UP Board Class 9 Science Notes For Chapter 3 Atoms And Molecules
In the present chapter, we shall discuss about the various laws (which explains how atoms combine to form molecule), symbols and formulae of atoms and molecules and various ways of expressing their masses.
UP Board Class 9 Science Notes For Chapter 3 Laws Of Chemical Combination
Whenever reactants react together to form the products or the elements combine together to form a compound, they do this according to certain laws. These laws are called laws of chemical combination.
Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws of chemical combination which are as follows:
1. Law Of Conservation Of Mass Definition
It states that ‘mass can neither be created nor be destroyed during a chemical reaction.5 This means that in any chemical reaction, the total mass of the reactants is equal to the total mass of the products and there is no change in mass during the chemical reaction.
Question 1. If 4.0 g of sodium carbonate reacts with 10 g of hydrochloric acid, it results in the formation of 2.5 g of carbon dioxide and 11.5 g of sodium chloride solution. Show that these results are by the law of conservation of mass.
Answer:
Given
If 4.0 g of sodium carbonate reacts with 10 g of hydrochloric acid, it results in the formation of 2.5 g of carbon dioxide and 11.5 g of sodium chloride solution.
⇒ \(\underset{(4.0 \mathrm{~g})}{\text { Sodium carbonate }}+\underset{(10.0 \mathrm{~g})}{\text { Hydrochloric acid }} \longrightarrow \underset{(2.5 \mathrm{~g})}{\text { Carbon dioxide }}+\underset{(11.5 \mathrm{~g})}{\text { Sodium chloride }}\)
Here, total mass of reactants = 4.0 + 10 = 14 g
Total mass of products = 2.5 + 11.5 = 14 g
Since the reactants and products have the same mass, this means that there was no loss or gain of mass after the reaction. Hence, the data is in agreement with the law of conservation of mass.
Read and Learn More Class 9 Science Notes
2. Law Of Constant Proportions/ Law Of Definite Proportions
According to this law, in a chemical substance (or compound), the elements are always present in definite proportions (or ratios) by mass.
For example., In a compound such as water, the ratio of the mass of hydrogen to the mass of oxygen is always 1:8, whatever the source of water. Thus, if 9 g of water is decomposed, 1 g of hydrogen and 8 g of oxygen are always obtained.
Similarly, carbon dioxide (CO2) always contains carbon and oxygen in the ratio of 3: 8. If a sample of CO2 contains 36 g of carbon then it is compulsory that the sample has 96 g of oxygen.
This is calculated as \(\frac{3}{8}=\frac{36}{x} ;\)
∴ \(x=\frac{36 \times 8}{3}=96 \mathrm{~g}\)
Question 2. Copper oxide was prepared by two different methods. In one case, 1.75 g of the metal gave 2.19 g of oxide. In the second case, 1.14 g of the metal gave 1.43 g of the oxide. Show that the given data illustrate the law of constant proportions.
Answer:
Given
Copper oxide was prepared by two different methods. In one case, 1.75 g of the metal gave 2.19 g of oxide. In the second case, 1.14 g of the metal gave 1.43 g of the oxide.
Case 1 Mass of copper = 1.75 g
And a mass of copper oxide = 2.19 g
So, mass of oxygen = Mass of copper oxide – Mass of copper
= 2.19-1.75 =0.44g
Now, in the first sample of the copper oxide compound.
Mass of copper: Mass of oxygen = 1.75: 0.44
⇒ \(\frac{1.75}{0.44}: 1\)
⇒ 3.93:1
Case 2 Mass of copper = 1.14 g
And, a mass of copper oxide = 1.43 g
So, mass of oxygen = Mass of copper oxide – Mass of copper
= 1.43 -1.14 =0.29 g
Now, in the second sample of the copper oxide compound.
Mass of copper: Mass of oxygen = 1.14: 0.29
⇒ \(\frac{1.14}{0.29}: 1\)
393:1 ≈ 4:1
From the above calculations, we can see that the ratio (or proportion) of copper and oxygen elements in the two samples of copper oxide compound is the same, i.e. 4:1. So, the given data verify the law of constant proportions.
Explanation Of Laws Of Chemical Combination: Dalton’s Atomic Theory
Dalton’s atomic theory explained the law of chemical combination. According to Dalton’s atomic theory, all matter (whether an element, a compound or a mixture), is composed of small particles, called atoms.
The Main Postulates Of Dalton’s Atomic Theory
- Every matter is made up of very small particles, called atoms.
- Atoms are indivisible particles which can neither be created nor be destroyed in a chemical reaction.
- Atoms of a given element are identical in mass as well as in chemical properties.
- Atoms of different elements have different masses and chemical properties.
- Atoms combine in the ratio of small whole numbers to form compounds.
- The relative numbers and kinds of atoms are constant in a given compound.
UP Board Class 9 Science Notes For Chapter 3 Atoms
Atoms are the smallest particles of an element which may or may not have independent existence but take part in a chemical reaction. These are the building blocks of all matter.
For example., atoms of hydrogen, oxygen, nitrogen etc., are not capable of independent existence whereas atoms of helium, neon etc., are capable of existing independently.
Size Of Atoms
Atoms are very small and their radius is measured in nanometres.
1/109 m = 1 nm or
lm = 109 nm
A hydrogen atom is the smallest atom and its radius is 0.1 nm.
Modern-Day Symbols Of Atoms Of Different Elements
In chemistry, symbols are the representation of an element. It is simple to use the symbol of an element rather than writing a whole word of an element. Dalton was the scientist who introduced symbols for representing elements for the first time.
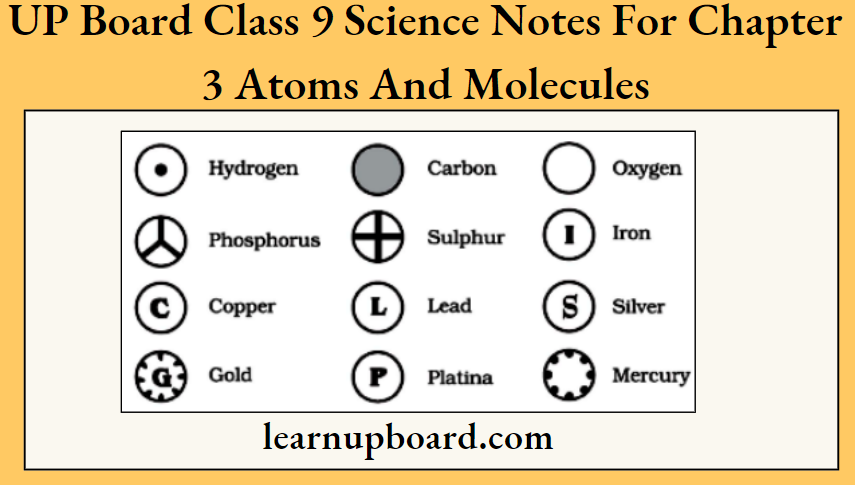
Symbols for some elements as proposed by Dalton
As Dalton’s symbols for elements were difficult to draw and inconvenient to use, modern symbols for the elements were introduced by J J Berzelius. These are defined as “a shorthand representation of the name of an element”.
- In the beginning, the names of elements were derived from the name of the place where they were found for the first time.
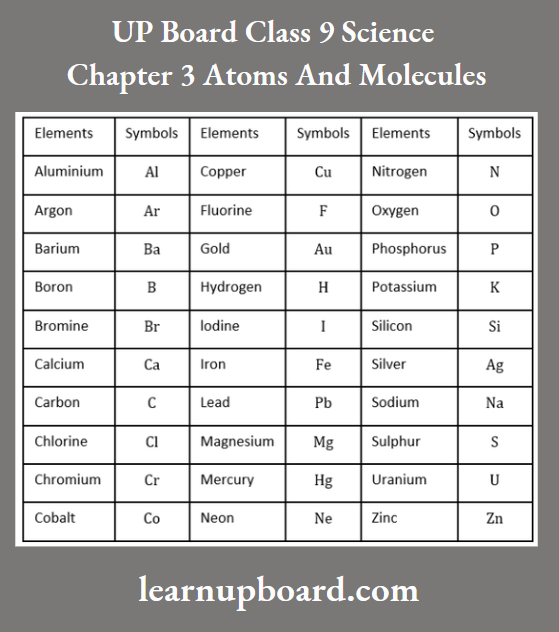
- Nowadays, it is the IUPAC (International Union of Pure and Applied Chemistry) that approves the names and symbols of the elements. Many of the symbols are the first one or two letters of the element’s name in English.
- The first letter of a symbol is always written in capital letters and the second letter is a small letter.
For example., chlorine (Cl), zinc (Zn) and aluminium (Al). Symbols of some elements have been taken from their names in different languages such as Latin, German, Greek etc.
For example.,
- Iron – Fe from Ferrum (Latin name)
- Gold – Au from Aurum (Latin name)
- Potassium – K from Kalium (Latin name)
- Chlorine – Cl from Chloros (Greek name)
- Cobalt – Co from Kobold (German name)
- Sodium – Na from Natrium (Latin name)
Symbols For Some Elements
Atomic Mass
According to Dalton, each element has a characteristic atomic mass. However, determining the mass of an individual atom was a relatively difficult task due to its very small size. Hence, their relative atomic masses were determined using the laws of chemical combinations and the compounds formed.
For this purpose, initially, 1/16 of the mass of an atom of naturally occurring oxygen was taken as a standard unit because of the following two reasons:
- Oxygen reacted with a large number of elements and formed compounds.
- This unit gave masses of most of the elements as whole numbers.
However, in 1961, carbon (C-12 isotope) was chosen as a standard reference for measuring atomic masses universally.
Relative Atomic Mass
It is defined as the number of times a given atom is heavier than 1/12th of the mass of 1 atom of carbon-12 (C-12) or it is the average mass of the atom as compared to l/12th the mass of one carbon-12 atom.
Atomic Mass Unit
It is defined as the mass unit equal to exactly 1/12th of the mass of one atom of the C-12 isotope. Earlier, it was abbreviated as amu but according to the latest recommendations of IUPAC, it is now written as ‘u’- unified mass.
Atomic Masses Of Few Elements
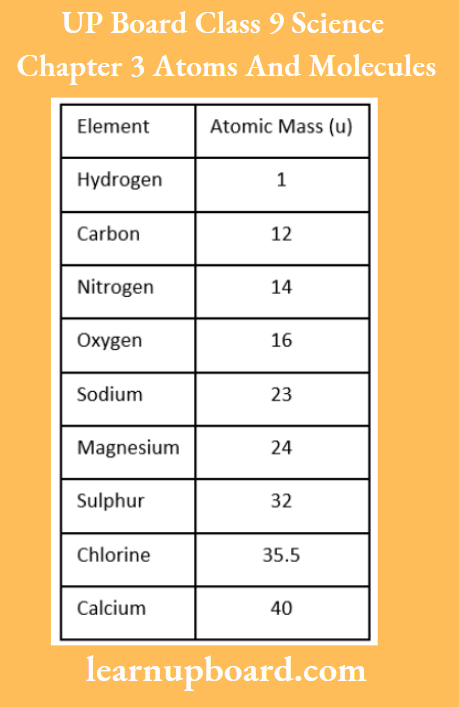
Note: Atoms of most of the elements are not able to exist independently. Atoms form molecules and ions. These molecules or ions aggregate in large numbers to form the matter that we can see, feel or touch.
UP Board Class 9 Science Notes For Chapter 3 Molecules
The smallest particle of an element or compound which is capable of independent existence and shows all the properties of that substance is called a molecule. In general, a molecule is a group of two or more atoms that are chemically bonded together. Atoms of the same element or different elements can join together to form molecules.
Molecules can be divided into two categories:
1. Molecules Of Elements
The molecules of an element contain the same type of atoms. Molecules of many elements are made up of only one atom of that element, for example., noble gases like argon (Ar), helium (He) etc.
The molecules of most of the non-metals are made up of more than one atom. for example., a molecule of oxygen (O2) consists of two atoms of oxygen and is known as a diatomic molecule, and ozone (O3) consists of three atoms of oxygen and is known as a triatomic molecule.
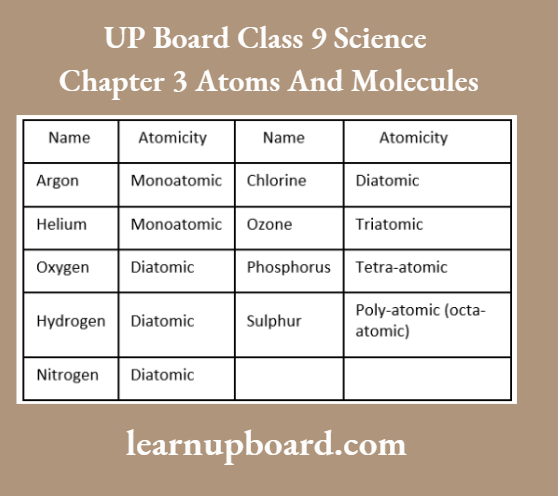
Atomicity
It is defined as the number of atoms present in a molecule. Based on atomicity, molecules can be classified as:
- Monoatomic molecules consist of only one atom.
- For example., He, Ne, Ar, Xe, Fe, Al etc.
- Diatomic molecules consist of two atoms.
- For example., H2, O2, N2, I2, Br2, Cl2, HCl, NaCl etc.
- Triatomic molecules consist of three atoms,
- For example., O3, CO2, NO2 etc.
- Tetra-atomic molecules consist of four atoms,
- For example., P4, H2O2 etc.
- Polyatomic molecules consist of more than four atoms,
- For example., CH4 (penta-atomic), S8 (octa-atomic) etc.
Atomicity of Some Elements (Non-metals):
2. Molecules Of Compounds
Atoms of different elements join together in definite proportions to form molecules of compounds.
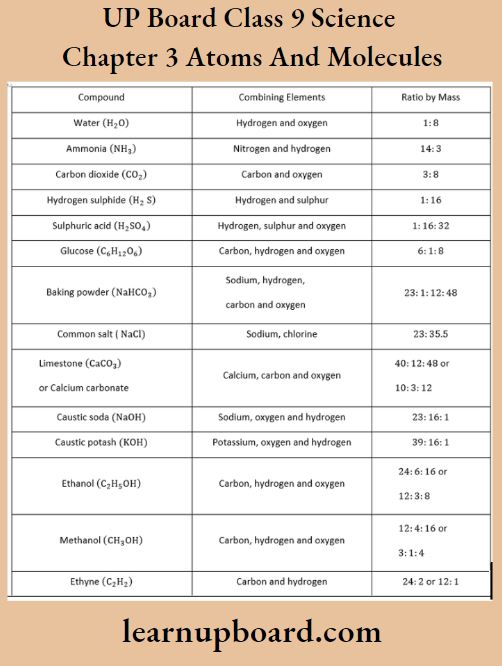
Prediction Of Number Of Atoms From Mass Ratio
To predict the number of atoms from the mass ratio, divide the given mass of each element by the atomic mass of the element and calculate the simplest ratio between the obtained moles, for example., we know that the mass ratio of nitrogen and hydrogen in an ammonia molecule is 14 : 3. The number of atoms of nitrogen and hydrogen present in the molecule of ammonia can be calculated as,
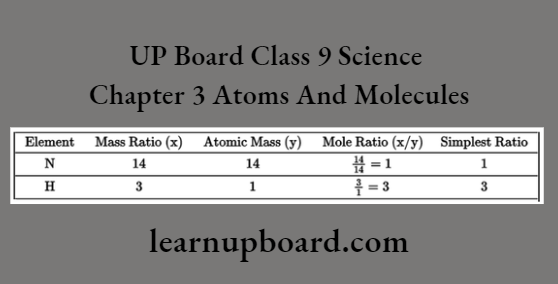
Thus, in an ammonia molecule, one N and three H-atoms are present hence, the formula of ammonia is NH3.
UP Board Class 9 Science Notes For Chapter 3 Ions
When atoms, groups of atoms or molecules lose or gain electron(s) they become charged. These charged species are known as ions. Atoms in solution generally exist in the form of ions. These can be negatively or positively charged and thus can be categorised into two groups.
Cations
The positively charged ions are known as cations, for example., Na+, K+, Ca2+, Al3+ etc. These are formed when elements lose electrons. Usually, metals form cations.
Anions
The negatively charged ions are known as anions, for example., Cl–, Br–, O2-, N3- etc. These are formed when elements gain electrons. Usually, non-metals form anions.
Poly-atomic Ion
A group of atoms carrying charge and acting as a single entity is known as a polyatomic ion. It carries a fixed charge, for example., NO–3 (nitrate ion), CO2-3 (carbonate ion) and SO2-4 (sulphate ion) etc.
Some Ionic Compounds
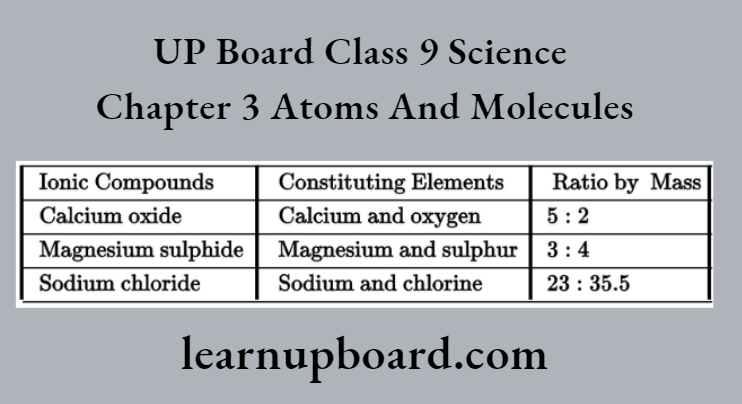
Note: Ionic compounds are formed by cations and anions, for example., sodium chloride or common salt (NaCl) consisting of a positively charged sodium ion (Na+ cation) and negatively charged chloride ion (Cl– anion).
UP Board Class 9 Science Notes For Chapter 3 Valency
The combining power (or capacity) of an element is called its valency. Valency can be used to find out how the atoms of an element will combine with the atom(s) of another element to form a chemical compound. The valency of an ion is equal to the charge of the ion.
Names, Symbols And Valency Of Some Ions
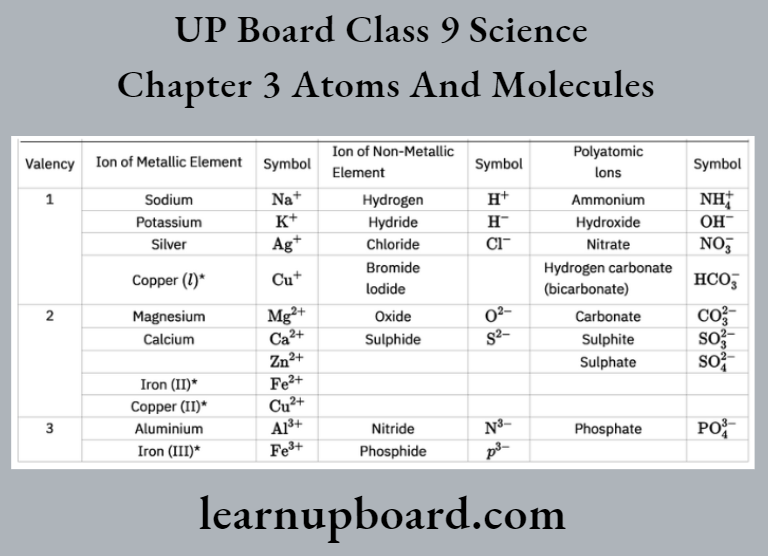
Note These elements show more than one valency. Here, the Roman numeral written in brackets shows their valency.
UP Board Class 9 Science Notes For Chapter 3 Writing Chemical Formulae
The shortest way to represent a compound with the help of symbols and the valency of elements is known as a chemical formula. The chemical formula of a compound shows its constituent elements and the number of atoms of each combining element.
In ionic compounds, the charge on each ion is used to determine the chemical formula of a compound.
There are some rules for writing the chemical formula:
- The valencies or charges on the ion must be balanced.
- When a compound consists of a metal and a non-metal, the symbol of the metal is written first and on the left whereas of non-metal on its right, for example., calcium oxide (CaO), sodium chloride (NaCl), iron sulphide (FeS), copper oxide (CuO) etc., where oxygen, chlorine, sulphur are non-metals and are written on the right, whereas calcium, sodium, iron and copper are metals and are written on left.
- When a compound is formed with polyatomic ions, the ion is enclosed in a bracket before writing the number to indicate the ratio, for example., Ca(OH)2. In case the number of polyatomic ions is one, the bracket is not required, for example., NaOH.
Formulae Of Simple Compounds
To write the chemical formula for simple compounds :
- Write the symbols of constituent elements and their valencies as shown below.
- Write the symbol of the cation first followed by the symbol of the anion.
- Then criss-cross their charges or valencies to get the formula.
- The positive and negative charges must balance each other and the overall structure must be neutral.
Note The simplest compounds made up of two different elements are also called binary compounds.
Hydrogen sulphide
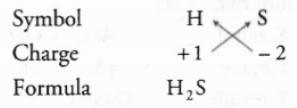
Note: When the subscript is number 1, subscript is not written.
Carbon tetrachloride
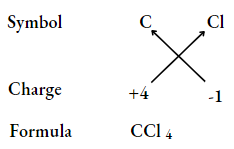
Magnesium chloride
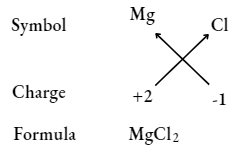
Calcium oxide
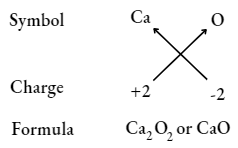
Note: When the valency of both elements is numerically equal, the subscripts are not written.
Aluminium oxide

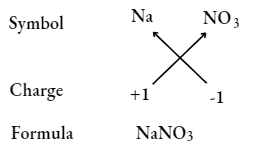
Sodium nitrate
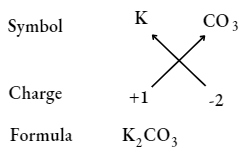
Potassium carbonate
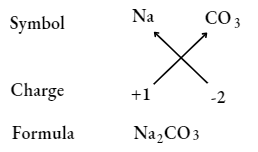
Sodium carbonate
We use brackets when we have two or more of the same polyatomic ions in the formulae, for example.,
Aluminium Hydroxide

Ammonium sulphate
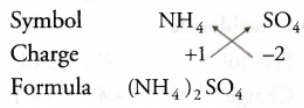
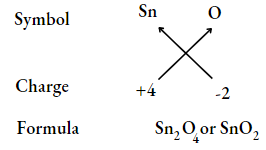
All subscripts must be reduced to the lowest term (except for molecule or covalent compound) for example., Tin oxide
UP Board Class 9 Science Notes For Chapter 3 Molecular Mass
The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of the substance. It is, therefore, the relative mass of a molecule expressed in atomic mass units (u).
For example., the relative molecular mass of water (H2O) is 18 u, which can be calculated as
the atomic mass of hydrogen = 1u
the atomic mass of oxygen = 16u
H2O contains two hydrogen atoms and one oxygen atom.
Therefore, the molecular mass of water is=2 × 1 + 1 × 6 = 18u
Question 3. Calculate the molecular mass of the following substances.
- Ammonia
- Hydrochloric acid
- Phosphorus molecule
- Hydrogen molecule
- Oxygen molecule
- Sulphur dioxide
Answer:
The molecular mass of ammonia (NH3)
= 1 × 14 + 3 × 1 =17 u
The molecular mass of hydrochloric acid (HCl)
= 1 × 1 + 1 × 35.3
=36.5 u
The molecular mass of phosphorus molecule (P4)
= 4 × 31
= 124 u
The molecular mass of hydrogen molecule (H2)
= 2 × 1
= 2u
The molecular mass of oxygen molecule (O2)
= 2 × 16
= 32 u
The molecular mass of sulphur dioxide (SO2)
= 32 + 2 × 16
= 64 u
Formula Unit Mass
It is the sum of the atomic masses of all atoms present in a formula unit of a compound. Formula unit mass is calculated in the same manner as we calculate the molecular mass, for example., formula unit mass for sodium chloride (NaCl)
= 1 × 23 +1 × 35.5
= 58.5 u
UP Board Class 9 Science Notes For Chapter 3 Activity 1
Objective
To understand that there is no change in mass when a chemical reaction takes place. (To understand the law of conservation of mass experimentally).
Procedure
1. Take any one of the following sets, X and Y of chemicals
- Copper sulphate, 1.25 g Sodium carbonate, 1.43 g
- Barium chloride, 1.22 g Sodium sulphate, 1.53 g
- Lead nitrate, 2.07 g Sodium chloride, 1.17 g
2. Prepare separate solutions of any one pair of substances listed under Xand Yeach in 10 mL water.
3. Take the solution of Y in a conical flask and the solution of X in a small test tube.
4. Hang the test tube in the flask carefully. Put a cork on the flask and weigh it.
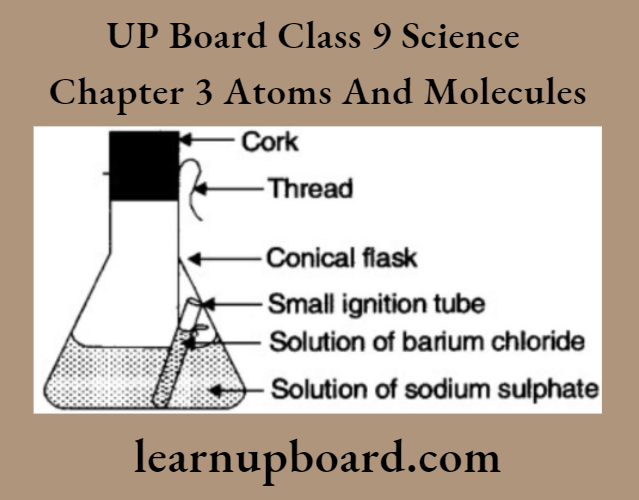
In (1), on weighing, its weight is (1.25 + 1.43 + x) g = (2.68 + x)g
In (2), on weighing, its weight is (1.22 + 1.53 + x)g= (2.75 + x)g
In (3), on weighing, its weight is (2.07+ 1.17+ x)g= (3.24 + x)g
x = combined weight of the set-up (conical flask, test tube, cork etc).
5. Now, tilt and swirl the flask, so that the solutions X and F get mixed. We should put a cork on the mouth of the flask so that no gas can pass out if formed.
6. Weigh again.
Observation
The sum of the weights of the products formed is the same as before the mixing of reactants.
In the reaction flask, the following chemical reactions take place (in respective cases):
- CuSO4 + Na2CO3 → Na2SO4 + CuCO3
- BaCl2 + Na2SO4 → BaSO4 + 2NaCl
- Pb(NO3)2 + 2NaCl → 2NaNO3 + PbCl2
Conclusion
The combined mass of the flask and its contents does not change which means mass remains conserved in the reaction. i.e. mass can neither be created nor be destroyed in chemical reactions.
Question 1. Which law is verified by this activity?
Answer: The law of conservation of mass is verified by this activity.
Question 2. When 20 g of BaCl2 is mixed with 10.6 g of Na2SO4, it produces 8.2 g of NaCI and some amount of BaSO4. What is the mass of BaSO4 produced?
Answer:
Total mass of reactants = 20+ 10.6
= 30.6 g
So, a mass of products should be 30.6 g
∴ Mass of BaSO4 = (30.6 – 8.2)
= 22.4 g
Question 3. What role does this law play in the balancing of chemical equations?
Answer: Chemical equations are balanced to satisfy this law.
Question 4. Who gave the law of conservation of mass in chemical reactions?
Answer: Antoine Lavoisier
Question 5. Matter can neither be created nor be destroyed is the law of…
Answer: Matter can neither be created nor be destroyed is the law of conservation of mass.
UP Board Class 9 Science Notes For Chapter 3 Activity 2
Objective
To understand how atoms of different elements join together in definite proportion to form molecules of compounds.
Procedure
1. The ratio by number of atoms for a water molecule can be found as follows:
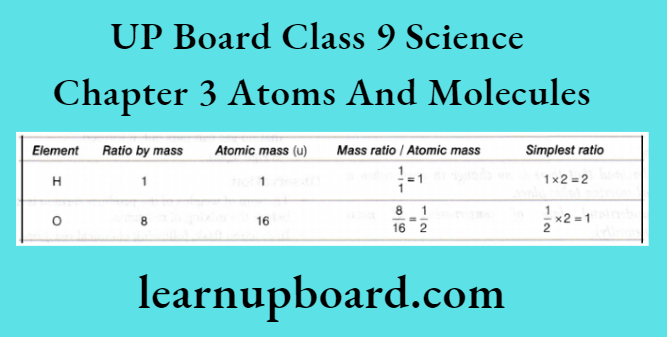
Thus, the ratio by number of atoms for water is H: O = 2: 1
2. The ratio by number of atoms for ammonia molecule can be found as follows:
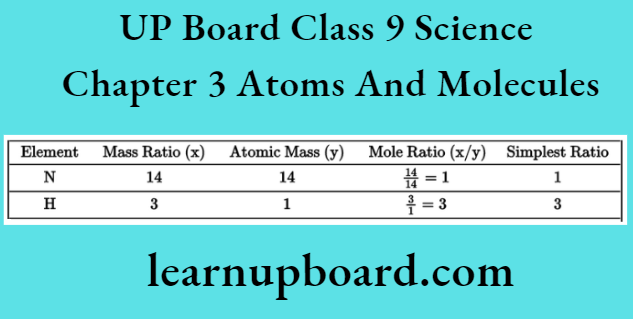
Thus, the ratio by number of atoms for ammonia is N: H = 1:3
3. The ratio by number of atoms for carbon dioxide molecule can be found as follows:
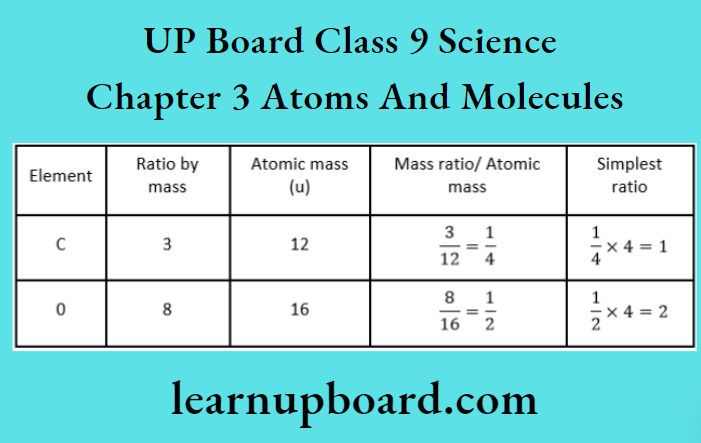
Thus, the ratio by number of atoms for carbon dioxide molecule is C: O = 1: 2.
Question 1. What is the atomic mass of nitrogen?
Answer: The atomic mass of nitrogen is 14.
Question 2. What is the ratio of N and H by mass in ammonia molecules?
Answer: The ratio of N and H by mass in ammonia molecule is 14 : 3.
Question 3. Find the ratio of C and O by mass in CO2.
Answer: The ratio of C and O by mass in CO2 is 3: 8.
Question 4. What is the simplest ratio by several atoms of C and O for CO2?
Answer: The simplest ratio for CO2 is 1: 2.
Question 5. Write the molecular formula of ammonia.
Answer: Ammonia is NH3
UP Board Class 9 Science Notes For Chapter 3 Atoms And Molecules Summary
Antoine L Lavoisier laid the foundation of chemical sciences by establishing two important laws of chemical combination which are:
- Law of Conservation of Mass It states that “mass can neither be created nor destroyed in a chemical reaction”.
- Law of Constant Proportion It states that “in a pure chemical substance, the elements are always present in definite proportions by mass”. It was given by J Proust and is also known as the law of definite proportions.
- Dalton’s Atomic Theory
- It states that matter is made up of very small indivisible particles called atoms.
- Atoms of a given element are identical but those of different elements have different masses and chemical properties.
- The major drawback of this theory is that atoms are no longer considered indivisible. Discoveries show that atoms are made up of electrons, protons and neutrons.
- Atom It is the smallest particle of matter which takes part in a chemical reaction.
- Symbols of elements are derived from one or two letters of the names of the elements in English, Greek, Latin, German etc. First letter is written in capital and the second one is small, for example., iron: Fe (from Ferrum).
- Relative Atomic Mass It is defined as the number of times a given atom is heavier than 1/12th of the mass of 1 atom of carbon-12.
- Atomic mass unit (amu) now called unified mass (u) is defined as the mass of l/12th of the mass of one atom C-12 isotope.
- Molecule It is an atom or group of bonded atoms that exist independently.
- Molecules of Elements These are made up of atoms of only one kind. for example., Ar, He, O2, O3, etc.
- Molecules of Compounds These are made up of atoms of different elements joined together in fixed ratios, for example., H2O, CH4, etc.
- Atomicity It is defined as the number of atoms present in a molecule of an element or a compound. Monoatomic (He, Ne etc.) diatomic (H2, HCl etc.) triatomic (O3, H2Oetc.) and polyatomic (P4, S8 etc.) molecules consist of one, two, three and more than three atoms respectively.
- Ions are the charged species and can be positively or negatively charged. Positive charged ions are called cations (for example Na+, K+ etc) and negative charged ions are called anions (for example., Cl–, O2- etc). Polyatomic ions consist of a group of atoms that carry a net charge on them. (for example., OH–, SO2-4 etc).
- Ionic Compounds These compounds are made up of cations and anions, for example., NaCl (Na+, Cl–).
- Valency It is the combining capacity of an element and it is equal to charge in the case of ions.
- Molecular Mass It is the sum of atomic masses of all the atoms present in a molecule. It is expressed in atomic mass unit (u). For example., H2O = 2 × 1 + 16 = 18 u
