Classification Of Elements NEET MCQs
NEET Chemistry For Polymers Multiple Choice Questions
Question 1. Which statement regarding polymers is not correct?
- Elastomers have polymer chains held together by weak intermolecular forces.
- Fibres possess high tensile strength.
- Thermoplastic polymers are capable of repeatedly softening and hardening m on heating and cooling respectively.
- Thermosetting polymers are reusable.
Answer: 4. Thermosetting polymers are reusable.
Thermosetting polymers on heating undergo extensive cross-linking and become infusible. Hence, these cannot be reused.
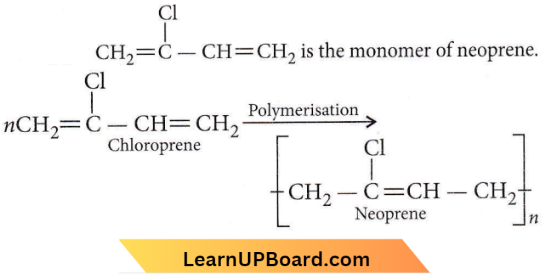
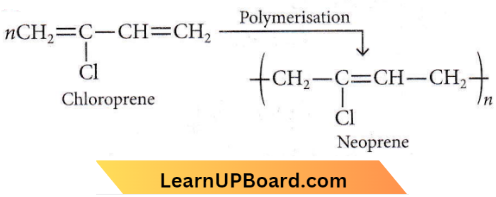
Question 2. Which of the following molecules on polymerization produces neoprene?
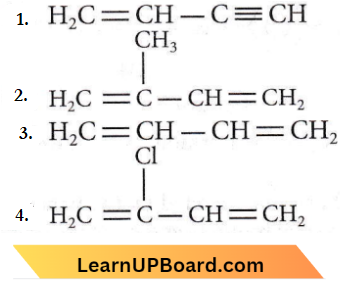
Answer: 4
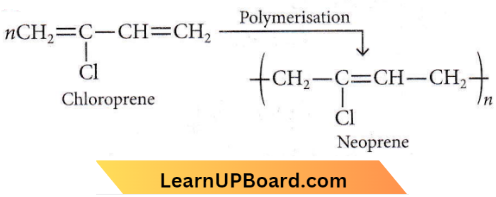
Neoprene or polychloroprene is formed by the free radical polymerisation of chloroprene.
Question 3. Which of the following polymers is prepared by addition polymerisation?
- Dacron
- Teflon
- Nylon-6,6
- Novolac
Answer: 2. Nylon-6,6
Among the given polymers tellon polymer is prepared by addition polymerisation.
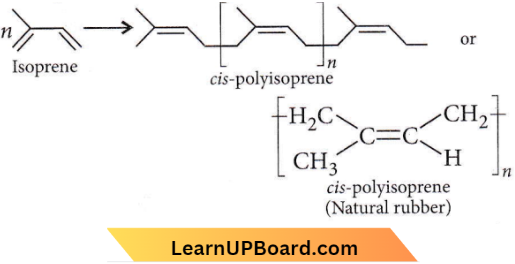
Question 4. Which of the following is a natural polymer?
- cis-1, 4-polyisoprene
- poly (Butadiene-styrene)
- polybutadiene
- poly (Butadiene-acrylonitrile)
Answer: 1. cis-1, 4-polyisoprene
Read and Learn More NEET MCQs with Answers
Classification of Elements NEET MCQs
Question 5. The polymer that is used as a substitute for wool in making commercial fibres is
- Melamine
- Nylon-6, 6
- Polyacrylonitrile
- Buna-N.
Answer: 3. Polyacrylonitrile
Question 6. Regarding cross-linked or network polymers, which of the following statements is incorrect?
- They contain covalent bonds between various linear polymer chains.
- They are formed from bi- and tri-functional monomers.
- Examples are bakelite and melamine.
- They contain strong covalent bonds in their polymer chains.
Answer: 4. They contain strong covalent bonds in their polymer chains.
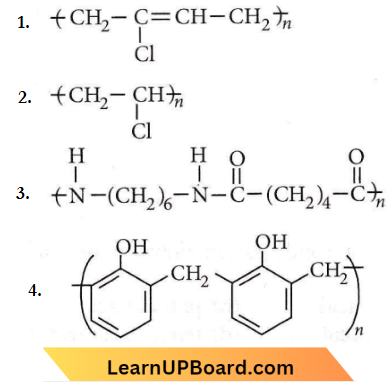
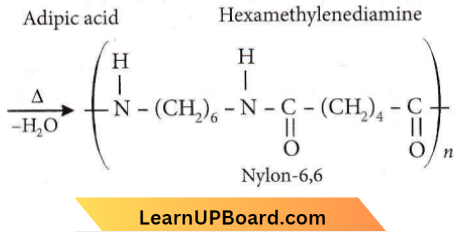
Question 7. Which one of the following structures represents nylon 6, 6 polymers?
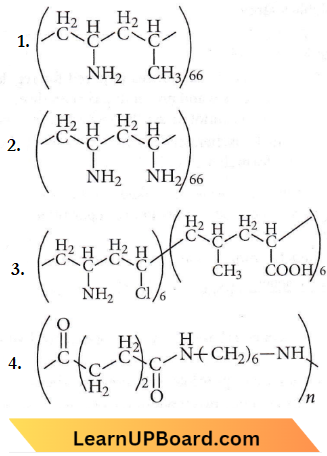
Answer: 4
Nylon-6,6 is obtained by condensing adipic acid (HOOC(CH2)4COOH) with hexamethylenediamine (H2N(CH2)6NH3).
Quetsion 8. Natural rubber has
- Alternate cis- and trans-configuration
- Random cis- and fraus-configuration
- All cis-configuration
- All fraus-configuration.
Answer: 3. All cis-configuration
Natural rubber is cls-polylsoprene.
Periodicity in Properties NEET questions
Question 9. Caprolactam is used for the manufacture of
- Teflon
- Terylene
- Nylon 6, 6
- Nylon 6.
Answer: 4. Nylon 6.
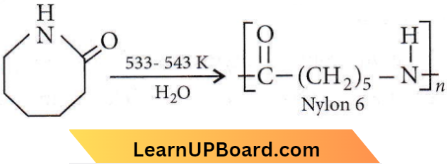
Question 10. Which one of the following is an example of a thermosetting polymer?
Answer: 4
- Neoprene rubber (elastomer)
- PVC (thermoplastic polymer)
- Nylon-6,6 (fibre)
- Novolac which further undergoes cross-linking to produce bakelite (thermosetting polymer)
NEET MCQs on Classification and Periodicity
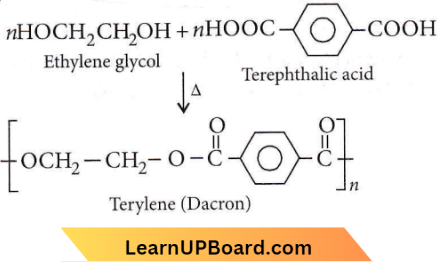
Question 11. Which of the following organic compounds polymerizes to form the polyester dacron?
- Propylene and para HO—(C6H4)—OH
- Benzoic acid and ethanol
- Terephthalic acid and ethylene glycol
- Benzoic acid and para HO—(C6H4)—OH
Answer: 3. Terephthalic acid and ethylene glycol
Question 12. Nylon is an example of
- Polyamide
- Polythene
- Polyester
- Polysaccharide.
Answer: 1. Polyamide
Question 13. Which is the monomer of neoprene in the following?
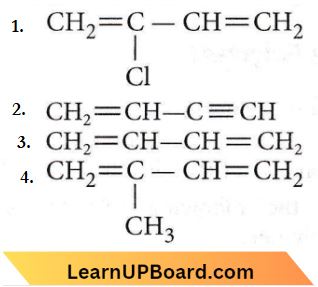
Answer: 1
NEET MCQs on Classification and Periodicity
Question 14. Which one of the following is not a condensation polymer?
- Melamine
- Glyptal
- Dacron
- Neoprene
Answer: 4. Neoprene
Neoprene is an additional polymer.
Question 15. Which of the following statements is false?
- Artificial silk is derived from cellulose.
- Nylon-6,6 is an example of an elastomer.
- The repeat unit in natural rubber is isoprene.
- Both starch and cellulose are polymers of glucose.
Answer: 2. Nylon-6,6 is an example of an elastomer.
Nylon-6,6 is an example of fibre.
Question 16. Of the following which one is classified as polyester polymer?
- Terylene
- Bakelite
- Melamine
- Nylon-6,6
Answer: 1. Terylene
Terylene (Dacron) is a polyester polymer because it is made by monomer units of ethylene glycol and terephthalic acid.
NEET practice questions on Periodic Table
Question 17. Which of the following structures represents neoprene polymer?
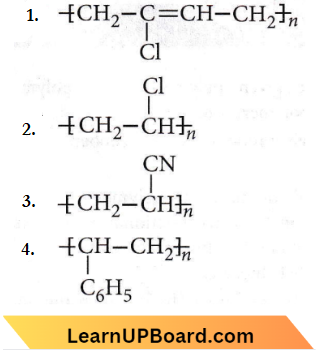
Answer: 1
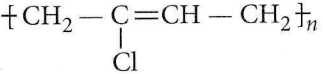
It is a polymer of chloroprene.
Question 18. Structures of some common polymers are given. Which one is not correctly presented
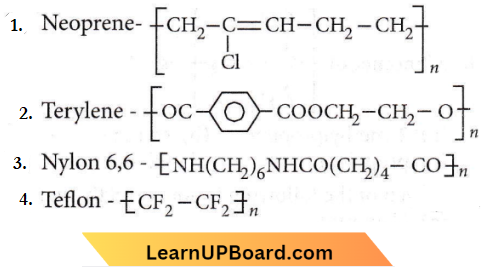
Answer: 1
Neoprene is a polymer of chloroprene.
The rest of the polymers are correctly represented.
Question 19. Which one of the following statements is not true?
- Buna-S is a copolymer of butadiene and styrene.
- Natural rubber is a 1,4-polymer of isoprene.
- In vulcanization, the formation of sulphur bridges between different chains makes rubber harder and stronger.
- Natural rubber has the trans-configuration at every double bond.
Answer: 4. Natural rubber has the trans-configuration at every double bond.
Natural rubber is cis-1,4-polyisoprene and has only cis-configuration about the double bond as shown below
whereas in Gutta-percha, only trans configuration exists about the double bond.
Chemistry MCQs on Periodic Classification for NEET
Question 20. Which one of the following polymers is prepared by condensation polymerisation?
- Teflon
- Natural rubber
- Styrene
- Nylon-6,6
Answer: 4. Nylon-6,6
Nylon-6,6 is a condensation polymer of adipic acid and hexamethylenediamine.
n HOOC-(CH2)4-COOH + n H2N – (CN2)6 – NH2
Periodic Table quiz for NEET
Question 21. ![]()
- Homopolymer
- Copolymer
- Addition polymer
- Thermosetting polymer.
Answer: 2. Copolymer
Formed by the condensation of adipic acid and hexamethylenediamine. It is a copolymer (a polymer made from more than one type of monomer molecule is referred to as a copolymer).
Question 22. The monomer of the polymer
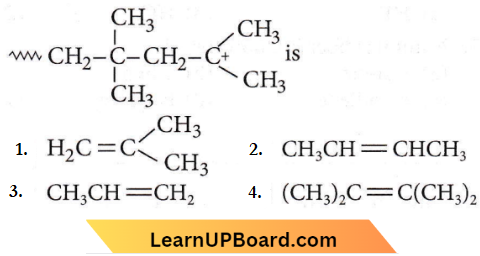
Answer: 1
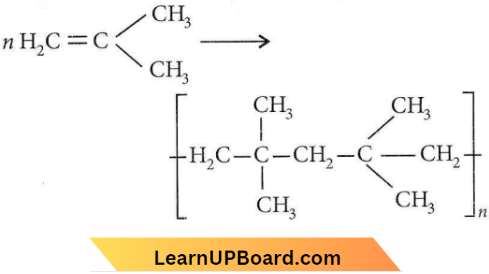
Question 23. Which one of the following is a chain-growth polymer?
- Starch
- Nucleic acid
- Polystyrene
- Protein
Answer: 3. Polystyrene
Chain-growth polymers involve a series of reactions each of which consumes a reactive particle and produces another similar one. The reactive particles may be free radicals or ions (cation or anion) to which monomers get added by a chain reaction. It is an important reaction of alkenes and conjugated dienes or indeed of all kinds of compounds that contain C – C double bonds.
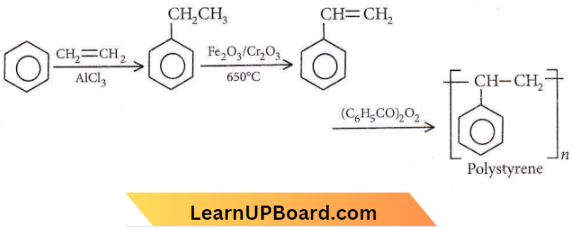
NEET question bank on Element Classification
Question 24. Acrilan is a hard, horny and a high melting material. Which one of the following represents its structure?
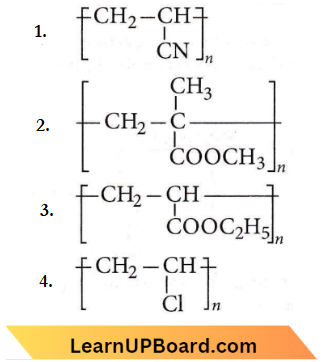
Answer: 1
Acrilan is an additional polymer of acrylonitrile.
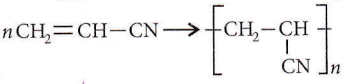
Question 25. Monomer of 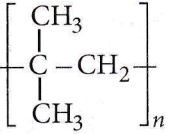
- 2-Methylpropene
- Styrene
- Propylene
- Ethene.
Answer: 1. 2-Methylpropene
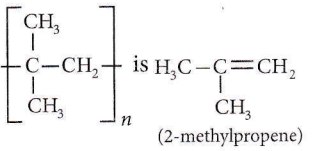
Quetsion 26. Which of the following is not correctly matched?
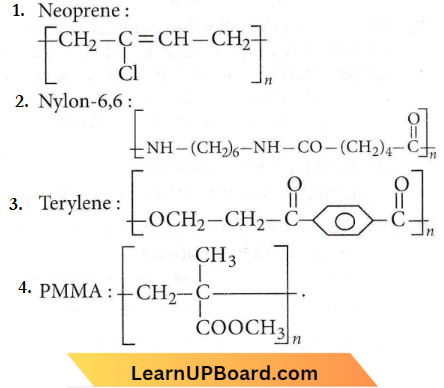
Answer: 3
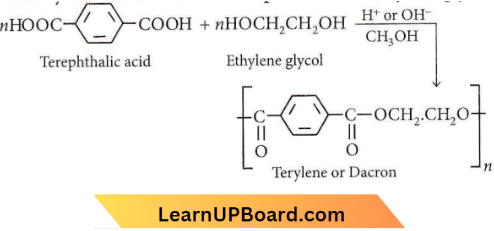
Terylene is an example of condensation po1ymer and the condensation of terephthalic and ethylene glycol.
NEET question bank on Element Classification
Quetsion 27. CF2 = CF2 is the monomer of
- Teflon
- Orlon
- Polythene
- Nylon-6.
Answer: 1. Teflon
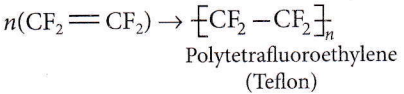
CF2 = CF2 is the monomer of Teflon
- Teflon
Question 28. Which compound forms linear polymer due to H-bond?
- H2O
- NH3
- HF
- HCl
Answer: 3. HF
H-F—H-F—H-F—H-F
Dotted lines represent hydrogen bonds between HF molecules and hence, it is a linear polymer. Due to the high electronegativity value of ‘F’ atom, it forms effective hydrogen bonding.
NEET question bank on Element Classification
Question 29. Natural rubber is a polymer of
- Styrene
- Ethyne
- Butadiene
- Isoprene.
Answer: 4. Isoprene.
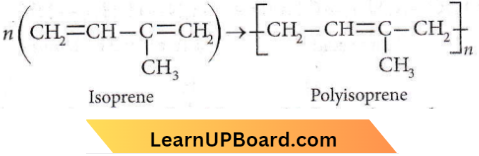
Polyisoprene is the natural rubber, which is the polymer of isoprene.
Question 30. Terylene is a condensation polymer of ethylene glycol and
- Salicylic acid
- Phthalic acid
- Benzoic acid
- Terephthalic acid
Answer: 4. Terephthalic acid
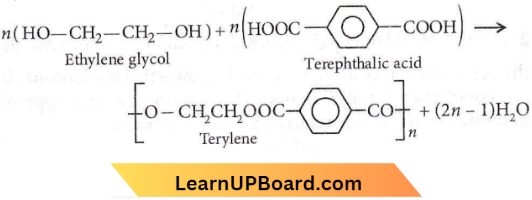
Terylene is the condensation polymer of ethylene glycol and terephthalic acid
Question 31. Which one of the following is used to make ‘non¬stick’ cookware?
- Polyethylene terephthalate
- Polytetrafluoroethylene
- PVC
- Polystyrene
Answer: 2. Polytetrafluoroethylene
Polytetrafluoroethylene or Teflon is a tough material, resistant to heat and a bad conductor of electricity. It is used for coating the cookware to make them non-sticky
Multiple choice questions on Periodicity in Properties for NEET
Question 32. The bakelite is prepared by the reaction between
- Phenol and formaldehyde
- Tetramethylene glycol
- Urea and formaldehyde
- Ethylene glycol.
Answer: 1. Phenol and formaldehyde
Phenol and formaldehyde undergo condensation polymerisation under two different conditions to give a cross-linked polymer called bakelite
Question 33. The biodegradable polymer is
- Buna-5
- Nylon-6,6
- Nylon-2-nylon 6
- Nylon-6.
Answer: 3. Nylon-2-nylon 6
Nylon-2-nylon-6 is a biodegradable polymer.
Multiple choice questions on Periodicity in Properties for NEET
Question 34. Which one of the following sets forms the biodegradable polymer?
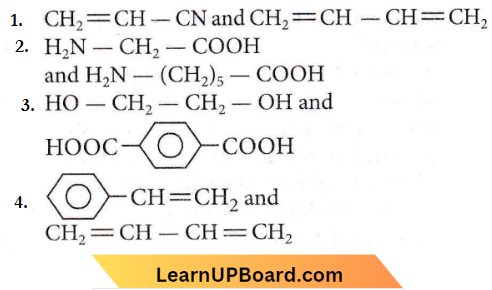
Answer: 2
Nylon-2-nylon-6 is an alternating polyamide copolymer of glycine (H2NCH2COOH) and aminocaproic acid (H2N(CH2)2COOH) and is biodegradable
