Output }}\)
= \(\frac{\mathrm{W}}{6 \times 10^4}\)
∴ \(\mathrm{~W} =\eta \times 6 \times 10^4 \)
= \(\frac{1}{5} \times 6 \times 10^4\)
= \(1.2 \times 10^4 \mathrm{cal}\).
Chapter-Wise Physics MCQs for NEET
Question 51. The efficiency of the Camot engine is 50% and the temperature of the sink is 500 K. If the temperature of the source is kept constant and its efficiency raised to 60%, then the required temperature of the sink will be:
- 100 K
- 600 K
- 400 K
- 500 K
Answer : 3. 400 K
⇒ \(\% \eta=\left(1-\frac{T_2}{T_1}\right) \times 100\)
For 50 \(\%, \frac{50}{100}=1-\frac{50}{11}\)
⇒ \(T_1=1000 \mathrm{~K}\)
aim for 60 \(\%, \frac{60}{100}=1-\frac{T_2}{1000}\)
∴ \(T_2=400 \mathrm{~K}\)
Question 52. The (W/Q) of a Camot engine is 1/6, now the temperature of the sink is reduced by 62°C, then this ratio becomes twice, therefore the initial temperature of the sink and source are respectively:
- 33°C, 67°C
- 37°C, 99°C
- 67°C, 33°C
- 97 K, 37 K
Answer: 2. 37°C, 99°C
⇒ \(\frac{1}{6}=-\frac{T_2}{T_1}{ or }\frac{5}{6}=\frac{T_2}{T_1}\)
Now,\(\frac{1}{3}=1-\frac{T_2-62}{T_1}=1-\frac{5}{6}+\frac{62}{T_1}\)
⇒ \(\mathrm{~T}_1=62 \times 6=372 \mathrm{~K}=99^{\circ} \mathrm{C}\)
and \(\mathrm{T}_2=310 \mathrm{~K}=37^{\circ} \mathrm{C}\)
Chapter-Wise Physics MCQs for NEET
Question 53. The efficiency of a Carnot engine operating between temperatures of 100°C and-23°C will be
- \(\frac{100-23}{273}\)
- \(\frac{100+23}{373}\)
- \(\frac{100+23}{100}\)
- \(\frac{100-23}{100}\)
Answer: 2. \(\frac{100+23}{373}\)
The efficiency of the Carnot engine is given by, \(\eta=1-\frac{T_2}{T_1}=\frac{T_1-T_2}{T_1}\)
Given, \(T_1\) = temperature of reservoir
= \(100+273=273 \mathrm{~K}\)
⇒ \(T_2\) = temperature of sink
=\(-23+273=250 \mathrm{~K}\)
Substituting in Eq. (1), we get,
\(\eta =\frac{373-250}{373}\)=\(\frac{100+23}{373}=\frac{123}{373}\)
