UP Board Class 9 Science Notes For Chapter 4 Structure Of The Atom
We have learned that atoms and molecules are the fundamental building blocks of matter. The existence of different kinds of matter around us is due to different types of atoms and molecules present in them.
- Dalton assumed that an atom is indivisible, i.e. it has no constituent particles. But, a series of experimental evidence revealed that an atom is not the smallest particle. Some other particles smaller than the atom are also present which are called sub-atomic particles, i.e. electrons, protons, and neutrons.
- The atoms of different elements differ in the number of electrons, protons, and neutrons.
- In this chapter, we will describe how electrons, protons, and neutrons were discovered and the various models that have been proposed to explain how these particles are arranged within the atom.
UP Board Class 9 Science Notes For Chapter 4 Charged Particles In Matter
The particles that carry an electric charge are called charged particles. Generally, on rubbing two objects together, they become electrically charged. It means that some charged particles are present within the atom or the atom is made up of some charged particles. Two such particles are electrons and protons.
Read and Learn More Class 9 Science Notes
Discovery Of Electrons
- It was known by 1900, that the atom was not a simple, indivisible particle but contained at least one sub-atomic particle—the electron, which was identified by J. J. Thomson when he performed a cathode ray experiment using a discharge tube.
- In the experiment, a gas at low pressure was taken in a discharge tube made up of glass At the ends of the discharge tube two electrodes (metal plates) were placed, connected to a battery for high voltage supply.
- The electrode connected to the negative end was known as the cathode and that to the positive is the anode. During this experiment, he found a beam of negatively charged particles, called cathode rays, as they originated from the cathode. These negatively charged particles were called electrons.
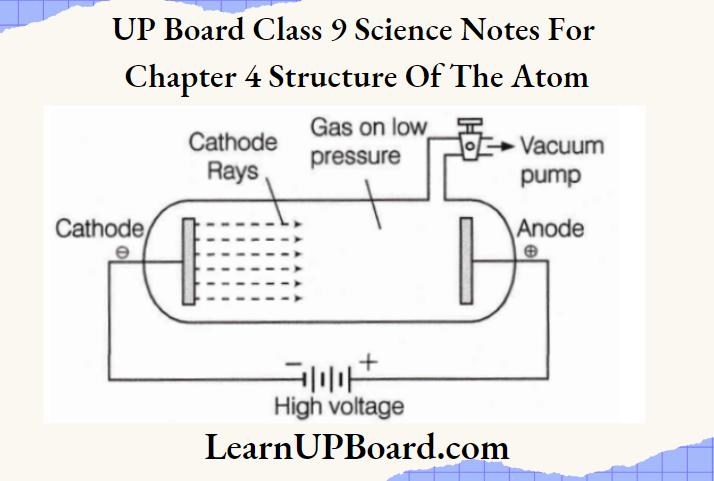
Electrons are negatively charged particles and are denoted by ‘e–‘ The charge present on an electron is equal to -1.6 × 10-19 Coulomb.
Since this charge is considered to be the smallest, therefore, charge on e– is taken as -1. The mass of an electron is equal to 9.1 × 10-31 kg.
Discovery Of Protons
An atom is electrically neutral but the formation of cathode rays has shown that all the atoms contain negatively charged electrons. So, atoms must also contain some positively charged particles to balance the negative charge of electrons. This was the basis of the discovery of protons.
- Before the identification of electrons, E. Goldstein in 1886, discovered the presence of new radiations known as canal rays or anode rays. These rays were positively charged radiations which are seen moving from the anode towards.
- Cathode in a specially designed discharge tube (with a porous cathode), when a high voltage is applied across the electrodes. A porous cathode is used to provide the path for passing anode rays. It led to the discovery of another sub-atomic particle, the proton.
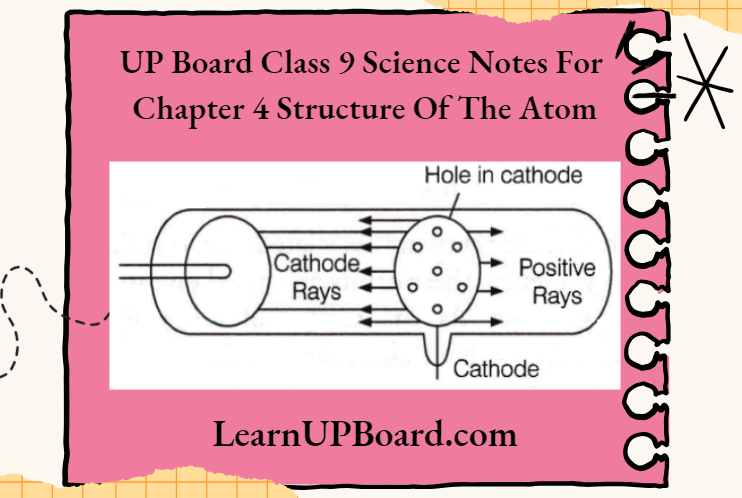
Protons are positively charged particles and are denoted by ‘p+’. The charge present on a proton is equal to +1.6 × 10-19 Coulomb and it is considered as +1.
The mass of a proton is equal to 1.6 × 10-27 kg. The mass of a proton is approximately 2000 times that of an electron.
Conclusion
The mass of a proton is taken as one unit and its charge is (+1), whereas the mass of an electron is considered to be negligible and its charge is (-1). It seems that an atom is composed of protons and electrons, mutually balancing their charges.
UP Board Class 9 Science Notes For Chapter 4 The Structure Of An Atom
According to Dalton’s atomic theory, the atom was indivisible and indestructible. Now, the discovery of two fundamental particles (electrons and protons) inside the atom, led to the failure of this aspect of Dalton’s theory. To know the arrangement of electrons and protons within an atom, many scientists proposed various atomic models.
Thomson’s Model Of An Atom
- J.J. Thomson was the first scientist to propose a model for the structure of an atom. Thomson’s model of an atom was similar to Christmas pudding. The electrons in a sphere of positive charge were like currants (dry fruits) in a spherical Christmas pudding.
- It can also be compared to a watermelon, in which, the positive charge in an atom is spread all over like the red edible part, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon.
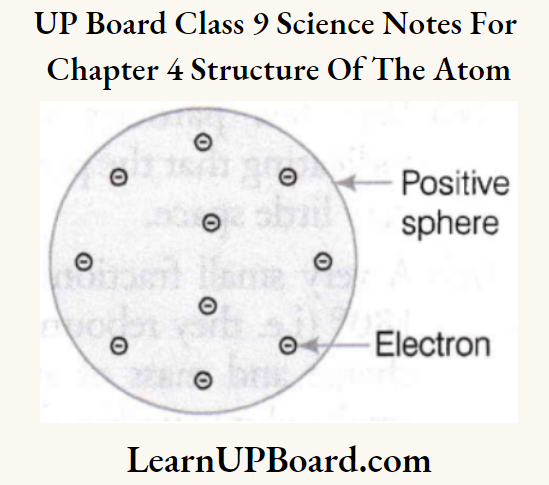
The following are the postulates of this model:
- Electrons are embedded in the sphere of positive charge.
- The negative and positive charges are equal in magnitude. Therefore, the atom as a whole is electrically neutral.
- The mass of an atom is assumed to be uniformly distributed throughout the atom.
Limitations of Thomson’s Model of an Atom
Limitations of J.J. Thomson’s model of an atom are:
- J.J. Thomson’s model could not explain the experimental results of other scientists such as Rutherford, as there is no nucleus in the atomic model proposed by Thomson.
- It could not explain the stability of an atom, i.e. how positive and negative charges could remain, so close together.
UP Board Class 9 Science Notes For Chapter 4 Rutherford’s Model Of An Atom
Ernest Rutherford designed an experiment to know how the electrons are arranged within an atom. He bombarded fast-moving a-particles (these are doubly charged helium ions having a mass of 4 u) on a thin sheet of gold foil. He selected a gold foil because he wanted a layer as thin as possible. This gold foil was about 1000 atoms thick.
The following observations were made by Rutherford:
- Most of the fast-moving a-particles passed straight through the gold foil.
- Some of the a-particles were deflected by the foil by small angles.
- Very few a-particles (one out of 12000) appeared to rebound.
Based on his experiment, Rutherford concluded that:
- Most of the space inside the atom is empty because most of the a-particles pass through the gold foil without getting deflected.
- Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
- A very small fraction of a-particles were deflected by 180° (i.e. they rebound), indicating that all the positive charge and mass of the atom were concentrated in a very small volume within the atom.
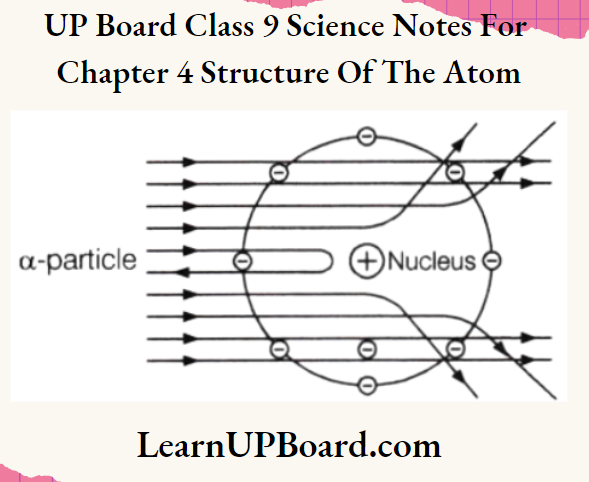
Based on his experiment, Rutherford put forward the nuclear model of an atom, having the following features:
- There is a positively charged, highly dense center in an atom, called a nucleus. Nearly, the whole mass of the atom resides in the nucleus.
- The electrons revolve around the nucleus in a circular path.
- The size of the nucleus (10-15m) is very small as compared to the size of the atom (10-10m).
Note: Rutherford suggested that his model of the atom was similar to that of the solar system. In the solar system, the different planets revolve around the Sun. Similarly, in an atom, the electrons are revolving around the nucleus. So, these electrons are also called planetary electrons.
Limitations Of Rutherford’s Model Of An Atom
Limitations of Rutherford’s model of an atom are:
- Any charged particle when accelerated is expected to radiate energy. To remain in an ill circular orbit, the electron. Electrons would need to undergo acceleration. Therefore, it would radiate energy.
- Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable. Therefore, matter would not exist, but we know matter exists. It means that atoms are quite stable.
- Thus, it could not explain the stability of an atom when charged electrons are moving under the attractive force of positively charged nucleus.
- Rutherford’s model could not explain the distribution of electrons in the extra nuclear portion of the atom.
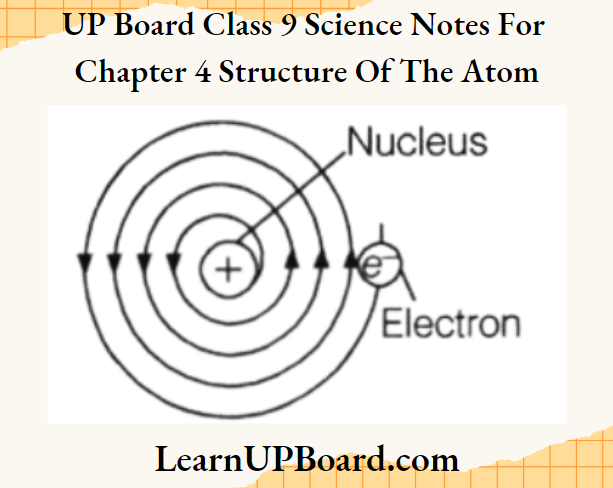
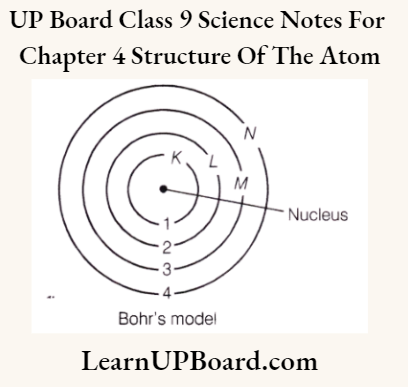
Bohr’s Model Of An Atom
To overcome the objections raised against Rutherford’s model of the atom, Neils Bohr put forward the following postulates about the model of an atom:
- Atom consists of a positively charged nucleus around which electrons revolve in discrete orbits, i.e. electrons revolve in certain permissible orbits and not just in any orbit.
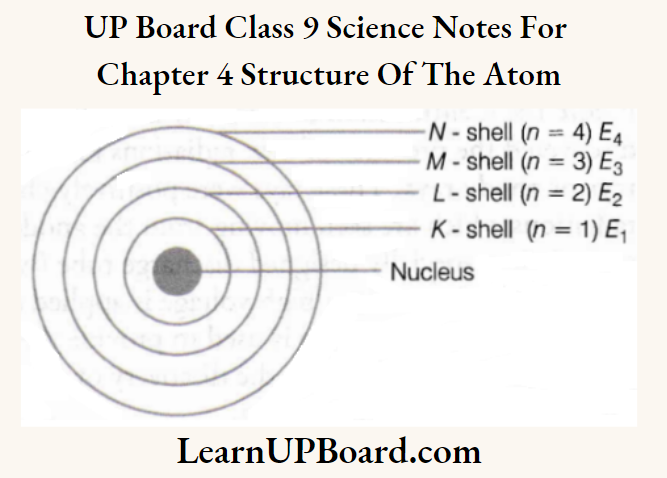
- Each of these orbits are associated with certain value of energy. Hence, these orbits are called energy shells or energy levels. As the energy of an orbit is fixed (stationary), orbit is also called stationary state.
- Starting from the nucleus, energy levels (orbits) are represented by numbers (1, 2, 3, 4, etc.) or by alphabets (K, Z, M, Netc.).
- The electrons present in the first energy level (Z^) have the lowest energy. Energies increases on moving towards outer energy levels.
- The energy of an electron remains the same as long as it remains in a discrete orbit and it does not radiate energy while revolving.
- When energy is supplied to an electron, it can go to higher energy levels. Wfiile an electron falls to lower energy level, when it radiate energy.
Neutrons (n)
- Neutron is another sub-atomic particle, discovered by J. Chadwick in 1932. It is represented by n. Neutrons are electrically neutral particles and are as heavy as protons (i.e. their mass is 1.67493 × 10-27kg) which is equal to that of protons.
- Neutrons are present in the nucleus of all atoms except hydrogen. The mass of an atom is given by the sum of the masses of protons and neutrons present in the nucleus.
Distribution Of Electrons In Different Orbits (Shells)
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury. For writing the number of electrons in different energy levels or shells, some rules are followed. These are:
1. The maximum number of electrons present in a shell is given by the formula 2n2, where, n is the orbit number or energy level, 1, 2, 3,….
Therefore, the maximum number of electrons in different shells is as follows:
First orbit or K-shell = 2 × (1)2 =2
Second orbit or Z-shell = 2 × (2)2 =8
Third orbit or Mshell = 2 × (3)2 = 18
Fourth orbit or Af-shell = 2 × (4)2 =32 and so on.
2. The maximum number of electrons that can be accommodated in the outermost orbit is 8.
3. Electrons are not accommodated in a given shell unless the inner shells are filled (i.e. the shells are filled in a stepwise manner).
Valency
The electrons present in the outermost shell of an atom are known as the valence electrons. They govern the chemical properties of atoms. The atoms of elements having completely filled outermost shell means which has eight electrons show little chemical activity, i.e. they are highly stable. Such elements are called inert elements.
- It means, their valency is zero. Of these inert elements, the helium atom has two electrons in its outermost shell and all other elements have atoms with eight electrons in the outermost shell.
- The tendency to react with atoms of the same or different elements to form molecules is an attempt to attain fully-filled outermost shell. It means, atoms react with other atoms in order to attain fully-filled outermost shell.
- An outermost-shell, which had eight electrons is called an octet. Atoms would thus react, so as to achieve an octet in the outermost shell. This was done by sharing, gaining or the loss of electrons. The number of electrons lost or gained or shared by an atom to become stable or to achieve an octet in the outermost shell is known as valency of that element.
- In other words, it is the combining capacity of the atom of an element with the atom(s) of other element(s) in order to complete its octet.
The valencies of elements of some groups are described below:
- Hydrogen (H), lithium (Li), sodium (Na) and potassium (K) atoms contain one electron each in their outermost shell, therefore, each one of them can lose one electron to become stable. Hence, their valency is 1.
- The valency of each of Mg, Ca and Be is 2 because all of these have 2 valence electrons and they can lose these 2 electrons to make the octet of electrons in the outermost shell or to become stable.
- The valency of boron and aluminium is 3 because each has 3 valence electrons.
- The valency of carbon and silicon is 4 because each has 4 valence electrons. Nitrogen and phosphorus each has 5 valence electrons, so their valency is 3 because they can gain 3 electrons (instead of losing five electrons) to become stable. Hence, their valency is determined by subtracting five electrons from the octet, i.e. 8-5 = 3. However, P can also share 5 electrons, hence it shows a valency of 5 along with 3.
- Oxygen and sulfur each have 6 valence electrons, therefore, their valency is 2 because they can gain 2 electrons or share 2 electrons to complete their octet.
- Similarly, fluorine and chlorine each has 7 valence electrons, their valency is 1 because they can gain 1 electron or share 1 electron to complete their octet.
- All the inert elements, i.e. He, Ne, Ar, etc., have filled outermost shells. Therefore, their valency is zero.
Note: For metals, valency = Number of valence electrons and for non-metals, valency = 8 – number of valence electrons.
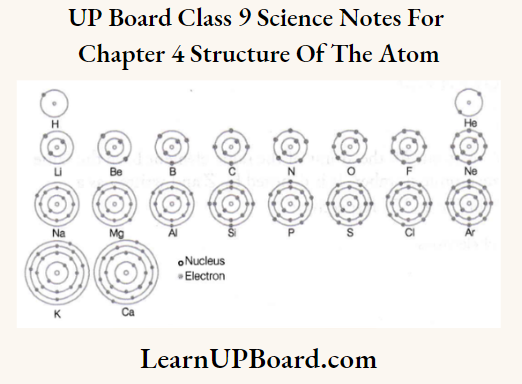
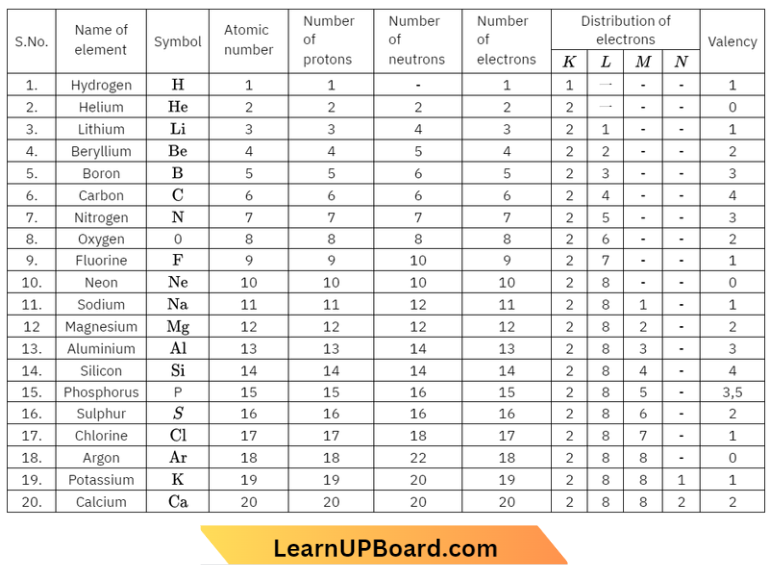
Composition of Atoms of the First Twenty Elements with Electron Distribution in Various Shells:
UP Board Class 9 Science Notes For Chapter 4 Atomic Number And Mass Number
Atomic Number
It is defined as the number of protons present in the nucleus of an atom. All the atoms of the same element have the same number of protons in their nuclei and hence, they have the same atomic number. It is denoted by Z and written as a subscript to the left of the symbol, for example., \({ }_2^4 \mathrm{He},{ }_3^7 \mathrm{Li} \text {, }\) Z =2 and 3 for He and Li respectively.
Note: In a neutral atom, atomic number = number of protons = number of electrons
Mass Number
It is defined as the sum of several protons and neutrons present in the nucleus of an atom. Protons and neutrons together are called nucleons. The mass number is denoted by A. Mass number = Number of protons + a number of neutrons, e.g. 2 He, 3 Li, A -4, and 7 for He and Li respectively.
Number of neutrons = Mass number – atomic number
(∵ Atomic number = Number of protons)
The mass number is written as a superscript to the left of the symbol.
In the notation for an atom, the atomic number, mass number, and symbol of the element are to be written as:

Question 1. An atom of an element A may be written as \({ }_{12}^{24} \mathrm{~A} \text {. }\)
- What does the superscript 24 indicate?
- What does the subscript 12 indicate?
- What are the number of protons, neutrons, and electrons in atom A?
- Write the symbol of an ion formed by an atom of element A.
Answer:
24 is the mass number of atom A.
12 is the atomic number of atom A.
Number of protons =12 Number of neutrons =12 Number of electrons = 12
Electronic configuration of given atom =\(\begin{aligned}
& K L M \\
= & 2, 8,2.
\end{aligned}\)
It can lose two electrons (and attain a stable configuration), therefore the symbol of its ion is A2+.
UP Board Class 9 Science Notes For Chapter 4 Different Atomic Species
Isotopes Definition
These are defined as the atoms of the same element, having the same atomic number but different mass numbers, e.g. there are 3 isotopes of the hydrogen atoms, namely protium (1H1), deuterium (2H1), and tritium (3H1).
- In other words, it can be said that isotopes have same number of protons but differ in the number of neutrons. Each isotope of an element is a pure substance.
- Since, chemical properties of elements largely depend on their electronic configuration or outermost electrons and as the isotopes of an element have similar electronic configuration, therefore, isotopes of an element have same chemical properties.
- We know that, masses of isotopes of elements are different. Since, physical properties such as density, light scattering etc., depend on mass therefore, these are different for isotopes of an element.
Average Atomic Mass
If an element has no isotopes, the mass of its atom would be the same as the sum of masses of protons and neutrons in it. But if an element occurs in isotopic forms, then from the percentage of each isotopic form, the average mass is calculated as:
The average atomic mass of an element [(Atomic mass of isotope 1 × percentage of isotope 1) + (Atomic mass of isotope 2 × percentage of isotope 2) +… ] e.g. the two isotopic forms of chlorine atom with masses 35u and 37u occur in the ratio of 3: 1.
Therefore, the average atomic mass of a chlorine atom can be calculated as:
The average atomic mass of a chlorine atom
⇒ \(\left(35 \times \frac{75}{100}+37 \times \frac{25}{100}\right)\)
⇒ \(\left(\frac{105}{4}+\frac{37}{4}\right)=\frac{142}{4}=35.5 \mathrm{u}\)
Here, 35.5 u is not the atomic mass of any one atom of chlorine but it shows that its given amount contains both the isotopes and their average atomic mass is 35.5 u.
Note: The fractional atomic masses of elements are due to the existence of their isotopes having different masses.
Applications Of Isotopes
- An isotope of uranium (U-235) is used as a fuel for the production of electricity in nuclear reactors.
- U-238 is used to determine the age of very old rocks and even the age of the earth.
- An isotope of cobalt (Co-60) is used in the treatment of cancer.
- An isotope of carbon (C-14) is used to determine the age of old specimens of wood or old bones of living organisms.
- An isotope of iodine (1-131) is used in the treatment of goiter.
Isobars Definition
Atoms of different elements with different atomic numbers but the same mass number are known as isobars. In other words, isobars are the atoms of different elements that have the same number of nucleons (protons + neutrons) but differ in the number of protons, for example., \({ }_{18}^{40} \mathrm{Ar} \text { and }{ }_{20}^{40} \mathrm{Ca}\) are isobars.
Since isobars have different atomic numbers as well as different electronic configurations. Thus, they also have different chemical properties.
Question 2. Consider the following pairs,
⇒ \((1) ${ }_{26}^{58} A,{ }_{28}^{58} B$
(2) ${ }_{35}^{79} \mathrm{X},{ }_{35}^{80} \mathrm{Y}$\)
- Which of the above pairs are isotopes and isobars?
- What factors are responsible for the change in superscripts, 79, 80 (in case II), though the element is the same?
- Give the nuclear composition of H A.
Answer:
Isobars:
- \(\text { Isobars: }{ }_{26}^{58} A \text { and }{ }_{28}^{58} B \text { Isotopes: }{ }_{35}^{79} X \text { and }{ }_{35}^{80} Y\)
- X and Y are pairs of isotopes. Isotopes have the same number of protons but differ in the number of neutrons (hence, their mass number differs, from each other because mass number is the sum of several protons and neutrons).
- Number of protons = 26, Number of electrons = 26 Number of neutrons = 58 – 26 = 32
UP Board Class 9 Science Notes For Chapter 4 Activity 1
Objective
To show the presence of charged particles in matter.
Procedure
- Comb dry hair.
- Bring the comb near the small pieces of paper.
- Rub a glass rod with a silk cloth and bring the rod near an inflated balloon.
Observation
The comb will attract the pieces of paper. Rod will also attract the inflated balloon. In both cases, it is concluded that on rubbing two objects together, they become electrically charged.
Conclusion
This shows that an atom is divisible and consists of charged particles.
Question 1. What happens when we rub two objects?
Answer: On rubbing two objects together, they become electrically
charged.
Question 2. What conclusion can you draw from the above activity?
Answer: The activity shows that an atom is divisible and consists of
charged particles.
Question 3. Name the charged particles present in an atom.
Answer: Electrons and protons are the charged particles, present in an
atom.
Question 4. Name the neutral particle present in an element.
Answer: A neutron is a neutral particle present in an element.
Question 5. What happens if two oppositely charged substances are placed near each other?
Answer: They attract each other.
UP Board Class 9 Science Notes For Chapter 4 Activity 2
Objective
To understand the composition of atoms of the first twenty elements.
Procedure
Make a static atomic model displaying the electronic configuration of the first twenty elements.
Electronic Configuration Of Some Elements:
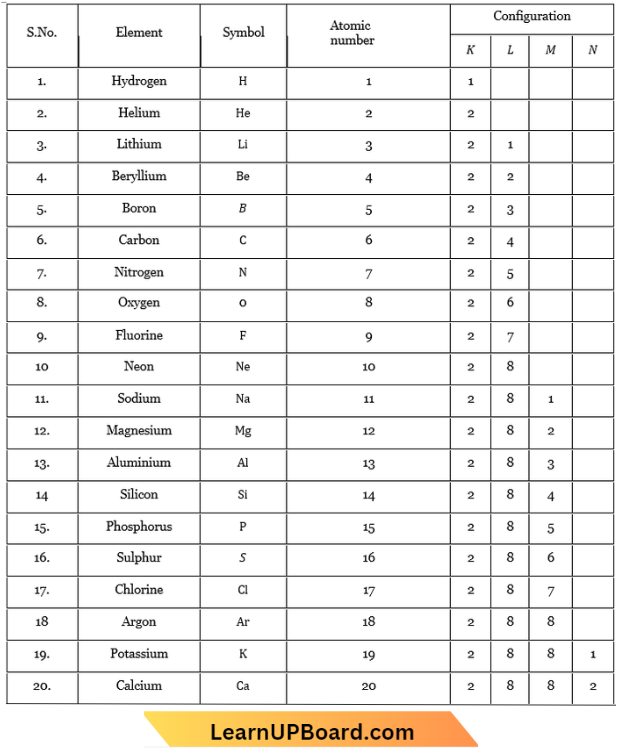
Question 1. What is the number of valence electrons in an atom of element having atomic number =16?
Answer: The electronic configuration of elements is 2, 8, 6. So, the number of
valence electrons is 6.
Question 2. Name the atom that shows two types of valencies.
Answer: P shows a valency of 3 and 5.
Question 3. The electronic configuration of an element Vis \(\begin{aligned}
& K L M N \\
& 2,8,8,1
\end{aligned}\). Name this element Y.
Answer: The element Y is potassium.
Question 4. There are four atoms A, B, C, and D with atomic numbers 9, 11,13, and 15 respectively. Which atom contains less than 8 electrons in an L-shell?
Answer: The electronic configuration of A (9) is 2, 7. So, atom A contains less than 8 electrons in the L-shell
Question 5. Calcium has twenty electrons which occupy K, L, M, and N-shells. Which of these is/are incomplete?
Answer: The electronic configuration of Ca is\(\begin{aligned}
& K L M N \\
& 2,8,8,2
\end{aligned}\). As N-shells can accommodate a maximum of 8 electrons thus, N-shells are incomplete.
UP Board Class 9 Science Notes For Chapter 4 Structure Of The Atom Summary
Discovery Of Electrons
- J.J. Thomson in 1990 discovered cathode rays (or electrons) originating or emitting from the cathode in a gas discharge tube. Electrons are the fundamental particles of all atoms.
- Cathode rays travel in a straight line. In the presence of an electric field, these get deflected towards the positive electrode. They produce fluorescence when strike on the walls of the discharge tube.
- The charge and mass of electrons are 1.6 ×10-19 C and 9.11 × 10-31 kg respectively.
Discovery Of Protons
- E. Goldstein in 1886, discovered the presence of new radiations known as canal rays or anode rays passing through holes or ‘canals’ of the cathode and moving towards the cathode in a discharge tube.
- Anode rays consist of positively charged particles, known as protons.
- Protons have a charge, equal in magnitude but opposite in sign to that of electrons. Its mass is about 1840 times that of the electron.
Thomson’s Model of an Atom: Postulates are:
- The mass of an atom is assumed to be uniformly distributed throughout the atom.
- An atom is considered to be a sphere of uniformly distributed positive charge in which electrons are embedded.
- The negative and positive charges balance each other, therefore, the atom as a whole is neutral.
Rutherford’s Model of an Atom After performing a-particle experiment, he suggested that:
- There is a positively charged, highly dense center in an atom, called the nucleus. Nearly the whole mass of the atom resides in it.
- The electrons revolve around the nucleus in circular paths. The size of the nucleus is very small compared to the size of the atom.
Bohr’s Model of an Atom: Postulates are:
- Only certain special orbits called discrete orbits or energy levels of electrons are allowed inside the atom.
- While revolving in discrete orbits, the electrons do not radiate energy.
- The orbits are represented by the letters K, L, M, and N or the numbers 1,2,3, and 4.
Neutrons
In 1932, J. Chadwick discovered another sub-atomic particle called neutrons. They are electrically neutral and are as heavy as protons. They are present in the nucleus of all atoms, except hydrogen.
Bohr and Bury Scheme for Distribution of Electrons in Different Energy Levels The maximum number of electrons in an energy level is equal to 2n2 where ‘n’ is the energy level of orbits or shells.
Valency It is the combining capacity of an element with the atom(s) of another element (s) to complete its octet.
Atomic Number It is defined as the number of protons present in the nucleus of an atom. It is also equal to the number of electrons in the case of a neutral atom. It is denoted by Z and written as a subscript, for example., 6C.
Mass Number It is defined as the sum of the numbers of protons and neutrons in the nucleus. It is denoted by A.
Isotopes They have the same atomic number but different mass numbers or the same number of protons but different numbers of neutrons, for example., 1H1,1H2, 1H3. Their chemical properties are the same due to the same atomic number.
Isobars They have different atomic numbers but the same mass number. Their physical and chemical properties are different, for example., \({ }_{18}^{40} \mathrm{Ar},{ }_{20}^{4 a} \mathrm{Ca} .\)
