UP Board Solutions For Class 9 Science Chapter 2 Is Matter Around Us Pure
Question 1.
- What are elements?
- What are the three main types of elements?
- Write a property of each type of element.
Answer:
- An element consists of only one type of atom. It is a basic form of matter that cannot be broken down into simpler substances by chemical reactions.
- Metals, non-metals and metalloids.
- Metals—Malleable and ductile Non-metals—Brittle Metalloids—Semiconductors
Question 2.
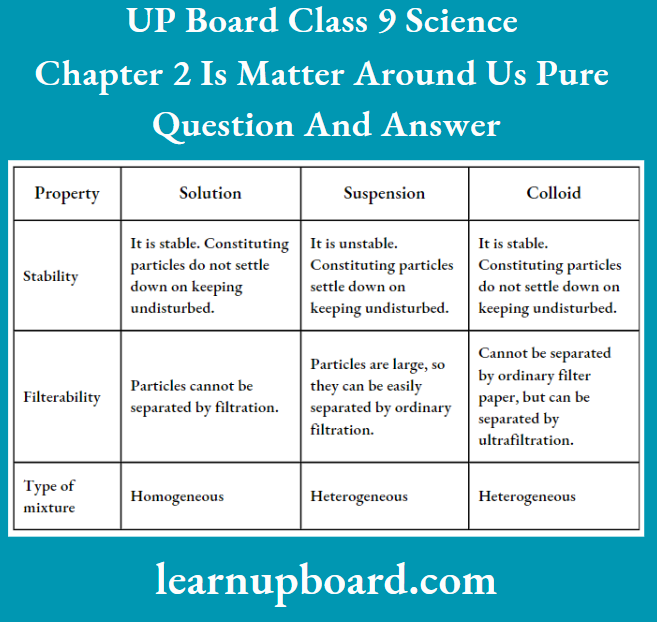
- Distinguish among the true solution, suspension and colloid in a tabular form under the following heads:
- Stability
- Filterability
- Type of mixture
- Give expression for the concentration of a solution. How will you prepare a 10% solution of glucose by mass in water?
Answer:
Distinctions between true solution, suspension and colloid are:
The methods by which the concentration of a solution can be expressed are:
Mass by mass% of solution
⇒ \(=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
Mass by volume% of solution
⇒ \(\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
A 10 per cent solution of glucose can be prepared by dissolving 10 g of glucose in 90 g of water.
Read and Learn More Class 9 Science Solutions
Question 3. Write your observations when the following processes take place:
- An aqueous solution of sugar is heated to dryness.
- A saturated solution of potassium chloride prepared at 608°C is allowed to cool at room temperature.
- A mixture of iron filings and sulphur powder is heated strongly.
- A beam of light is passed through a colloidal solution.
- Dilute HCl is added to the mixture of iron and sulphur.
Answer:
- Sugar remains as a residue in the form of a solid mass.
- Potassium chloride crystallises out.
- A black-coloured compound is formed.
- The path of the light becomes visible.
- A colourless gas has evolved.
Question 4.
- Pond water contains sand grains, clay particles, salt, pieces of paper and some air bubbles. Select from amongst these, an example each of a solvent, solute, colloid and suspension.
- Give one example of each of the following:
- A solution of gas in liquid
- A solution of two solids
- A solution of two gases
Answer:
- Solvent-Water Solute-Salt, pieces of paper, air bubbles Colloid-Mixture of air bubbles and water Suspension-
- A mixture of water and sand grains
- A mixture of water and clay particles
- Aerated drinks,
- Brass,
- Air
Question 5. Classify each of the following as a physical or a chemical change. Give reasons.
- Drying of a shirt in the sun.
- Rising of hot air over a radiator.
- Burning of kerosene in a lantern.
- Change the colour of black tea by adding lemon juice to it.
- Churning of milk cream to get butter.
Answer:
- Physical change Because the evaporation of water takes place, but no change occurs in the composition of the substance.
- Physical change It involves ing only the movement of with it, no change in the composition of air.
- Physical as well as chemical change Physical change occurs when kerosene vapourises. After that, the burning of kerosene is a chemical change because, during burning, kerosene oil gets converted into carbon dioxide and water both of which are new compounds.
- Chemical change The acid present in lemon juice will react with the constituent (for example., caffeine) present in black tea.
- Physical change As there is no change in composition. Only the separation of components takes place by the physical phenomenon of centrifugation. However, butter will not change to milk easily.
Question 6. Iron filings and sulphur were mixed and divided into two parts, A and B. Part A was heated strongly while part B was not heated. Dilute hydrochloric acid was added to both the parts and the evolution of gas was seen in both cases. How will you identify the gases that evolved?
Answer:
⇒ \(\underbrace{\mathrm{Fe}+\mathrm{S}}_{\text {Part } A} \stackrel{\Delta}{\longrightarrow} \mathrm{FeS}\)
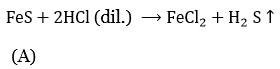
Part B is not heated, so the reaction will be as such
⇒ \(\underset{\text { (In Part B) }}{\mathrm{Fe}}+2 \mathrm{HCl} \text { (dil.) } \longrightarrow \mathrm{FeCl}_2+\mathrm{H}_2 \uparrow\)
In part A, H2S gas is produced, which is identified by its characteristic smell of rotten eggs. In part B, H2 gas is produced.
Hydrogen gas is tested by bringing a burning matchstick near the mouth of the test tube. It burns with a pop sound and water is formed.
Question 7. Rama tested the solubility of four substances at different temperatures and found a gram of each substance dissolved in 100 g of water to form a saturated solution.
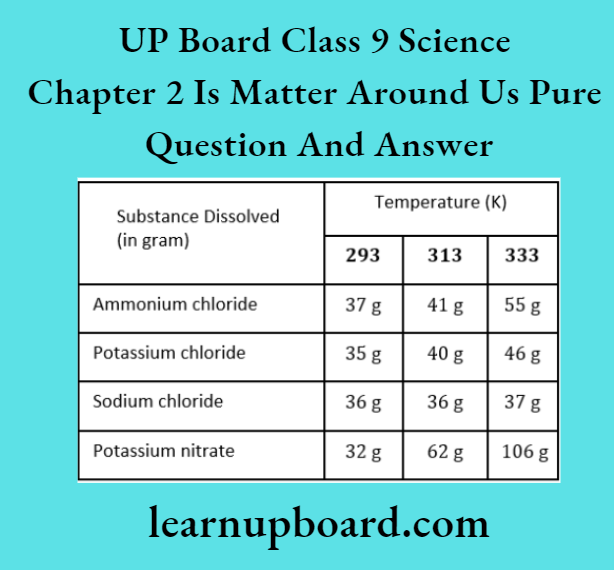
- Which solution is least soluble at 293 K?
- Which substance shows a maximum change in its solubility when the temperature is raised from 293 K to 313 K?
- Find the amount of ammonium chloride that will separate when 55 g of its solution at 333 K is cooled to 293 K.
- What is the effect of temperature on the solubility of a salt?
- What mass of sodium chloride would be needed to make a saturated solution in 10 g of water at 293 K?
Answer:
- Potassium nitrate.
- Potassium nitrate.
- At 333 K, ammonium chloride dissolved per 100 g = 55g.
- At 293 K, ammonium chloride dissolved per 100 g = 37g
- When a solution is cooled from 333 K to 293 K, the amount of ammonium chloride that will separate =55 -37= 18g
- The solubility of a salt (sold) increases with the rise in temperature and vice-versa.
- At 293K, the amount of NaCl dissolved in 100 g of water = 36 g,
∴ At 293 K, the amount of NaCl dissolved in 10 g water = \(\frac{36}{100} \times 10=3.6 \mathrm{~g}\)
Question 8. During an experiment, the students were asked to prepare a 10% (mass/mass) solution of sugar in water. Ramesh dissolved 10 g of sugar in 100 g of water while Sarika prepared it by dissolving 10 g of sugar in water to make 100 g of the solution.
- Are the two solutions of the same concentration?
- Compare the mass % of the two solutions.
Answer:
- No.
- Rameshs solution concentration
Mass % = \(\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
⇒ \(\frac{10 \mathrm{~g}}{(10+100) \mathrm{g}} \times 100\)
⇒ \(\frac{10}{110} \times 100\)
⇒ \(\frac{100}{11}=9.09 \%\)
⇒ \(9.1 \%\)
Sarika s solution concentration
⇒ \(\text { Mass } \%\)
⇒ \(\frac{10}{100} \times 100=10 \%\)
The solution prepared by Ramesh has less percentage by mass than that of Sarika.
Question 9.
- Under which category of mixtures will you classify alloys and why?
- Whether a solution is always liquid or not. Comment.
- Can a solution be heterogeneous?
Answer:
- Alloys are homogeneous mixtures of metals, or non-metals because
- It shows the properties of its constituents, and
- It has variable composition, for example., brass is considered a mixture because it shows the properties of its constituents, copper and zinc; and it has a variable composition.
- A solution is generally a liquid, not always, for example., alloys are known to be solid solutions.
- The term solution is generally used for ‘true solution. In this case, the solution is always homogeneous.
- In the case of a colloidal solution, that is not a true solution, the solution is heterogeneous.
Question 10. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 g of water to form a saturated solution).
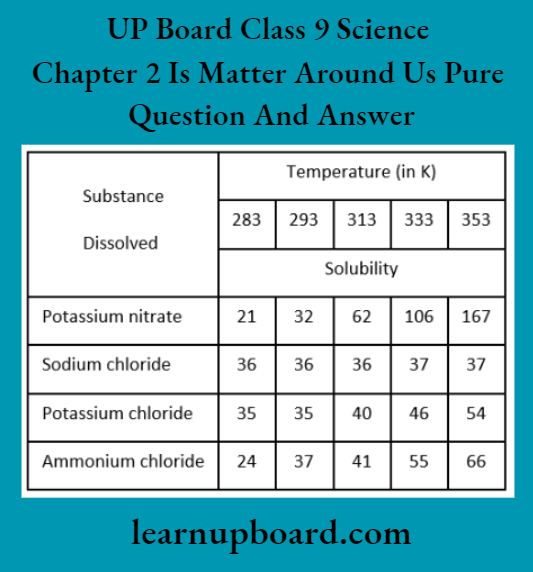
- What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 g of water at 313 K?
- Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
- Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
- What is the effect of a change in temperature on the solubility of a salt?
Answer:
1. At 313 K, solubility of potassium nitrate in 100 g of water = 62
Solubility = \(\frac{\text { Mass of solute }}{\text { Mass of solvent }} \times 100\)
Mass or solute = \(\frac{\text { Solubility } \times \text { Mass of solvent }}{100}\)
∴ Mass of potassium nitrate = \(\frac{62 \times 50}{100}=31 \mathrm{~g}\)
Hence, 31g of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 30 g of water at 313 K.
2. The amount of potassium chloride that should be dissolved in water to make a saturated solution increases with temperature.
Thus, as the solution cools, some of the potassium chloride will precipitate out of the solution and form crystals.
3. The solubility of the salts at 293 K are:
Potassium nitrate 32; Sodium chloride 36, Potassium chloride 33; Ammonium chloride 37 Thus, ammonium chloride has the maximum solubility (37) at 293 K.
4. The solubility of a solid (salt) decreases with a fall in temperature, while it increases with a temperature rise.
Class 9 Science Chapter 2 Is Matter Around Us Pure Short Answer Type Questions
Question 1. An element is sonorous and highly ductile. Under which category would you classify this element? What other characteristics do you expect the element to possess?
Answer:
This element is categorised as a ‘metal’ because metals are sonorous and ductile. Other properties or characteristics possessed by this element are expected to be the following:
- They possess metallic lustre.
- They are good conductors of heat and electricity.
- They are malleable.
- They have high tensile strength.
- They have high densities and high melting points/boiling points too.
Question 2. Classify the following as elements, compounds and mixtures.
- Gold
- Marble
- Air
- Milk
- Sugar
Answer:
- Element – Gold
- Compound – Marble and sugar
- Mixture – Air and milk
Question 3. How will you prove that water is a compound?
Answer:
Water is considered to be a compound due to the following reasons:
- Hydrogen and oxygen are present in a fixed ratio of 1:8 by mass in water.
- Water has a fixed boiling point, i.e. 100°C at 1 atmospheric pressure.
- The constituents of water (H and O) cannot be separated from it by simple physical methods.
- The properties of water are entirely different from those of its constituents, i.e. hydrogen and oxygen.
Question 4. Sucrose (sugar) crystals obtained from sugarcane and beetroot are mixed. Will it be a pure substance or a mixture? Give reasons for the same.
Answer:
It is a pure substance because the chemical composition of sugar crystals is the same irrespective of its source.
Question 5. Define a solution. Give an example of
- Gas in liquid solution.
- Gas in gas solution.
Answer:
Solution Definition:
The solution is a homogeneous mixture of two or more substances, for example., salt in water.
- CO2 dissolved in water
- Air
Question 6. What types of mixtures are represented by the following?
- Carbon dioxide gas dissolved in water.
- Air containing suspended particles.
- Soap bubbles formed by blowing air into the soap solution.
- Water in milk.
Answer:
- Homogeneous
- Heterogeneous
- Heterogeneous
- Heterogeneous
Question 7. When egg albumin is added to water the clear solution becomes turbid. How would you test to confirm that it is a colloidal solution?
Answer:
Filter the contents of test tubes. No residue is left on the filter paper, but the filtrate obtained is translucent. Since a colloid cannot be separated by filtration, it is a colloid.
Question 8. Give some examples of the Tyndall effect observed in your surroundings.
Answer:
Examples of the Tyndall effect
- Sunlight passes through the canopy of a dense forest.
- A fine beam of light enters a room through a small hole.
Question 9. Why does the sky appear blue?
Answer:
So The blue colour of the sky is due to the scattering of light by fine dust particles (i.e. Tyndall effect) which are present in the atmosphere.
Question 10. Give reasons:
- The path of a beam of light is not visible through a true solution.
- Particles of suspension can be seen with the naked eye.
Answer:
- The path of a beam of light is not visible through a true solution because particles of a true solution are not large enough to scatter light.
- The particle size of the suspension is greater than 100 nm. So, they can be seen with the naked eye.
Question 11. On dissolving chalk powder in water, a suspension is obtained. Give any four reasons to support the fact that the mixture so obtained is a suspension only.
Answer:
So It is supported by the following reasons:
- White particles of chalk powder can be seen with the naked eye.
- The particles can be separated by ordinary filter paper.
- Upon shaking, white turbidity reappears in the solution.
- Light can be passed through this solution, which suggests that it shows the Tyndall effect.
Question 12. Smoke and fog both are aerosols. In what way are they different?
Answer:
In both smoke and fog, the dispersion medium is the same, i.e. gas, but they differ in the dispersed phase. In smoke, the dispersed phase is solid while in fog, the dispersed phase is liquid.
Question 13. How do sol and gel differ from each other? Give one example for each.
Answer:
- The sol is a colloid in which tiny solid particles are dispersed in a liquid medium. Examples of sol are ink and soap solution.
- The gel is a semi-solid colloid in which liquid particles are dispersed in a solid. Examples of gel are jellies and gelatin.
Question 14. Classify the following as physical or chemical properties.
Answer:
- The composition of a sample of steel is 98% iron, 1.5% carbon and 0.5% other elements.
- Zinc dissolves in hydrochloric acid with the evolution of hydrogen gas.
- Metallic sodium is soft enough to be cut with a knife.
- Most metal oxides form alkalis on interacting with water.
Answer:
- Physical
- Chemical
- Physical
- Chemical
Question 15. On heating, calcium carbonate gets converted into calcium oxide and carbon dioxide.
- Is this a physical or a chemical change?
- Can you prepare one acidic or one basic solution by using the products formed in the above process? If so, write the chemical equation involved.
Answer:
The phenomenon given in question is a chemical change because the composition of the product formed is different from the substance taken.
The reaction involved is \(\mathrm{CaCO}_3→{\Delta} \mathrm{CaO}+\mathrm{CO}_2\)
Yes, 1. \(\mathrm{CaO}+\mathrm{H}_2 \mathrm{O} \longrightarrow \underset{\text { Calcium hydroxide } \\\text { (Basic solution) }}{\mathrm{Ca}(\mathrm{OH})_2}\)
2. \(\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O} \longrightarrow \underset{\text { Carbonic acid } \\ \text { (Acidic solution) }}{\mathrm{H}_2 \mathrm{CO}_3}\)
Question 16. A child eats chocolate and digests it. In doing so, some physical and chemical changes take place. Identify the changes.
Answer:
Physical change Breaking of chocolate into small pieces.
Chemical change Digestion of chocolate.
Question 17. Explain why particles of colloidal solution do not settle down when left undisturbed, while in the case of a suspension, they do.
Answer:
Colloidal particles are smaller in size. The force of gravity which is acting on colloidal particles is encountered and they do not settle down. However the particles of suspension are larger, so they settle down under the influence of gravity.
Question 18. A solution made by dissolving 50 g of glucose in 250 g of water. Calculate the concentration of this solution in mass percentage.
Answer:
Mass percentage \(=\frac{\text { Mass of glucose }}{\text { Mass of glucose }+ \text { Mass of water }} \times 100\)
⇒ \(\frac{50}{50+250} \times 100\)
⇒ \(\frac{50}{300} \times 100=\frac{50}{3}=16.66 \%\)
Question 19. What volume of ethyl alcohol and water must be mixed to prepare 250 mL of 60% by volume of alcohol in water?
Answer:
Let the volume of ethyl alcohol be x mL
Concentration of solution
⇒ \(\frac{\text { Volume of solvent (ethyl alcohol) }}{\text { Volume of solution }} \times 100\)
⇒ \(60=\frac{x}{250} \times 100\)
⇒ \(x=\frac{250 \times 60}{100}=150 \mathrm{~mL}\)
Question 20. Calculate the mass of potassium sulphate required to prepare its 10 per cent (mass per cent) solution in 100 g of water.
Answer:
∴ \(\text { Mass } \%=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
Let the mass of potassium sulphate (solute) = xg
Then, mass of solution = (100 + x) g
⇒ Mass %=10= \(\frac{x}{(x+100)} \times 100\)
⇒ \(1=\frac{x \times 10}{(x+100)} \Rightarrow x+100=10 x\)
⇒ 9x = 100
∴ \(x=\frac{100}{9}=11.1 \mathrm{~g}\)
Question 21. Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non-sonorous, non-malleable and are coloured.
- Name a lustrous non-metal.
- The allotropic form of a non-metal is a good conductor of electricity. Name the allotrope.
- Name a non-metal which is known to form the largest number of compounds.
- Name a non-metal other than carbon which shows allotropy
- Name a non-metal which is required for combustion.
- Name a non-metal that forms common salt with sodium.
Answer:
- Iodine
- Graphite (Carbon)
- Carbon
- Phosphorus
- Oxygen
- Chlorine
Question 22. Classify the substances given below into elements and compounds.

Answer:
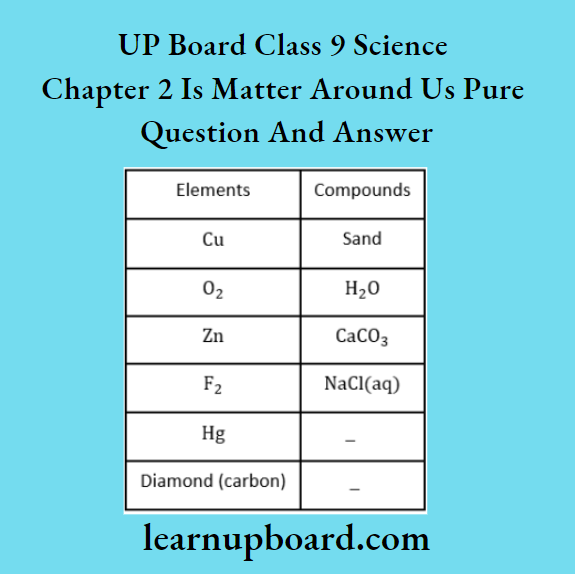
Question 23. Which of the following are not compounds?
- Chlorine gas
- Potassium
- Iron
- Iron sulphide
- Aluminium
- Iodine
- Carbon
- Carbon monoxide
Answer:
- Chlorine gas
- Potassium
- Iron
- Aluminium
- Iodine
- Carbon
Substances mentioned above are the elements, not compounds. Cl2 gas is a molecular element.
Question 24. Classify the following mixtures as homogeneous and heterogeneous.
- Tincture of iodine
- Smoke
- Brass
- Sugar solution
Answer:
- Homogeneous
- Heterogeneous
- Homogeneous
- Homogeneous
Question 25. Tell whether each of the following properties describes a homogeneous mixture, a solution, a heterogeneous mixture, a compound or an element.
- A homogeneous liquid which leaves a solid residue on boiling.
- A cloudy liquid which after some time appears more cloudy towards the bottom.
- A colourless liquid which boils at a definite temperature and can be decomposed into simpler substances.
Answer:
- A solution (a solid like salt dissolved in water from which water evaporates on boiling, leaving a solid residue, salt).
- A heterogeneous mixture in which suspended particles start settling down at the bottom (muddy water).
- A compound.
Question 26. What would you observe when
- A saturated solution of potassium chloride prepared at 60°C is allowed to cool to room temperature.
- A mixture of iron filings and sulphur powder is heated strongly.
Answer:
- Crystals of potassium chloride are formed because solubility decreases with a decrease in temperature.
- The compound iron sulphide is formed.
Answer:
Question 27. Why copper sulphate solution in water does not show the Tyndall effect, but a mixture of water and milk shows?
Answer:
- The solution of copper sulphate in water is a true solution. In a true solution, the solute particles are so small that they cannot scatter light falling on them.
- Hence, copper sulphate solution in water does not show the Tyndall effect.
- A mixture of water and milk is a colloid and in a colloidal solution, the particles are big enough to scatter light. So, the mixture of water and milk shows the Tyndall effect.
Question 28. Determine whether each of the following changes is physical or chemical. Give a reason for your answer.
- A balloon filled with hydrogen gas explodes upon contact with a spark.
- Copper turns green on exposure to air and water.
Answer:
- Chemical change Hydrogen gas burns to form water.
- Chemical change Copper combines with oxygen from air and water to form a copper oxide which has a green colour.
Question 29. Can physical and chemical changes occur together? Illustrate your answer.
Answer:
- In some cases, physical and chemical changes occur together. One such example is the burning of candles. The solid wax present in the candle first changes into a liquid state and then into a vapour state.
- Both these changes are physical. The wax vapours then combine with oxygen in the air to form a mixture of carbon dioxide and water. This involves a chemical change.
- The unburnt wax vapours again change first to the liquid state and finally to the solid state. This interconversion of states is a physical change. Thus, the burning of a candle involves both physical and chemical changes.
Question 30. Calculate the mass of sodium sulphate required to prepare its 20% (mass per cent) solution in 100 g of water.
Answer:
Mass % of sodium sulphate solution = 20%
Mass of the solvent = 100g
Let the mass of solute (sodium sulphate) = xg
Applying the formula,
⇒ \(\text { Mass } \%=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
⇒ \(20=\frac{x \mathrm{~g}}{(x+100) \mathrm{g}} \times 100\)
⇒ 20 (x+ 100) =100x
⇒ 20x + 2000 =100 xH2x
⇒ 100x – 20x = 2000
⇒ 80x = 2000
∴ \(x=\frac{2000}{80}=25 \mathrm{~g}\)
Mass of sodium sulphate = 25 g
Question 31. 110 g of salt is present in 550 g of solution. Calculate the mass percentage of the solution.
Answer:
Given
110 g of salt is present in 550 g of solution.
Mass percentage of solution
⇒ \(=\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
Mass of solute = 110 g [given]
Mass of solution = 550 g [given]
Mass percentage = \(\frac{110}{550} \times 100\)
= 20 %
Question 32. List the points of difference between homogeneous and heterogeneous mixtures. Or Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:
The main points of differences between the homogeneous and heterogeneous mixtures are as follows:

Question 33. To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Mass of solute (NaCl) =36 g
Mass of solvent (H2O) = 100 g
Mass of solution = 36 +100=136 g
Concentration (by mass) of the solution
⇒ \(\frac{\text { Mass of solute }}{\text { Mass of solution }} \times 100\)
⇒ \(\frac{36}{136} \times 100\)
= 26.47%
Question 36. Classify the following as chemical or physical changes:
- Cutting of trees,
- Melting of butter in a pan,
- Rusting of almirah,
- Boiling of water to form steam,
- The passing of electric current through water and the water breaking down into hydrogen and oxygen gases,
- Dissolving common salt in water,
- Making a fruit salad with raw fruits, and
- Burning of paper and wood.
Answer:
Physical changes
- Cutting of trees,
- Melting of butter in a pan, b
- Oiling of water to form steam,
- Dissolving common salt in water, and
- Making a fruit salad with raw fruits
Chemical changes
- Rusting of almirah
- The passing of electric current through water and breaking down of water into hydrogen and oxygen gases.
- Burning of paper and wood.
Question 35. Classify each of the following as a homogeneous or heterogeneous mixture. Soda water, wood, air, soil, vinegar, filtered tea.
Answer:
Homogeneous mixtures of Air, soda water, vinegar, and filtered tea.
Heterogeneous mixtures of Wood, and soil.
Note: Homogeneous mixtures have the same composition throughout, while the composition of heterogeneous mixtures is not uniform.
Question 36. How would you confirm that a colourless liquid given to you is pure water?
Answer:
- Take a given colourless liquid in a beaker and suspend a thermometer into it. Place the beaker on a wire gauze and start heating with the help of a burner.
- Note down the temperature at which water begins to boil. If the given liquid boils at 100°C, the liquid will be pure water as the boiling point of pure water is 100°C.
Question 37. Which of the following materials fall in the category of pure substance?
- Ice
- Milk
- Iron
- Hydrochloric acid
- Calcium oxide
- Mercury
- Brick
- Wood
- Air
Answer:
Ice, iron, calcium oxide and mercury are pure substances as they have definite composition. Hydrochloric acid is a mixture of hydrogen chloride gas and water, so it is a mixture and not a pure substance.
Question 38. Identify the solutions among the following mixtures.
- Soil
- Seawater
- Air
- Coal
- Soda water
Answer:
Sea water, air and soda water, as these are homogeneous mixtures of two or more substances.
Note: Sea water is also considered a heterogeneous solution.
Question 39. Which of the following will show the Tyndall effect?
- Salt solution
- Milk
- Copper sulphate solution
- Starch solution
Answer:
Milk and starch solution being a colloid will show the Tyndall effect, while salt solution and copper sulphate solution are true solutions that will not show the Tyndall effect due to the small size of their particles.
Question 40. Classify the following into elements, compounds and mixtures.
- Sodium
- Soil
- Sugar solution
- Silver
- Calcium carbonate
- Tin
- Silicon
- Coal
- Air
- Soap
- Methane
- Carbon dioxide
- Blood
Answer:
Elements Sodium, silver, tin and silicon.
Compounds Calcium carbonate, methane, carbon dioxide and soap.
Mixtures Soil, sugar solution, coal, air, blood and soap. Soap is a compound, but to make it suitable for specific purposes, various substances are added to it. Then, it becomes a mixture.
Question 41. Which of the following are chemical changes?
- Growth of a plant
- Rusting of iron
- Mixing of iron filings and sand
- Cooking of food
- Digestion of food
- Freezing of water
- Burning of a candle
Answer:
GrowtlTof a plant, rusting of iron, cooking of food, digestion of food and burning of a candle are chemical changes.
Question 42. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:
Method Of Preparation Of Tea
- Take some water (solvent) in a pan and heat it.
- Add some sugar (solute) and boil to dissolve the sugar completely, the obtained homogeneous mixture is called a solution.
- Add tea leaves (or tea) to the solution and boil the mixture.
- Now add milk and boil again.
- Filter the mixture through the tea stainer and collect the filtrate or soluble substances, i.e. tea in a cup. The insoluble tea leaves are left behind as residue in the strainer.
Class 9 Science Chapter 2 Is Matter Around Us Pure Very Short Answer Type Questions
Question 1. State any one difference between pure and impure substances.
Answer:
The pure substance contains only one kind of particle. All the elements and compounds are pure substances. An impure substance contains two or more different kinds of particles. All the mixtures are impure substances.
Question 2. Identify homogeneous mixtures from the following. Soda water, soil, vinegar, unfiltered tea.
Answer: Soda water and vinegar.
Question 3. Is ice water a homogeneous or heterogeneous substance? Is it a pure or impure substance?
Answer:
Ice water is a heterogeneous, but pure substance as ice is made up of water only, which contains only one kind of particle.
Question 4. The ‘sea water’ can be classified as a homogeneous as well as a heterogeneous mixture. Comment.
Answer:
Sea water is called a homogeneous mixture as it contains dissolved salts in it. It may be called a heterogeneous mixture as it contains various insoluble components too such as sand, microbes, shells made up of calcium carbonate and so many other things.
Question 5. Is fresh air free of dust particles and impurities of all other kinds, a pure substance?
Answer:
No, only elements and compounds are pure substances. Air is a mixture of gases and so, not a pure substance.
Question 6. Give one test to show that brass is a mixture and not a compound.
Answer:
The melting point of brass is not definite. So, it is not a compound but it is an alloy (mixture) of copper and zinc.
Question 7. Tincture of iodine has antiseptic properties. How is it prepared?
Answer: A tincture of iodine is prepared by dissolving iodine in alcohol.
Question 8. What are the two components of a solution?
Answer:
The two components of a solution are:
- Solute
- Solvent
Question 9. What is meant by the concentration of a solution?
Answer:
The amount of solute present in a given amount (mass or volume) of solution (or solvent) is known as the concentration of solution.
Question 10. Based on which factor, a solution is said to be dilute, concentrated or saturated?
Answer: Amount of solute present in it.
Question 11. Why particles in a true solution cannot be seen with the naked eye?
Answer: Particles of a true solution are very small in size (less than 1nm), hence they are not visible.
Question 12. The particle size of a substance in water is 200 nm. What is the nature of the solution?
Answer:
As the particle size of a substance is greater than 100 nm, the solution will be a suspension.
Question 13. A student mixes the white of an egg with water and stirs it well. After some time, what did he observe?
Answer: He observed that a cloudy solution is formed.
Question 14. Do suspensions show the property of the Tyndall effect?
Answer:
Yes, suspensions show the property of the Tyndall effect because their particles are too large and scatter the light.
Question 15. State which of the following solutions exhibits the Tyndall effect. Starch solution, sodium chloride solution, tincture of iodine, smoke.
Answer: Starch solution and smoke.
Question 16. ‘Tyndall effect can be observed when sunlight passes through the canopy of dense forest’. Explain, how this occurs.
Answer:
This is because the forest contains mist which in turn contains tiny droplets of water that act as particles of colloid dispersed in air.
Question 17. What are the favourable qualities given to gold when it is alloyed with copper or silver to make ornaments?
Answer:
When alloyed with copper or silver, the gold becomes harder and stronger and its brittleness decreases and becomes suitable for making ornaments.
Question 18. Which of the tubes in Figures (1) and (2) will be more effective as a condenser in the distillation apparatus?

Answer:
Condenser (1) will be more effective in the distillation apparatus because the beads present will provide more surface area for cooling and condensation of the vapour occurs repeatedly by passing through it.
Question 19. How will you justify that the rusting of iron is a chemical change?
Answer:
In a chemical change, a new substance is formed and rust is different from iron. Iron is an element while rust is hydrated oxide of iron (Fe2O3 xH2O). Thus, the formation of rust from iron is a chemical change.
Question 20. Choose the chemical change out of the following. Digestion of food, freezing of water, glowing of electric lamp and a mixture of iron filings with sulphur.
Answer: Digestion of food.
Question 21. What is meant by a substance?
Answer:
Substance
A substance which is made up of a single type of particle is called a pure substance, For example., hydrogen, oxygen, sulphur, etc.
Question 22. Try segregating the things around you as pure substances or mixtures.
Answer:
Pure substance- Sugar, common salt, rubber. Mixture- Wood, coal, milk, soap, soil. The soap that we use in our daily lives is a mixture.
