UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Concepts
- Electric Current
- Chemical Ejects of Electric Current
- Electroplating
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Abstract
- Based on electrical conductivity, materials can be classified as conductors or insulators.
- Substances that allow an electric current to pass through them are called conductors, for example, copper, iron, etc.
- Substances that do not allow electric current to pass through them are called insulators, for example, wood, rubber, etc.
- Under certain circumstances, most of the substances can conduct electricity. Hence, substances through which electric current can flow easily are good conductors, while the substances through which electric current can pass negligibly or in a very small quantity are poor conductors.
- The passage of electric current through conducting liquids causes chemical changes in the conducting liquid.
- This phenomenon is called the chemical effect of electric current.
- The process of depositing a layer of desired metal on another material with the help of electricity is called electroplating.
Read and Learn More UP Board Notes for Class 8 Science
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric CurrentImportant Terms And Definitions
Anode: The electrode which is connected to the positive terminal of a batter/ is known as the anode.
Cathode: The electrode which is connected to the negative terminal of a battery is known as the cathode.
Electrodes: Both anode and cathode are collectively called electrodes.
| Class 10 Science | Class 11 Chemistry |
| Class 11 Chemistry | Transformation of Sentences |
| Class 8 Maths | Class 8 Science |
Electrolysis: The process of decomposition of chemicals in a solution or a liquid by passing electric current through it is called electrolysis.
Electrolytes: The substances which give ions in a solution are called electrolytes.
Light Emitting Diode (LED): It glows even when a small amount of current passes through the electric circuit
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric CurrentElectric Current
Electric current or electricity can flow through different types of substances including liquids. Most of the liquids that conduct electricity are solutions of acids, bases and salts.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 1
Aim: To test whether lemon juice or vinegar is a good or bad conductor of electricity
Procedure:
1. Collect a few small plastics or rubber caps from the discarded bottles and clean them.
2. Pour one teaspoon of lemon juice or vinegar into one cap. Then, bring your tester over this cap and let the ends of the tester dip into lemon juice or vinegar as shown below.
3. Take care that the ends are not more than I cm apart but at the same time, they do not touch each other.
4. Check to see if the bulb of the tester glows or not
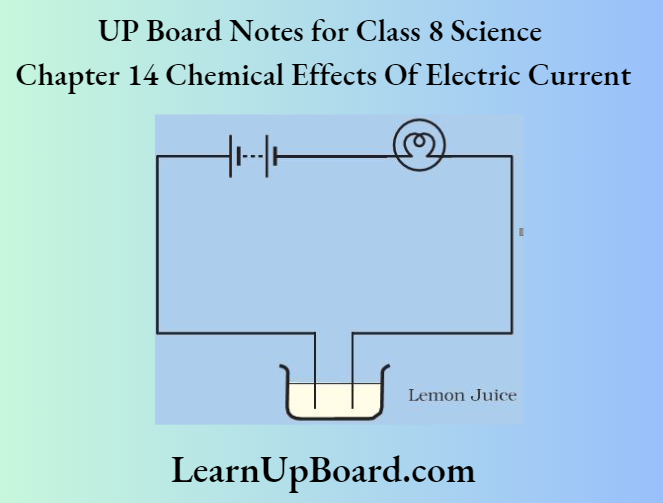
Observation: The bulb glows when dipped in the image here lemon juice or vinegar.
Conclusion: Lemon juice or vinegar is a good conductor of electricity.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 2
Aim: To prepare an electromagnetic tester based on the magnetic effect of current Precaution: Wash and wipe dry the ends of the test- after testing each liquid.
Procedure:
1. Take the tray of a discarded matchbox
2. Wrap an electric wire a few times around the tray and place a small compass needle inside it
3. Connect one free end of the wire to the terminal of a battery.
4. Leave the other end free. Take another piece of the wire and connect it to the other terminal of the batter.
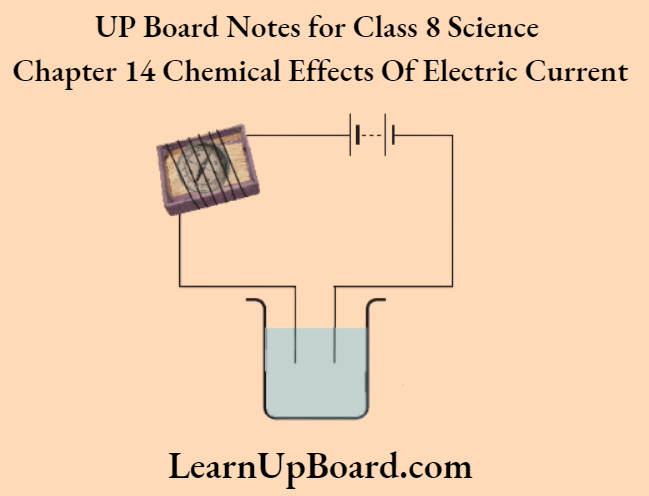
5. Join the free ends of two wires momentarily. The compass needle should show deflection. Your tester with two free ends of the wire is ready.
6. Repeat ‘Activity 2’ above using this tester. You will notice that there is a deflection in the compass needle the moment the free ends of the tester are dipped in the lemon juice.
7. Take out the ends of the tester from the lemon juice. Dip them in water and then wipe them dry.
8. Repeat the activity with other liquids such as milk, honey, etc.
9. In each case, observe whether the magnetic needle shows deflection or not.
Observation:
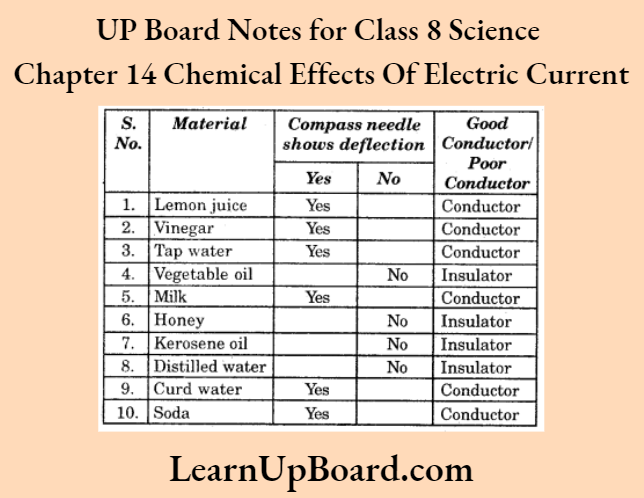
Conclusion: Lemon juice, vinegar, tap water, milk, curd water and soda are all good conductors of electricity. However, vegetable oil, honey, kerosene oil and distilled water are all bad conductors of electricity.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 3
Aim: To show that distilled or pure water is a poor conductor of electricity
Procedure:
- Take about two teaspoons full of distilled water in a clean, dry plastic or rubber cap of a bottle.
- Use the tester to test if distilled water conducts electricity.
- Dissolve a pinch of common salt in the distilled water and test again.
Observation:
- The bulb of the tester does not glow when the tester is put into distilled water.
- When a pinch of salt is dissolved in the distilled water and the tester is immersed in it, the bulb glows.
Conclusion: Distilled or pure water is a poor conductor of electricity.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 4
Aim: To show that water containing any salt, acid or alkali is a good conductor of electricity
Procedure:
- Take three clean plastic or rubber caps of bottles.
- Pour about two teaspoonfuls of distilled water into each of them.
- Add a few drops of lemon juice or dilute hydrochloric acid to the distilled water in the first cap.
- In the second cap, add a few drops of a base such as caustic soda or potassium iodide.
- Add a small amount of sugar to the distilled water in the third cap and dissolve it.
- Test to see which of these solutions conduct electricity.
Observation: Solutions in the first and second cap conduct electricity, while the one in the third does not conduct electricity.
Conclusion: Water containing any salt, acid or alkali is a good conductor of electricity.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Objective Type Questions
1. State whether the following statements are true or false.
- All liquids allow electric current to flow through them.
- The deflection of the needle of a magnetic compass kept near an electric circuit indicates the flow of electric current.
- An LED glows even when a small amount of electric current is passed through it.
- Adding sugar to distilled water makes it a good conductor of electricity.
Answers:
- False
- True
- True
- False
2. Multiple Choice Questions.
1. Which of the following does not allow the passage of electric current through them?
- Tap water
- Vinegar
- Distilled water
- Seawater
Answer: 3. Distilled water
2. ‘Which of these substances is a poor conductor of electricity?
- Wood
- Iron
- Copper
- Graphite
Answer: 1. Wood)
3. Fill in the blanks.
1. Materials that do not allow electric current to easily pass through them are called__________
conductors of electricity.
2. Tap water is a__________ conductor of electricity.
3. An LED consists of two wires called_________.
Answers:
- Poor
- Good
- Leads
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 4 Short Answer Type Questions
Question 1. What are electrodes?
Answer
Electrodes
Electrodes are electrical conductors that are used to make contact with the non-metallic part of a circuit such as a conducting liquid, air or a semiconductor.
Question 2. Explain how an LED should be connected to a circuit
Answer
LED or light emitting diode comprises a bulb with two wires (called leads), where one lead is longer than the other. The longer lead is connected to the positive terminal of the battery and the shorter lead is connected to the negative terminal of the battery.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current
The passage of electric current through conducting liquids causes chemical changes in the liquids. This phenomenon is called the chemical effect of electric current. The chemical reactions or changes vary depending on the type of solution and electrodes used.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 5
Aim: To show the production of gases during electrolysis of water
Procedure:
1. Carefully take out carbon rods from two discarded batteries.
2. Clean their metal caps with sandpaper.
3. Wrap copper wires around the metal caps of the carbon rods and connect them to a battery.
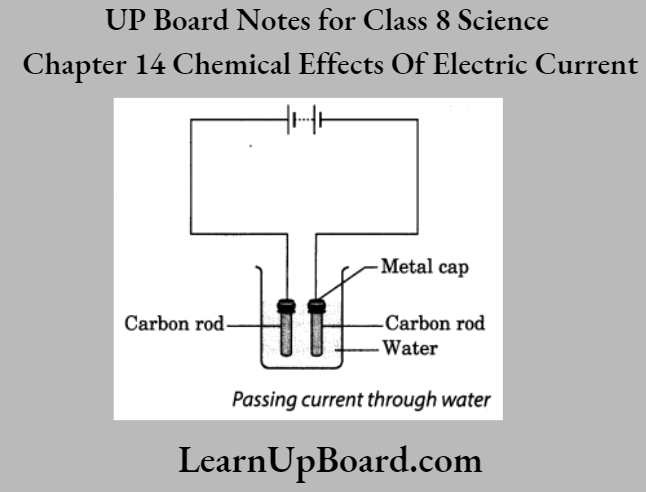
Note: Instead of carbon rods, you may take bio-iron nails about 6 cm long.
4. Pour a cupful of water into a glass or a plastic bowl.
5. Add a teaspoonful of salt or a few drops of lemon juice to the water to make it more conducting.
6. Immerse the electrodes in this solution. Ensure that the metal caps of the carbon rods are outside the water.
7. Wait for 3-4 minutes.
8. Observe the electrodes.
Observation: Gas bubbles are seen at the ends of the electrodes.
Conclusion:
1. Due to the passage of electric current, water is broken down into its constituent gases, i.e., hydrogen and oxygen. This process of breaking up a*i electrolyte by passing an electric current through it is known as electrolysis.
2. Oxygen bubbles are formed on the positively charged electrode (anode) and hydrogen bubbles are formed on the negatively charged electrode (cathode).
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Objective Type Questions
1. State whether the following statements are true or false.
- When electric current passes through copper sulphate solution, copper gets deposited at the anode.
- Electric current can bring a chemical change in a liquid solution.
Answers:
- False
- True
2. Multiple Choice Questions.
1. ‘What does a positively charged electrode know as?
- Cathode
- Anode
- Electrolyte
- Electrolysis
Answer: 2. Anode
2. When electrodes are immersed in water and electric current is passed through them, on which terminal of the battery oxygen bubbles are formed?
- Positive terminal
- Negative terminal
- Both the terminals
- Neither of the terminal
Answers: 1. Positive terminal
3. Fill in the blanks.
- When electrodes are immersed in water and an electric current is passed through them, bubbles of
__________and_________are produced. - The closed path through which electric current ‘lows is called a________.
- The negative terminal of a batter)’ or negatively charged electrode is also known as the_______.
Answers:
- Oxygen, hydrogen
- Circuit
- Cathode
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current
Activity 5 Short Answer Type Questions
Question 1. During electroplating, the copper deposited on the plate connected to the negative terminal comes from the solution. How is this loss of copper from the solution compensated?
Answer
The loss of copper from the solution is compensated by the other plate. An equal amount of copper gets dissolved in the solution and the process keeps on going. This means that copper gets transferred from one plate to another.
Question 2. Solid NaCI does not conduct electricity but when dissolved in water, it conducts electricity. Why?
Answer
Solid NaCI consists of ions that cannot move. However, when solid NaCI is dissolved in water, the ions separate and can move easily. Thus, solid NaCI cannot conduct electricity, whereas NaCI solution can conduct electricity.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Electroplating
- Electroplating is the process of depositing a layer of a desired metal on another material with the help of electricity. Artificial jewellery is made with coatings of less expensive metals such as iron and aluminium with costlier metals, such as silver and gold to give them a rich look.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 6
Aim: To demonstrate the process of electroplating
Materials Required: Two copperplates approximately 10 cm 4 cm in size, copper sulphate, distilled water, a beaker, dilute sulphuric acid, sandpaper and batter)’
Procedure:
1. Take 250 ml of distilled water in a clean and dry beaker.
2. Dissolve two teaspoonfuls of copper sulphate in rt. Also, add a few drops of dilute sulphuric acid to the copper sulphate solution to make it more conducting.
3. Clean copper plates with a sandpaper.
4. Rinse the plates with water and let them dry/.
5. Connect the copper plates to the terminals of a batter)- and immerse them into the copper sulphate solution.
6. Allow the electric current to pass through the circuit for abo it 15 minutes. Now, remove the electrodes from the solution and observe them.
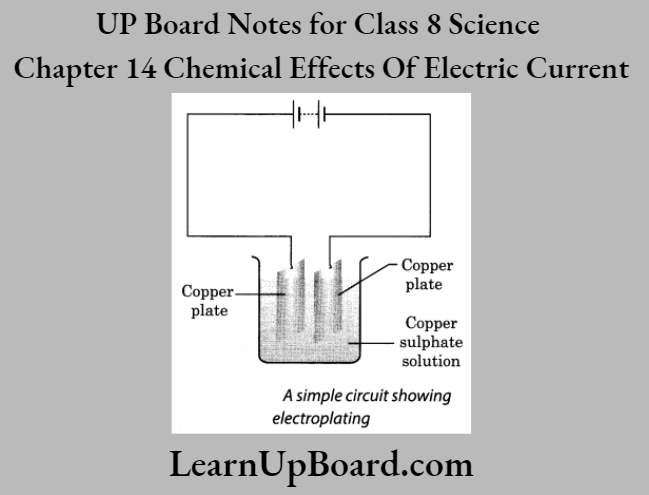
Observation: Copper metal gets deposited on the plate at the negative terminal of the battery.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Objective Type Questions
1. State whether the following statements are true or false.
- During electroplating, the object to be electroplated is connected to the positive terminal of the batter).
- Copper and iron are the only metals that can be used for electroplating purposes.
- A coating of zinc is deposited on iron to protect it from corrosion.
Answers:
- False
- False
- True
2. Multiple Choice Questions.
1. Electroplating is based on which of these effects of electricity?
- Magnetic effect
- Physical effect
- Heating effect
- Chemical effect
Answer: 4. Chemical effect
2. Which of these is true about electroplating?
- It is a method of making metal plates.
- It is the process of coating a layer of metal over another metal by using an electric current.
- It is the technique of making plates that do not conduct electricity,
- None of these
Answer: 2. ft is the process of coating a layer of metal over another metal by using electric current
3. Fill in the blanks.
1.__________is used to coat bath taps, bicycle handlebars, etc., and give them a shiny look.
2.________is one of the most common applications of chemical effects of electric current.
Answers:
- Chromium
- Electroplating
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Activity 6 Textbook Exercises
Question 1. Fill in the blanks.
- Most liquids that conduct electricity are solutions of_________,__________ and_________
- The passage of an electric current through a solution causes__________effects.
- If the current passes through a copper sulphate solution, copper gets deposited on the plate connected
to the__________terminal of the battery. - The process of depositing a layer of any desired metal on another material using electricity
is called____________.
Answers:
- Acids, bases, salts
- Chemical
- Negative
- Electroplating
Question 2. When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
Answer
The needle shows deflection because the solution is a good conductor of electricity and electric current has a magnetic effect
Question 3. Name three liquids, which when tested in the manner given below, may cause the magnetic needle to deflect.
Answer: Tap water, lime water and vinegar cause the magnetic needle to deflect.
Question 4. The bulb does not glow in the setup shown in the figure given below. List the possible reasons. Explain your answer.
Answer:
In the given setup, if the bulb does not glow, it may be because the bulb may be fused or the connection of the wires may be loose. Replace the bulb and check once again. If the bulb still does not glow, check the connection of the wires. However, after tightening the connections and testing the bulb, if it still does not glow, then the only possible reason can be that the solution is a poor conductor of electricity.
Question 5. A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightfor liquid A, while it glows very dimly for liquid B. You would conclude that
- liquid A is a better conductor than Liquid B
- liquid B is a better conductor than Liquid A
- both liquids are equally conducting
- conducting properties of liquids cannot be compared in this manner
Answer:
- liquid A is a better conductor than liquid B.
Question 6. Does pure water conduct electricity? If not, what can we do to make it conduct?
Answer
No, pure water does not conduct electricity. However, adding some salt, acid or alkali can make pure water a good conductor of electricity.
Question 7. In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Answer
Tap water is a good conductor of electricity. Hence, to avoid electric shock, firemen shut off the main electrical supply before using water hoses.
Question 8. A child staying in a coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Answer
Drinking water is generally free of salts and minerals, whereas seawater is rich in salts and minerals. Thus, seawater is a better conductor of electricity than drinking water. Hence, the compass needle deflects more in the case of seawater as compared to drinking water
Question 9. Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpours?
Answer
Explain. No. it is not at all safe to perform outdoor electrical repairs when it is raining. Rather, it is dangerous because the electrician may get an electric shock as rainwater is a good conductor of electricity.
Question 10. Paheli had heard that rainwater is as good as distilled water. So she collected some rainwater in a clean glass tumbler and tested it using a tester To her surprise she found that the compass needle showed deflection. What could be the reason?
Answer
Though rainwater is as pure as distilled water it may be contaminated by the impurities suspended in the atmosphere. These impurities make rainwater a better conductor of electricity. This is the reason that the compass needle showed deflection.
Question 11. Prepare a list of objects around you that are e electroplated.
Answer
Bicycle rims, door handles, taps, metallic pens, artificial jewellery, utensils, belts, and buckles.
Question 12. The process that you saw in Activity 7 is used for the purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod j is sought to be transferred to the thin copper plate. Which electrode should be attached to the • positive terminal of the battery and why?
Answer
The impure copper rod should be attached to the positive terminal because copper ions will get drawn towards the negative terminal and will be deposited there. So, the copper from the impure rod will get deposited on the pure copper plate.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Hots Corner
Question 1. We are advised not to touch electrical appliances with wet hands. Why?
Answer
Small quantities of mineral salts are naturally present in water. The salts make water a good conductor of electricity. Therefore, it may be dangerous to touch any electrical appliance with wet hands because of the potential risk of being electrocuted.
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Practice Exercise Objective Type Questions
1. Give one word for the following.
- Substances that do not allow an electric current to pass through them
- The metal rods dipped in liquids to which batteries are attached in a circuit
- Substances that conduct electricity in a liquid state or when dissolved in water and break up chemically during the process
Answers:
- Insulators
- Electrodes
- Electrolytes
2. State whether the following statements are true or false.
- Electric bulbs glow due to the chemical effect of electricity.
- Some liquids are good conductors of electricity and some are poor conductors.
- Electrolysis is a chemical change.
- Bath taps are electroplated to prevent them from corrosion.
- A solution in which very few mobile ions are present is a strong electrolyte.
Answers:
- False
- True
- True
- True
- False
3. Circle the odd one out.
- Plastic, copper, aluminium, graphite
- Lemon juice, tap water, vegetable oil, and common salt solution
Answers:
- Plastic
- Vegetable oil
D. Fill in the blanks.
- Electroplating __________rusting of objects.
- is an electrolyte and____________ is a non-electrolyte.
- Distilled water is a____________ conductor of electricity.
- Carbonic acid is a____________ electrolyte.
Answers:
- Prevents
- Sulphuric acid, ethyl alcohol
- Poor
- Weak
UP Board Notes For Class 8 Science Chapter 14 Chemical Effects Of Electric Current Short Answer Type Questions
Question 1. Name four liquids that can conduct electricity.
Answer
Sea water, vinegar, soda and milk
Question 2. A pencil sharpened at both ends can be used as a conductor in laboratories. Why?
Answer
A pencil sharpened at both ends can be used as a conductor because pencil lead is made up of graphite and graphite is a good conductor of electricity.
Question 3. Why high voltage electric current is dangerous for our body?
Answer
The brain and heart send tiny electrical impulses generated within the body. If high voltage electric current passes through our body, the brain or heart could stop working. Thus, high-voltage electric current is dangerous for our bodies.
- Chapter 1 Crop Production and Management
- Chapter 2 Microorganisms: Friend and Foe.
- Chapter 3 Synthetic Fibres and Plastics
- Chapter 4 Materials: Metals and Non-Metals
- Chapter 5 Coal and Petroleum
- Chapter 6 Combustion and Flame
- Chapter 7 Conservation of Plants and Animals
- Chapter 8 Cell: Structure and Functions
- Chapter 9 Reproduction in Animals
- Chapter 10 Reaching the Age of Adolescence
- Chapter 11 Force and Pressure
- Chapter 12 Friction
- Chapter 13 Sound
- Chapter 15 Some Natural Phenomena
- Chapter 16 Light
- Chapter 17 Stars and the Solar System
- Chapter 18 Pollution of Air and Water
