UP Board Class 9 Science Notes For Is Matter Around Us Pure
Matter:
In chemistry, when we say a substance is pure, it means that the substance is made up of only one type of constituent particle. In other words, a substance is a pure single form of matter.
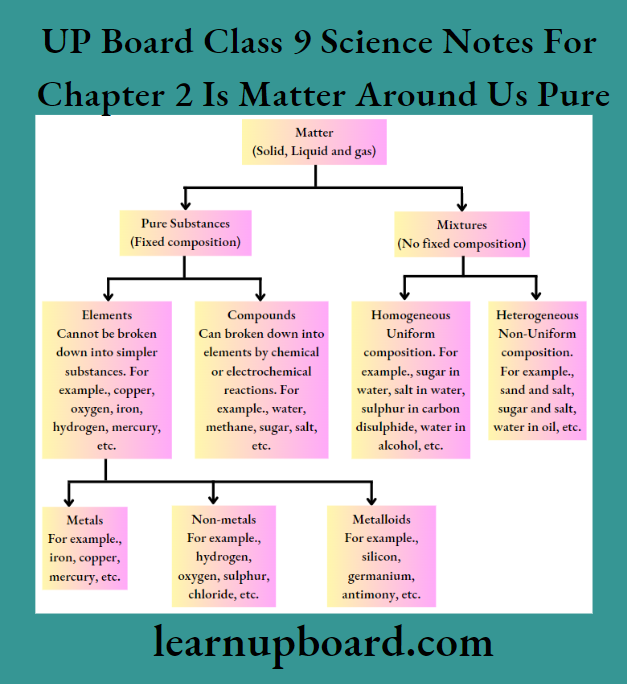
Depending upon the chemical composition, matter is classified into elements, compounds, (i.e. pure substances that are non-separable by physical methods) and mixtures (separable by physical methods like sublimation, etc).
UP Board Class 9 Science Notes For Chapter 2 Pure Substance
A substance that consists of only a single type of constituent particles is called pure substance, for example., gold, water, etc. Based on the nature of the constituent particles, a pure substance is of two types, i.e. elements and compounds.
1. Elements
The term element was first used by Robert Boyle in 1661. According to Antoine Laurent Lavoisier, a French chemist, (1743-94), ‘an element is a basic form of matter that cannot be broken down into simpler substances by chemical reactions’.
Read and Learn More Class 9 Science Notes
An element is a pure substance. Till now 118 elements have been discovered, out of these 92 are natural elements and others are man-made. Based on variation in properties, elements can be broadly classified as metals, non-metals and metalloids.
- Metals
- A metal is an element that is malleable (i.e. can be hammered into thin sheets), ductile (i.e. can be drawn into wires), sonorous (i.e. make a ringing sound when hit), and conduct heat and electricity.
- They are lustrous (shine) and have silvery-grey or golden- yellow colour, for example., gold, silver, copper, iron, sodium, potassium, etc.
- Mercury is the only metal that is liquid at room temperature. Gallium and caesium because of their very low melting points remain in liquid state at a temperature slighdy above room temperature (303K).
- Non-metals
- A non-metal is an element that is neither malleable nor ductile and does not conduct heat and electricity.
- They display a variety of colours, for example., hydrogen, oxygen, iodine, carbon (coal, coke), bromine, chlorine, etc.
- Bromine is the only non-metallic element that exists in a liquid state at normal conditions of temperature and pressure.
- Metalloids
- Elements having intermediate properties between those of metals and non-metals are called metalloids, for example., boron, silicon, germanium, etc.
2. Compounds Definition
A compound is a substance composed of two or more elements, chemically combined in a fixed proportion, for example., water (H2O), methane (CH4), carbon dioxide (CO2), ammonia (NH3), sodium chloride (NaCl), etc.
UP Board Class 9 Science Notes For Chapter 2 Mixtures
Mixtures are constituted by more than one kind of pure form, known as a substance. Most of the matter around us exist as mixtures of two or more pure components, for example., sea water, minerals, soil, etc, are all mixtures.
Types of Mixtures: Depending upon the nature of the components that form a mixture, we have two types of mixtures:
- Homogeneous Mixture
- A mixture in which the constituents are uniformly distributed throughout i.e. without any clear boundary of separation is called homogeneous mixture.
- Here, the constituents cannot be seen with naked eyes or under a microscope.
- Some of the examples of homogeneous mixtures are salt solution, sugar solution, air, soft drinks, petroleum, biogas, alloys, etc.
- Note Air is a homogeneous mixture of gas. Its two major constituents are oxygen (21 %) and nitrogen (78%) and other gases in small quantities.
- Heterogeneous Mixture
- A mixture that does not have uniform composition, i.e. has visible boundaries of separation between its constituents is called heterogeneous mixture.
- Here, the constituents of a heterogeneous mixture can be seen by naked eyes or under a microscope.
- Examples of heterogeneous mixtures are sugar and sand mixture, salt and sand mixture, polluted air, muddy water, etc.
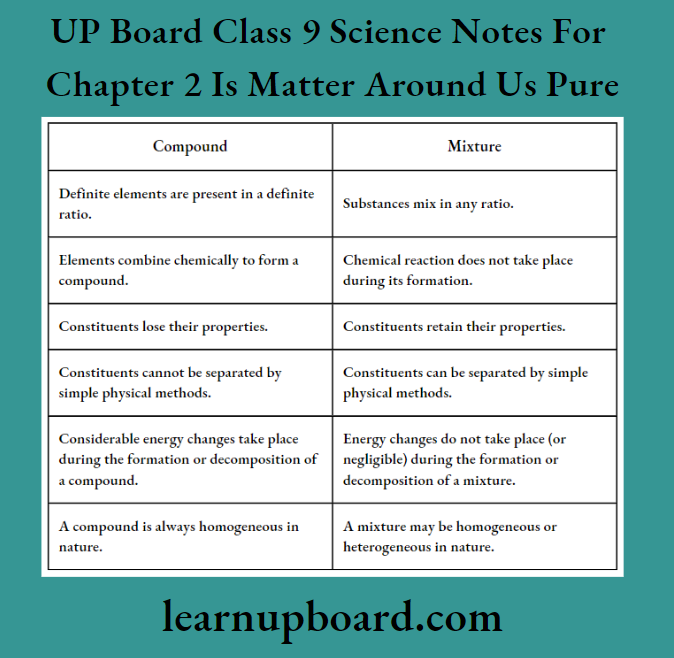
Differences Between Compounds And Mixtures:
UP Board Class 9 Science Notes For Chapter 2 Solution
A homogeneous mixture of two or more substances is called a solution. A solution is sometimes also called a true solution.
- Lemonade, soda water, salt solution, sugar solution, etc., all are examples of solutions.
- In a solution, there is homogeneity at the particle level, i.e. the particles of dissolved substances are evenly distributed in the solution and are indistinguishable from one another.
There are two main components of a solution:
- Solvent (Dissolving Phase) The component (usually present in larger amounts) of the solution that dissolves the other component in it, is called the solvent.
- Solute (Dissolved Phase) The component (usually present in lesser quantity) of the solution that is dissolved in the solvent is called the solute.
Some Common Examples Of Solution:
- In sugar solution, sugar is the solute and water is the solvent.
- A solution of iodine in alcohol known as tincture of iodine, has iodine (solid) as the solute and alcohol (liquid) as the solvent.
- Aerated drinks like soda water, etc., are gas in liquid solutions. CO2 (gas) as solute and water (liquid) as solvent. Solid solutions (alloys) and gaseous solutions (air)
Alloys
- Alloys are mixtures of two or more metals or a metal and a non-metal and cannot be separated into their components by physical methods.
- But still, an alloy is considered as a mixture because it shows the properties of its constituents and can have variable composition, for example., brass is a mixture of approximately 30% zinc and 70% copper.
UP Board Class 9 Science Notes For Chapter 2 Properties Of A Solution
Some important properties of a solution are as follows:
- A solution is a homogeneous mixture.
- The particles of a solution are smaller than 1 nm (10-9 m) in diameter. Therefore, they cannot be seen by the naked eye.
- Due to very small particles, they do not scatter a beam of light passing through the solution. So, the path of light is not visible in a solution.
- A solution is stable, i.e. the solute particles do not settle down when left undisturbed. The solute particles cannot be separated from the mixture by the process of filtration.
Concentration Of A Solution
- The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution, or the amount of solute dissolved in a given mass or volume of the solvent. In a solution, the relative proportion of the solute and solvent can be varied.
- Depending upon the amount of solute present in a given amount of solvent, it can be classified as under:
Saturated solution A solution in which no more amount of solvent can be dissolved at a given temperature, is called a saturated solution. The amount of the solute present in the saturated solution at this temperature is called solubility.
Solubility =\(\frac{\text { Mass of solute }}{\text { Mass of solvent }} \times 100\)
Unsaturated solution If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
Concentration of solution
⇒ \(=\frac{\text { Amount of solute }}{\text { Amount of solution }}=\frac{\text { Amount of solute }}{\text { Amount of solvent }}\)
Expressing The Concentration Of A Solution: The methods by which the concentration of a solution can be expressed are:
Mass by mass percentage of a solution
⇒ \(=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
Mass by volume percentage of a solution
∴ \(=\frac{\text { Mass of solute }}{\text { Volume of solution }} \times 100\)
Question1. A solution contains 50 g of common salt in 450 g of water. Calculate the concentration of the solution.
Answer:
Given
A solution contains 50 g of common salt in 450 g of water.
Concentration of solution = \(\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
Mass of common salt (solute) = 50 g
Mass of water = 450 g
Mass of solution = 50 + 450 = 500 g
Concentration of solution = \(\frac{50}{500} \times 100=10 \%\)
Example 2. 4 g of a solute is dissolved in 40 g of water to form a saturated solution at 25°C. Calculate the solubility of the solute at 25°C.
Answer:
Given
4 g of a solute is dissolved in 40 g of water to form a saturated solution at 25°C.
Solubility = \(\frac{\text { Mass of solute }}{\text { Mass of solvent }} \times 100\)
Mass of solute = 4g,
Mass of solvent = 40 g,
Solubility = \(\frac{4(\mathrm{~g})}{40(\mathrm{~g})} \times 100\)
= 10g
UP Board Class 9 Science Notes For Chapter 2 Suspension
A suspension is a heterogeneous mixture in which the solute particles do not dissolve, but remain suspended throughout the bulk of the medium, for example., a mixture of chalk powder in water, a mixture of sand in water, smoke coming out of a chimney of a factory.
Properties of Suspension
Some important properties of suspension are as follows:
- Suspension is a heterogeneous mixture.
- Its particles can be seen by the naked eye.
- Its particles scatter a beam of light passing through it and make its path visible (Tyndall effect).
- It is unstable, i.e. the solute particles settle down when suspension is left undisturbed. They can be separated by the process of filtration. When the particles settle down, the suspension breaks and it does not scatter light any more.
Note: The insoluble particles in a suspension are called ‘suspended particles’, whereas the solvent is referred to as ‘medium’.
UP Board Class 9 Science Notes For Chapter 2 Colloidal Solution
A colloid (or colloidal solution) is a mixture that is heterogeneous but appears to be homogeneous as the particles are uniformly spread throughout the solution, for example., milk, shaving cream, cheese, etc. Colloidal solutions are also called colloidal sols.
Properties Of A Colloid: Some important properties of a colloid are as follows :
- A colloid is a heterogeneous mixture.
- The size of particles of a colloid is too small to be individually seen by the naked eye.
- Colloids are big enough to scatter a beam of light passing through it and make its path visible.
- The colloids are quite stable. Particles do not settle down when a colloid is left undisturbed.
- Particles of colloid can pass through filter paper, therefore a colloid cannot be separated by filtration. However, they get separated by a special technique called centrifugation.
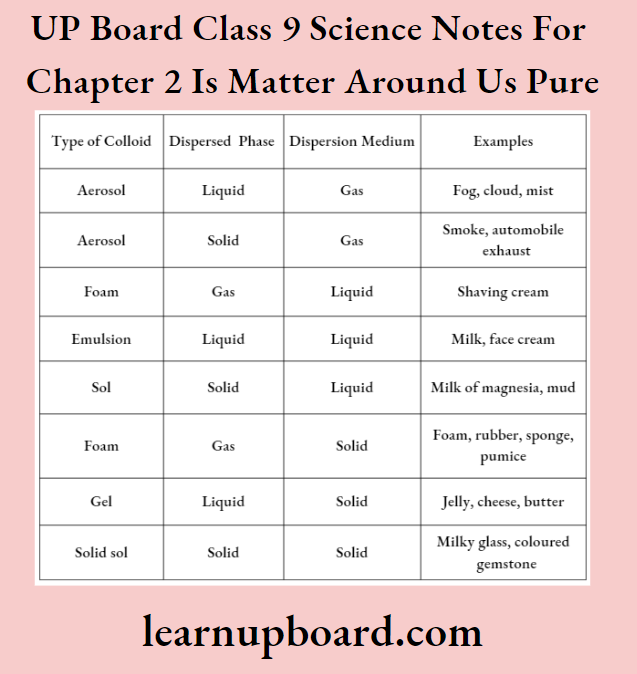
Common Examples Of Colloids
Colloids are classified according to the state (solid, liquid or gas) of the dispersion medium and the dispersed phase.
Types Of Colloids
Tyndall Effect Definition
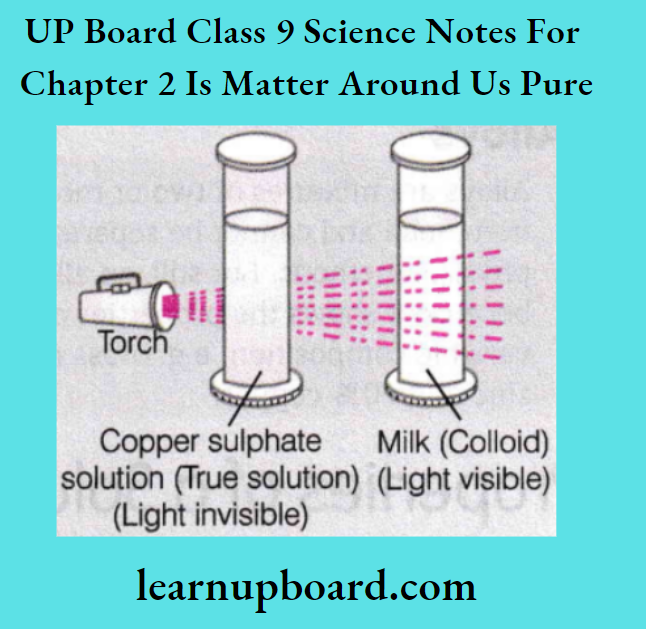
The scattering of light by colloidal particles is known as the Tyndall effect. In a true solution, the solute particles are so small that they cannot scatter light falling on them.
- In a colloidal solution, the particles are big enough to scatter light. Tyndall effect can also be observed in the following situations:
- When a fine beam of light enters a room through a small hole (due to scattering of the beam of light by the particles of dust and smoke in the air).
- When sunlight passes through the canopy of a dense forest (as the mist containing tiny droplets of water scatters it). The components of a colloidal solution are the dispersed phase and the dispersion medium.
- The solute-like component or the dispersed particles in a colloid form is the dispersed phase and the component in which the dispersed phase is suspended, is known as the dispersion medium.
UP Board Class 9 Science Notes For Chapter 2 Physical And Chemical Changes
Physical Changes
The properties that can be observed and specified like colour, hardness, rigidity, fluidity, density, melting point, boiling point, etc., are physical properties.
- The changes which occur without a change in composition and the chemical nature of the substance are called physical changes.
- The interconversion of states is a physical change, for example., change of water in ice is a physical change because chemically, ice and liquid water both are the same.
- Although ice, water and water vapour all look different and display different physical properties chemically they are the same.
Chemical Changes
In chemical changes, one substance reacts with another substance to change chemical composition. Chemical changes bring a change in the chemical properties of matter and a new substance is obtained.
- A chemical change is also called a chemical reaction, for example., both water and cooking oil are liquid, but their chemical characteristics are different. They differ in odour and inflammability.
- Oil burns in air whereas water extinguishes fire, i.e. it is the chemical property of oil that makes it different from water.
Note:
- Burning is a chemical change.
- During the burning of a candle, both physical and chemical changes take place.
UP Board Class 9 Science Notes For Chapter 2 Activity 1
Objective
To prepare homogeneous and heterogeneous mixtures.
Materials Required
Four beakers, copper sulphate powder, common salt, sulphur powder, iron filings, water, spatula, etc.
Procedure
- Label the beakers as A, B, C and D respectively.
- In beakers A and B, take 50 mL of water.
- Now, add one spatula of copper sulphate powder in beaker A and two spatula of copper sulphate powder in beaker B.
- Stir the solutions and observe the changes.
- Now, in beakers C and D, take equal quantity of common salt (sodium chloride).
- In beaker C, add iron filings and in beaker D, add sulphur powder.
- Mix them with the help of a glass rod and observe the changes.
Observation
In beakers A and B, the composition of the mixture is the same throughout but the intensity of the colour of the solutions is different. In beakers C and Z), the obtained mixture has physically distinct parts and a non-uniform composition.
Conclusion
Beakers A and B have homogeneous mixtures of variable composition. Beakers C and D have heterogeneous mixtures of variable composition.
Question 1. What are heterogeneous mixtures?
Answer: The mixtures in which composition are not uniform throughout and which have physically distinct parts are called heterogeneous mixtures.
Question 2. What name is given to a mixture having a uniform composition and no distinct parts?
Answer: Such mixtures are called homogeneous mixtures.
Question 3. On mixing iron filings with sulphur in a beaker, what type of mixture is obtained?
Answer: Heterogeneous mixture is obtained when iron filings are mixed with sulphur.
Question 4. If the substances present in the beaker as given in Q-3 are heated, what do you observe?
Answer: On heating iron with sulphur, we get black coloured iron
sulphide which is a compound, not a mixture as its properties are quite different from the properties of iron as well as sulphur.
Question 5. Give an example of homogeneous mixture.
Answer: Solution of sugar or salt in water.
UP Board Class 9 Science Notes For Chapter 2 Activity 2
Objective
To study the properties of solution, suspension and colloidal solution.
Materials Required
Three beakers, glass rod, copper sulphate crystals, chalk powder (or wheat flour) and few drops of milk or ink.
Procedure
- Label the beakers as A, B and C.
- In beaker A, take 20 mL of water and add copper sulphate crystals.
- In beaker B, take 20 mL of water and add chalk powder (or wheat flour).
- In beaker Q take 20 mL of water and add few drops of milk or ink.
- Stir the solutions with glass rod and observe the changes.
- Now, direct a beam of light from a torch through all the beakers containing the mixtures and observe from the front.
- Note your observations.
- Leave all the solutions undisturbed for a few minutes and observe the changes.
- Filter the solutions and observe whether there is a residue on the filter paper or not.
Observations
- In beaker A, solution of uniform composition is obtained. It does not affect the beam of light and no change is observed on keeping it undisturbed for a few minutes. Further, there is no residue on the filter paper in this case.
- In beaker B, solution of non-uniform composition is obtained. It scatters the beam of light, so its path become visible. On keeping undisturbed, the solute, i.e. chalk powder settle down and if we filter it, the chalk powder comes on filter paper as residue.
- In beaker C, solution of uniform composition is obtained, but it scatters the beam of light and thus, makes the path visible. If left undisturbed, these remain unaffected. Moreover, no residue left over the filter paper.
Conclusion
- Beaker A contains a true solution. True solutions are homogeneous and stable. They do not show the Tyndall effect and are not separated by filtration.
- Beaker B contains suspension. Suspensions are heterogeneous, opaque, unstable and exhibit the Tyndall effect. They are separated by filtration.
- Beaker C contains the colloidal solution. Colloids are also heterogeneous, translucent, stable and exhibit Tyndall effect. They are not separated by filtration.
Question 1. Why is the Tyndall effect not shown by true solutions?
Answer: This is because the particles of true solutions are very small in size (<1 nm) and hence, these are not able to scatter a beam of light.
Question 2. Give a technique through which colloids can be separated.
Answer: Colloids can be separated by centrifugation.
Question 3. Why do colloidal solutions exhibit the Tyndall effect?
Answer: Because the size of their particles is large enough to scatter light.
Question 4. Why filtration technique does not apply to the separation of a true solution?
Answer: Because the pore size of filter paper is much larger than the size of particles of true solutions.
Question 5. Which of the two will scatter light-soap solution or salt solution?
Answer: Soap solution will scatter light because soap solution is a colloid. A salt solution is a true solution so, it will not scatter light.
UP Board Class 9 Science Notes For Chapter 2 Activity 3
Objective
To demonstrate that the different substances in a given solvent have different solubilities.
Materials Required
Beakers, salt, sugar/barium chloride, water, burner, glass rod, etc.
Procedure

- Take approximately 50 mL of water each in two separate beakers.
- Add common salt in the first beaker and sugar or barium chloride in the second beaker with continuous stirring.
- Continue the addition till it stops dissolving.
- When no more solute (salt/barium chloride) can be dissolved, heat the contents of the beakers.
- Start adding the solute again.
- Take a saturated solution at a certain temperature and cool it slowly.
Observations
- When no more salt or barium chloride can be dissolved in a solution or they stop dissolving, a saturated solution of salt or barium chloride is obtained.
- On heating, when more solute (i.e. salt or barium chloride) is added to the saturated solution, then this solution becomes unsaturated.
- When a saturated solution at a certain temperature is cooled, the solubility of a solute decreases and the amount of the solute which exceeds the solubility at the lower temperature crystallises out of the solution.
Conclusion
Different substances in a given solvent have different solubilities at the same temperature. In general, the solubility decreases as the solution is cooled and the extra amount crystallises out.
Question 1. What is a saturated solution?
Answer: A solution in which no more solute can be dissolved at any fixed temperature is called a saturated solution.
Question 2. What is an unsaturated solution?
Answer: A solution in which some more solute could be
dissolved at any fixed temperature is called an unsaturated solution.
Question 3. Write the effect of heating on a saturated solution.
Answer: If a saturated solution at a particular temperature is heated
to a higher temperature, then it becomes unsaturated.
Question 4. What happens when a saturated solution is allowed to cool?
Answer: If a saturated solution available at a particular temperature is cooled to a lower temperature, then some of its dissolved solutes will separate in the form of solid crystals.
Question 5. What is a supersaturated solution?
Answer: Any solution containing more solute than the required amount to prepare a saturated solution at any fixed temperature is called a supersaturated solution.
Question 6. How can you convert a saturated solution into an unsaturated solution?
Answer: By adding more solvent or by applying heat.
UP Board Class 9 Science Notes For Chapter 2 Summary
A substance that consists of only a single type of constituent particles is called a pure substance.
- An element is a basic form of matter that cannot be broken down into simpler substances by chemical reactions.
- A metal is an element that is malleable, ductile, and sonorous and conducts heat and electricity.
- A non-metal is an element that is neither malleable nor ductile and does not conduct heat and electricity.
- Metalloids are intermediate properties between those of metals and non-metals.
- A compound can be defined as a substance composed of two or more elements, chemically combined in a fixed proportion.
- Mixtures are constituted by more than one substance mixed in any proportion.
Depending upon the nature of the components that form a mixture, we have two types of mixtures:
- Homogeneous Mixture A mixture in which the constituents are uniformly distributed throughout i.e. without any clear boundary of separation is called a homogeneous mixture.
- Heterogeneous Mixture A mixture that does not have uniform composition, i.e. has visible boundaries of separation between its constituents is called a heterogeneous mixture.
- A solution is a homogeneous mixture of two or more substances. There are two main components of a solution; solvent and solute.
- The concentration of a solution is the amount of solute present in a given amount (mass or volume) of a solution, or the amount of the solute dissolved in a given mass or volume of the solvent.
- Depending upon the amount of solute present in a given amount of solvent, it can be classified as under:
- Saturated Solution A solution in which no more amount of solvent can be dissolved at a given temperature, is called saturated solution.
- Unsaturated Solution If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
⇒ \(\text { Concentration of solution }=\frac{\text { Amount of solute }}{\text { Amount of solution }}\)
⇒ \(=\frac{\text { Amount of solute }}{\text { Amount of solvent }}\)
- A suspension is a heterogeneous mixture in which the solute particles do not dissolve, but remain suspended throughout the bulk of the medium.
- A colloid is a mixture that is actually heterogeneous, but appears to be homogeneous as the particles are uniformly spread throughout the solution.
- The scattering of light by colloidal particles is known as Tyndall effect.
- In physical changes, only physical properties of the substance changes.
- In chemical changes, one substance reacts with another substance to undergo a change in chemical composition.
